Beneficial autoimmunity improves cancer prognosis
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
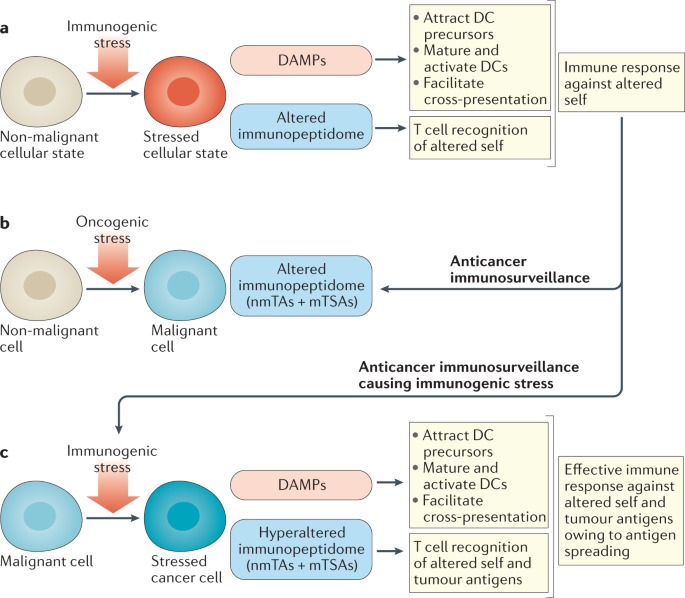
Many tumour antigens that do not arise from cancer cell-specific mutations are targets of humoral and cellular immunity despite their expression on non-malignant cells. Thus, in addition to
the expected ability to detect mutations and stress-associated shifts in the immunoproteome and immunopeptidome (the sum of MHC class I-bound peptides) unique to malignant cells, the immune
system also recognizes antigens expressed in non-malignant cells, which can result in autoimmune reactions against non-malignant cells from the tissue of origin. These autoimmune
manifestations include, among others, vitiligo, thyroiditis and paraneoplastic syndromes, concurrent with melanoma, thyroid cancer and non-small-cell lung cancer, respectively. Importantly,
despite the undesirable effects of these symptoms, such events can have prognostic value and correlate with favourable disease outcomes, suggesting ‘beneficial autoimmunity’. Similarly, the
occurrence of dermal and endocrine autoimmune adverse events in patients receiving immune-checkpoint inhibitors can have a positive predictive value for therapeutic outcomes. Neoplasias
derived from stem cells deemed ‘not essential’ for survival (such as melanocytes, thyroid cells and most cells in sex-specific organs) have a particularly good prognosis, perhaps because the
host can tolerate autoimmune reactions that destroy tumour cells at some cost to non-malignant tissues. In this Perspective, we discuss examples of spontaneous as well as therapy-induced
autoimmunity that correlate with favourable disease outcomes and make a strong case in favour of this ‘beneficial autoimmunity’ being important not only in patients with advanced-stage
disease but also in cancer immunosurveillance.
We thank V. Carbonnier (Centre de Recherche des Cordeliers, Paris, France) for help in preparing Fig. 2. L.Z. and G.K. receive support from the Agence National de la Recherche (ANR) –
Projets blancs; AMMICa US23/CNRS UMS3655; Association pour la recherche sur le cancer (ARC); Association “Ruban Rose”; Cancéropôle Ile-de-France; Chancelerie des universités de Paris (Legs
Poix), Fondation pour la Recherche Médicale (FRM); a donation by Elior; European Research Area Network on Cardiovascular Diseases (ERA-CVD, MINOTAUR); Gustave Roussy Odyssea, the European
Union Horizon 2020 Project Oncobiome; Fondation Carrefour; High-end Foreign Expert Program in China (GDW20171100085); Inserm (HTE); Institut National du Cancer (INCa); Institut Universitaire
de France; LeDucq Foundation; the LabEx Immuno-Oncology (ANR-18-IDEX-0001); Ligue contre le Cancer (équipe labellisée); RHU Torino Lumière; Seerave Foundation; SIRIC Stratified Oncology
Cell DNA Repair and Tumour Immune Elimination (SOCRATE); and SIRIC Cancer Research and Personalized Medicine (CARPEM). This study contributes to the IdEx Université de Paris
ANR-18-IDEX-0001. C.P. receives support from the Canadian Cancer Society (#705604) and the Canadian Institutes of Health Research (FDN 148400). O.J.F. receives support from the University of
Pittsburgh Medical Center (UPMC) Immune Transplant and Therapy Center (ITTC) and the US National Cancer Institute (grant 5R35CA210039).
Gustave Roussy Comprehensive Cancer Institute, Villejuif, France
Université Paris Saclay, Faculty of Medicine, Le Kremlin-Bicêtre, France
Equipe labellisée par la Ligue contre le cancer, Villejuif, France
Center of Clinical Investigations in Biotherapies of Cancer (CICBT) BIOTHERIS, Villejuif, France
Suzhou Institute for Systems Medicine, Chinese Academy of Medical Sciences, Suzhou, China
Institute for Research in Immunology and Cancer, Université de Montréal, Montréal, QC, Canada
Department of Immunology, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA
Equipe labellisée par la Ligue contre le cancer, Université de Paris, Sorbonne Université, INSERM U1138, Centre de Recherche des Cordeliers, Institut Universitaire de France, Paris, France
Metabolomics and Cell Biology Platforms, Institut Gustave Roussy, Villejuif, France
Pôle de Biologie, Hôpital Européen Georges Pompidou, AP-HP, Paris, France
Karolinska Institute, Department of Women’s and Children’s Health, Karolinska University Hospital, Stockholm, Sweden
G.K. researched data for the manuscript, L.Z. and G.K. wrote it with the input of C.P. and O.J.F., and all authors edited and reviewed the manuscript before submission.
L.Z. and G.K. are founders of EverImmune. G.K. is a founder of Samsara Therapeutics and Therafast Bio. O.J.F. is a consultant for Biovelocita, GeoVax, Immodulon, Iaso Therapeutics and PDS
Biotech. C.P. declares no competing interests.
Nature Reviews Clinical Oncology thanks S. Karagiannis, R. Liblau, H-G. Rammensee and J. Yewdell for their contribution to the peer review of this work.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Expansion of the immune response to secondary epitopes that were not part of the initial recognition process.
(DAMPs). Protein exposed on the surface of stressed cells or metabolite or protein released from stressed or dying cells that alerts immune effectors.
Self-molecules that undergo changes in levels of expression and post-translational modifications in cells infected with pathogens, subjected to acute inflammation or during malignant
transformation.
Sum of peptides bound to MHC class I molecules that can be recognized by T cell receptors.
(irAEs). Undesired adverse events of immune-checkpoint inhibitors caused by excessive activation of autoimmune responses.
Myasthenia-like condition in which an autoimmune response to the neuromuscular junctions causes progressive muscle weakness. Half of the diagnoses occur in patients with small-cell lung
cancer.
(mTECs). Specialized cells that present self-antigens to induce clonal deletion of self-specific T lymphocytes, thus assuring central tolerance.
Specialized cells found in the basal skin layer that produce melanin and transfer this pigment in the form of melanosomes into the cytoplasm of adjacent keratinocytes.
Compendium of clinical signs provoked by a cancer at a distant location, generally as a result of autoimmune responses.
Close-to-irreversible blockade of the cell cycle coupled to a major loss of cell function that occurs in response to injury or as a result of aging.
Antigens that can be found on non-malignant cells but whose (over)expression on the surface of cancer cells enables the immune system to distinguish such cells from their non-transformed
counterparts.
Antigens that are uniquely present within or on a cancer cell. They can arise via both mutational (mutated tumour-specific antigens) and non-mutational (non-mutated tumour-specific antigens)
mechanisms.
Also known as leukoderma. White patches of the skin caused by the autoimmune destruction of melanocytes.
Anyone you share the following link with will be able to read this content: