Caudate and hippocampal volumes, intelligence, and motor impairment in 7-year-old children who were born preterm
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Children who survive very preterm birth without major disability have a high prevalence of learning difficulty, attention deficit, and minor motor impairment (MMI). To determine
whether these difficulties are associated with structural brain abnormalities, we studied 105 preterm children (<32 wk) at 7 y with tests of IQ and MMI (Movement ABC) and detailed
magnetic resonance brain scans. Scans were assessed qualitatively for visible cerebral lesions. Volume measurements of the caudate nuclei and hippocampal formations were made. Total brain
volume (TBV) was estimated from the head circumference. Qualitative assessment of the scans showed evidence of cerebral lesions in 20 (19%), which were associated with lower IQ and more
frequent MMI. IQ correlated with right and left caudate volume (Spearman's ρ 0.304 and 0.349; _p_ < 0.01). This association persisted (except for verbal IQ) when caudate volume was
expressed as a percentage of estimated TBV to allow for overall brain size. No significant correlations with hippocampal volumes were observed. These differences persisted when only scans
from children without visible lesions on scan were considered. MMI was significantly associated only with TBV and was more common in children with evidence of thinning of the posterior
corpus callosum, although most children with MMI have a normal corpus callosum. Lower IQs in children who were born preterm are related to poorer development of the caudate relative to the
rest of the brain, independent of other lesions. These findings suggest abnormal brain development after perinatal injury or postnatal nutritional deficits is responsible for cognitive
deficits in preterm children. SIMILAR CONTENT BEING VIEWED BY OTHERS CORPUS CALLOSUM LONG-TERM BIOMETRY IN VERY PRETERM CHILDREN RELATED TO COGNITIVE AND MOTOR OUTCOMES Article Open access
15 January 2024 LANGUAGE PERFORMANCE AND BRAIN VOLUMES, ASYMMETRY, AND CORTICAL THICKNESS IN CHILDREN BORN EXTREMELY PRETERM Article Open access 03 November 2023 ASSOCIATION BETWEEN CORTICAL
THICKNESS AND COGNITIVE ABILITY IN VERY PRETERM SCHOOL-AGE CHILDREN Article Open access 29 January 2024 MAIN Ten to 15% of children who were born in the United Kingdom at very low birth
weight (<1500 g) will have major physical impairments that usually require special educational provision, but the majority will enter mainstream school (1). Studies of these children have
shown that as many as 40% may show learning difficulties, often associated with problems of visuospatial perception, minor motor impairments (MMIs), and behavioral difficulties (2, 3). The
origin of these difficulties has been attributed to a delay in neurologic maturation, early cerebral injury, social factors, or poor early growth (4). Magnetic resonance imaging (MRI) has
proved to be a useful tool for the investigation of anomalies of cerebral maturation in a minimally invasive way. Very low birth weight (VLBW) infants show a relatively high prevalence of
MRI-defined cerebral lesions later in childhood (5). Most of these represent degrees of periventricular leukomalacia or more diffuse white matter atrophy. Although evidence of extensive
white matter damage correlates well with major neurologic impairment, there seems to be little if any correlation between signs of lesser damage and intellectual, motor, and behavioral
problems at school age (5). With the introduction of volumetric methods to MRI during the past decade, correlations have been noted between the volumes of parts of the brain and school
performance in children. Associations between the volumes of the caudate nuclei and the hippocampus and learning difficulties and attention deficit have been demonstrated in school-age
children who were born at term (6, 7). Results in children who were born very preterm have been conflicting and have described differences in cerebellar, basal ganglia, total brain, and gray
matter volumes (8–12). This study was designed to test the hypothesis that children who were born very preterm and have cognitive deficits and associated MMIs at school age would show
different caudate and hippocampal volumes from those who were doing well. METHODS As part of a study of school performance of all very preterm children who were born to mothers who resided
in a geographically defined area of Merseyside, UK, during a 2-y period, parents were asked whether they would allow their child to have a cerebral MRI scan (13). The study was approved by
the Local Children's Research Ethics Committee, and written informed parental consent was obtained. All children who were born in 1991 and 1992, whose mothers resided in Liverpool
postal districts, and who were born at <32 completed weeks of gestation were included in the study. Those who were known to be alive at discharge from any of the eight hospitals that
serve the area were traced through their general practitioner to confirm their current health status before their parents were approached. Children who were attending mainstream schools,
with or without special provision, and whose parents agreed to their being studied were matched by sex, age, and first language to a full-term classmate control. Depending on parental and
school wishes, the children were tested at 7–8 y of age, at school or at the Institute of Child Health, Royal Liverpool Children's Hospital. Intelligence was tested using the short form
of the Wechsler Intelligence Scales for Children Version III (14). Motor impairments were tested for using the Movement ABC (15). Measurements of height, weight, and head circumference and
a standardized neurologic examination were done. These tests were performed by an occupational therapist and a graduate psychologist who were used as research assistants for the project. A
parental questionnaire collected demographic data, and a perinatal data sheet was completed from the clinical case notes by a clerical assistant. Cranial ultrasound results were as reported
by the local consultant radiologists. A score below the fifth percentile on the Movement ABC was considered indicative of MMI. MRI scans were performed on a subset of the subjects in the
main study whose parents consented to scanning in addition to the other studies. Payment of travel expenses only was made. Magnetic resonance brain scans were performed on a Philips Gyroscan
NT5 scanner with a field strength of 0.5 Tesla (Philips Medical Systems, Best, Netherlands). The scans consisted of five sequences: sagittal T1 weighted spin-echo, axial T1-weighted
spin-echo, axial T2-weighted turbo spin-echo, coronal fluid-attenuated inversion recovery, and a T1-weighted fast field echo volume acquisition. The fast-field echo sequence (TR = 200 ms, TE
= 30 ms, TI = 13 ms, flip angle = 30 degrees) was used to produce a coronal volume acquisition with a slice thickness of 2 mm and a 1-mm overlap. From this sequence, caudate and hippocampal
volumes were measured on a Easivision workstation (Philips Medical Systems). For reducing scan times during volume acquisition only, the scans did not always include the entire brain, so
total brain volume (TBV) could not be measured from MRI. Instead, an estimate of TBV was calculated using the head circumference measured at the time of testing. We used a published formula
that relates brain size at postmortem examination to occipitofrontal head circumference (OFC) (16). This cannot allow for differences in extracerebral space or ventricular volume but is an
approximation. Caudate and hippocampal volumes could then be expressed as a percentage of estimated TBV to allow for differences in brain size. QUALITATIVE ASSESSMENT OF MAGNETIC RESONANCE
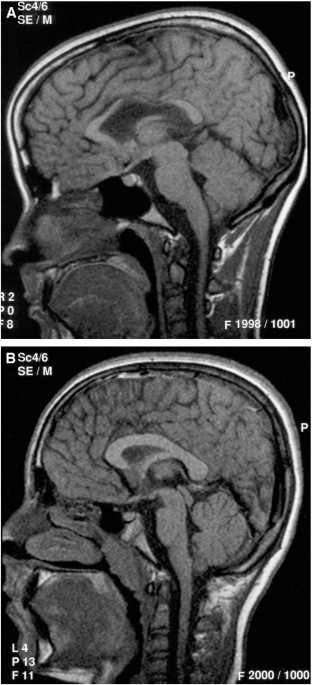
BRAIN SCANS. Scans were systematically assessed for visible lesions consistent with thinning of the corpus callosum (Fig. 1), ventricular enlargement (Fig. 2), periventricular leukomalacia
(Fig. 3), and porencephaly (Fig. 4) by an experienced pediatric radiologist (L.A.). Periventricular leukomalacia was recognized by a characteristic triad of abnormalities: abnormally high
signal within the periventricular white matter on T2-weighted turbo spin-echo and fluid-attenuated inversion recovery images; loss of periventricular white matter, particularly in the
periatrial regions; and compensatory focal ventricular enlargement adjacent to regions of abnormal signal intensity (17). Porencephaly was recognized as a well-defined cavity within the
brain substance, communicating with the lateral ventricle and containing fluid with identical signal characteristics to cerebrospinal fluid (CSF). QUANTITATIVE ANALYSIS OF MAGNETIC RESONANCE
BRAIN SCANS. Hippocampal volumes were measured using the technique previously described by Jack _et al._ (18, 19). The anterior and posterior boundaries were defined, including the whole of
the head of the hippocampus anteriorly and using the crus of the fornix as the posterior anatomic landmark. The in-plane boundaries were then traced sequentially on each slice. The head of
the hippocampus was separated from the amygdala by the uncal recess of the temporal horn of the lateral ventricle; in cases in which the uncal recess was not patent, an arbitrary horizontal
line was drawn through the most superomedial portion of the temporal horn. The boundaries of the hippocampal body and tail were defined laterally by CSF in the temporal horn of the lateral
ventricle, superiorly by CSF in the choroid fissure, medially by CSF in the uncal and ambient cistern, and inferiorly by the interface between the gray matter of the subiculum and the white
matter of the parahippocampal gyrus (Fig. 5). These anatomic landmarks have been shown to define reliably 90–95% of the total hippocampal volume (20). Volumes of the caudate nuclei were
measured using a similar technique. The boundaries of the caudate nucleus were defined anteriorly and laterally by the interface with the white matter of the frontal lobe and the anterior
limb of the internal capsule, and medially by CSF in the lateral ventricle (Fig. 6). The posterior boundary was arbitrarily defined by the most anterior coronal section to show the crus of
the fornix, as used to define the posterior boundary of the hippocampus. The slender tail of the caudate nucleus, which extends around the posterior part of the lateral ventricle, was not
measured. Once boundaries had been drawn on each coronal image, volumes were calculated automatically by the workstation using a volume of interest function. The ratio of the volumes of the
structures (left:right) was calculated as a percentage. The reliability of this technique was assessed as follows. Volume measurements on a convenience sample of 25 scans were made
independently by two observers. Intraobserver and interobserver variation was calculated by the Bland-Altman method (21). For volume measurements of the hippocampal formations, 95%
confidence intervals of agreement (intraobserver) were between −161 and 165 mm3. For interobserver variation, 95% confidence intervals of agreement were between −145 and 163 mm3. These
results (<±7%) are comparable with those in previous studies (18, 20) that have indicated that MRI-based measurements of these brain structures can be made with a high degree of
reproducibility. All volume measurements were made by one of the authors (L.A.), who was blind to the clinical assessments. Statistical analysis of the data were performed using SPSS version
10. RESULTS A total of 382 children had been discharged from the eight hospitals that serve the area during the period covered by the study, and 335 were alive and attending mainstream
school at 7 y. A total of 286 parents permitted their children to be tested at school, and 126 parents initially consented to their children's undergoing MRI scans; 15 later changed
their minds or failed to attend, three were unable to cooperate and satisfactory scans were not obtained, scan data were lost for two children because of a technical failure, and in one
child artifact from fixed dental braces prevented accurate volume measurement. A total of 105 children had satisfactory scans. No sedation was used. Scans were completed within 6 mo of
testing at school. Mean age at testing was 7 y 4 mo. The perinatal characteristics of children who were scanned are shown in Table 1. The characteristics of the children who were scanned and
not scanned at age 7 y are similar, although children who were scanned had a significantly smaller head circumference and higher performance IQ (PIQ; Table 2). Of the 105 preterm children
scanned, 20 (19%) showed a cerebral lesion on MRI. Lesions consistent with periventricular leukomalacia were present in 14 (12%), any ventricular dilation in 14 (11%), thinning of the
posterior part of the corpus callosum in eight (9%), and with porencephaly in two (2%) (Table 3). Several children had more than one lesion type. Mean and median values for IQ and ABC scores
are given in Table 2. Mean estimated TBV was 1,225,000 (138,000) mm3. TBV was correlated with ABC (_r_ = −0.233, _p_ = 0.02) but not when children with visible MRI abnormalities were
excluded. When only children with “normal” scans were considered, TBV was correlated with PIQ (_r_ = 0.252, _p_ = 0.029). Mean (SD) right and left caudate volumes were 4633 (807) and 5425
(789) mm3, and right and left hippocampal volumes were 2906 (490) and 2813 (429) mm3, respectively. Median (interquartile range) right and left caudate volumes as a percentage of TBV were
3.8 (3.4–4.1) and 3.7 (3.2–4.1) and for the right and left hippocampus were 2.4 (2.1–2.6) and 2.3 (2.0–2.6). Table 4 shows the mean values for volumes in children with IQs above and below
80, and Table 5 shows the mean values in children with ABC scores above and below the fifth percentile. Table 6 shows the correlations between the volume data and ABC and IQ scores for all
children, and Table 7 shows the correlations for only children who did not have a visible lesion on MRI scan. Significant correlations between IQ scores and caudate volumes are seen, which
largely persist when children without visible lesions alone are considered. Significant correlations for caudate volumes as a percentage of TBV persist only for PIQ. No significant
correlations between hippocampal volumes and ABC or IQ scores were observed. This was also true when hippocampal volumes were correlated with subsets of the IQ test used. DISCUSSION In our
previous work (5), we described the results of MRI brain scans in a cohort of adolescents (age 15–17 y) who had a history of VLBW (<1500 g) but no major disability and were born between
1980 and 1983. There was a high prevalence (42.5%) of structural brain abnormalities, although these abnormalities showed poor correlation with IQ and attention-deficit/hyperactivity
disorder (12). Similar results have been found in other studies. Skranes _et al._ (8) found no correlation between MRI abnormality and IQ in VLBW children without disability at 6 y of age,
and Krageloh-Mann _et al._ (9) found no difference in performance in intelligence tests in very preterm children, with and without MRI abnormalities. Stewart _et al._ (10) and Rushe _et al._
(11) reported the results of MRI brain scans and neuropsychological outcome at age 14–15 y of a cohort of 75 participants who were born at <33 wk gestation; 55% of this group had
structural brain abnormalities demonstrated on MRI brain scans, but those with abnormal MRI brain scans did not perform less well on any measure of cognitive function. There was also a lack
of correlation of gestational age and birth weight with neuropsychological outcome but a significant correlation between abnormal MRI scans and behavioral problems. In the group of VLBW
children that we studied at age 15–17 y, we found positive associations between full-scale IQ and caudate volumes, and attention-deficit/hyperactivity disorder and hippocampal volumes (12).
The subjects of our present study were born in 1991 and 1992. The selection of subjects was geography and gestation based rather than hospital and birth weight based. The prevalence of
structural brain abnormalities was 18.5%, much lower than in our previous study. The reason for this difference is uncertain. Recruitment criteria for the two groups were different, and
improvements in neonatal care may have had an effect on the prevalence of perinatal brain injury. Table 1 shows the perinatal characteristics of the present cohort, which shows that most
were not particularly ill in the neonatal period, but they are more likely to represent the population as a whole than a hospital-based cohort from a referral center. In both studies, there
was self-selection, in that the children and their parents had to consent to be scanned. Table 2 suggests that those who were scanned were broadly similar at age 7 y to the rest, although
those who were scanned had significantly smaller OFC and higher PIQ. In this cohort, volumes of the right and left caudate nuclei were significantly correlated with IQ but not with ABC
scores (Tables 4–6). There was no significant correlation between IQ and ABC scores and hippocampal volume measurements. The relationship between IQ and caudate volume could simply represent
the well-known correlation between brain size and IQ. When caudate volumes were expressed as a percentage of estimated TBV, the correlations remained significant except for that between
verbal IQ and right caudate volume percentage, suggesting that this was not the case (Table 6). When only the 85 children with apparently normal MRI scans were examined, the correlations
were weaker yet remained significant for most comparisons (Table 7). Volumes as a percentage of TBV remained significantly correlated only with PIQ (Table 7). Isaacs _et al._ (22) reported
the results of a study of 11 adolescents with a history of prematurity (<30 wk gestation). They had significantly smaller hippocampal volumes bilaterally and showed specific defects in
certain aspects of everyday memory. Regression analyses indicated that hippocampal volume was the best predictor of performance in tests of everyday memory. Some of the subscales of the IQ
test that we used involve aspects of short-term memory, but we were unable to show a correlation between them and hippocampal size. The hippocampal volumes measured in our present cohort
were not smaller than would be expected for an age of 7 y. Peterson _et al._ (23) studied a group of 25 preterm children at 8 y of age with quantitative MRI scans and found that regional
cortical volumes and basal ganglia and hippocampal volumes were smaller in preterm children than in control subjects. When corrected for TBV, basal ganglia and hippocampal volumes seemed to
be disproportionately reduced. In this group, full-scale IQ seemed to correlate most strongly with regional cortical volume, although some association with basal ganglia volumes was
demonstrated. This is in keeping with our findings in this cohort. The selection criteria for this group differed from our present study in that children were selected on the basis of birth
weight (<1250 g), and children with MMI were not excluded. Our results suggest that the final volume attained by key cerebral structures such as the caudate nuclei are important
determinants of cognitive function in preterm children. We were not able to study many other regions of the brain volumetrically, although recent studies in children who were born preterm
have highlighted volume differences in the cerebellum (24) and in total and gray matter volumes that have correlated with later cognitive function. Any of these differences may represent
evidence for disordered development of the preterm brain after perinatal injury, rather than a focal lesion affecting cognitive function. The hippocampus and basal ganglia are particularly
susceptible to injury in preterm infants; hippocampal abnormalities were found in 67% of autopsies of preterm infants (25). Hypoxia, metabolic disorders, nutritional impairment, and
infections all may affect the developing brain. The hippocampus is known to be selectively vulnerable to hypoxic-ischemic injury (26), hypoglycemia (27), and hypothyroidism (28). Lucas _et
al._ (29) showed that IQ in preterm children at age 7–8 y showed significant differences depending on nutritional intervention shortly after birth. Brain growth is most rapid around the time
of term in human infants, and the period between birth and term is one of relative malnutrition for many preterm infants. Nutritional impairment of these vulnerable infants at this time may
be of particular importance in limiting the development of key cerebral structures. Neonatal interventions to counteract this might lead to improved function later in this group of infants.
ABBREVIATIONS * CSF: cerebrospinal fluid * MMI: minor motor impairment * MRI: magnetic resonance imaging * PIQ: performance IQ * TBV: total brain volume * VLBW: very low birth weight
REFERENCES * Cooke RW 1993 Annual audit of 3-year outcome in very low birth weight infants. _Arch Dis Child_ 69: 295–298. Article CAS Google Scholar * Botting N, Powls A, Cooke RW, Marlow
N 1998 Cognitive and educational outcome of very low birth weight children in early adolescence. _Dev Med Child Neurol_ 40: 661–666. Google Scholar * Botting N, Powls A, Cooke RW, Marlow N
1997 Attention deficit hyperactivity disorders and other psychiatric outcomes in very low birth weight children at 12 years. _J Child Psychol Psychiatry_ 38: 931–941. Article CAS Google
Scholar * Powls A, Botting N, Cooke RW, Marlow N 1996 Growth impairment in very low birth weight children at 12 years: correlation with perinatal and outcome variables. _Arch Dis Child_
75:F152–F157. Article CAS Google Scholar * Cooke RW, Abernethy LJ 1999 Cranial magnetic resonance imaging and school performance in very low birth weight infants in adolescence. _Arch Dis
Child_ 82:F116–F121. Article Google Scholar * Castellanos FX, Geidd JN, Eckburg P, Marsh WL, Vaituzis AC, Kaysen D, Hamburger SD, Rapoport JL 1994 Quantitative morphology of the caudate
nucleus in attention deficit hyperactivity disorder. _Am J Psychiatry_ 151: 1791–1796. Article CAS Google Scholar * Hynd GW, Semrud-Clikeman M, Lorys AR, Novey ES, Elopulos D 1990 Brain
morphometry in developmental dyslexia and attention deficit hyperactivity disorder. _Arch Neurol_ 47: 919–926. Article CAS Google Scholar * Skranes JS, Vik T, Nilsen G, Smevik O,
Andersson HW, Brubakk AM 1997 Cerebral magnetic resonance imaging and mental and motor function of very low birth weight children at six years of age. _Neuropediatrics_ 28: 149–154. Article
CAS Google Scholar * Krageloh-Mann I, Toft P, Lunding J, Andresen J, Pryds O, Lou HC 1999 Brain lesions in preterms: origin, consequences and compensation. _Acta Paediatr_ 88: 897–908.
Article CAS Google Scholar * Stewart AL, Rifkin L, Amess PN, Kirkbride V, Townsend JP, Miller DH, Lewis SW, Kingsley DP, Moseley IF, Foster O, Murray RM 1999 Brain structure and
neurocognitive and behavioural function in adolescents who were born preterm. _Lancet_ 353: 1653–1657. Article CAS Google Scholar * Rushe TM, Rifkin L, Stewart AL, Townsend JP, Roth SC,
Murray RM 2001 Neuropsychological outcome at adolescence of very preterm birth and its relation to brain structure. _Dev Med Child Neurol_ 43: 226–233. Article CAS Google Scholar *
Abernethy LJ, Palaniappan M, Cooke RWI 2000 Quantitative magnetic resonance imaging of the brain in survivors of very low birth weight. _Arch Dis Child_ 87: 279–283. Article Google Scholar
* Foulder-Hughes L, Cooke RW 2003 Motor, cognitive and behavioural disorders in children born very preterm. _Dev Med Child Neurol_ 45: 97–103. Article CAS Google Scholar * Wechsler D
1992 _Wechsler Intelligence Scale for Children, 3rd Ed_. The Psychological Corporation, Harcourt Brace and Co, London Google Scholar * Henderson SE, Sugden DA 1992 Movement ABC. The
Psychological Corporation. Harcourt Brace and Co, London * Cooke RW, Lucas A, Yudkin PL, Pryse-Davies J 1977 Head circumference as an index of brain weight in the fetus and newborn. _Early
Hum Dev_ 1: 145–149. Article CAS Google Scholar * Baker LL, Stevenson DK, Enzmann DR 1988 End-stage periventricular leukomalacia: MR evaluation. _Radiology_ 168: 809–815. Article CAS
Google Scholar * Jack CR, Bentley MD, Twomey CK, Zinsmeister AR 1990 MR imaging-based volume measurements of the hippocampal formation and anterior temporal lobe; validation studies.
_Radiology_ 176: 205–209. Article Google Scholar * Jack CR 1994 MRI based hippocampal volume measurements in epilepsy. _Epilepsia_ 35:S21–S29. Article Google Scholar * Watson C,
Andermann F, Gloor P, Jones-Gotman M, Peters T, Evans A, Olivier A, Melanson D, Leroux G 1992 Anatomic basis of amygdaloid and hippocampal volume measurement by magnetic resonance imaging.
_Neurology_ 42: 1743–1750. Article CAS Google Scholar * Bland JM, Altman DG 1986 Statistical methods for assessing agreement between two methods of clinical measurement. _Lancet_ 1:
307–310. Article CAS Google Scholar * Isaacs EB, Lucas A, Chong WK, Wood SJ, Johnson CL, Marshall C, Vargha-Khadem F, Gadian DG 2000 Hippocampal volume and everyday memory in children of
very low birth weight. _Pediatr Res_ 47: 713–720. Article CAS Google Scholar * Peterson BS, Vohr B, Staib LH, Cannistraci BA, Dolberg A, Schneider KC, Katz KH, Westerveld M, Sparrow S,
Anderson AW, Duncan CC, Makuch RW, Gore JC, Ment LR 2000 Regional brain volume abnormalities and long-term cognitive outcome in preterm infants. _JAMA_ 284: 1939–1947. Article CAS Google
Scholar * Allin M, Matsumoto H, Santhouse AM, Nosarti C, Al Asady MH, Stewart AL, Rifkin L, Murray RM 2001 Cognitive and motor function and the size of the cerebellum in adolescents born
very preterm. _Brain_ 124: 60–66. Article CAS Google Scholar * Fuller PW, Guthrie D, Alvord EC 1983 A proposed neuropathological basis for learning disabilities in children born
prematurely. _Dev Med Child Neurol_ 25: 214–231. Article CAS Google Scholar * Schmidt-Kastner R, Freund TF 1991 Selective vulnerability of the hippocampus in brain ischaemia.
_Neuroscience_ 40: 599–636. Article CAS Google Scholar * Fujoka M, Okuchi K, Hiramatsu KI, Salaki T, Sakaguchi S, Ishil Y 1997 Specific changes in the human brain after hypoglycaemic
injury. _Stroke_ 28: 584–587. Article Google Scholar * Madiera MD, Sousa N, Lima-Andrade MT, Calheiros F, Cadate-Leite A, Paula-Barbosa MM 1992 Selective vulnerability of the hippocampal
pyramidal neurons to hypothyroidism in male and female rats. _J Comp Neurol_ 322: 501–518. Article Google Scholar * Lucas A, Morley R, Cole TJ 1998 Randomised trial of early diet in
preterm babies and later intelligence quotient. _BMJ_ 317: 1481–1487. Article CAS Google Scholar Download references ACKNOWLEDGEMENTS We thank Mrs. G. Hughes and Mrs. D. Garlick, Mrs. J.
Glynn, and Mrs. H. Tyrer for their skill and patience in performing the MRI scans; Mr. David Campling from Philips Medical Systems and Dr. Peter Cole from IRS for technical advice; Dr. M.
Palaniappan for assistance with MRI measurements; and Dr. Gillian Lancaster, Lecturer in Medical Statistics at the University of Liverpool, for statistical advice. Dr. Lynda Foulder-Hughes
and Dr. Lisa Thompson tested the children at school. Special thanks are due to the parents and children who gave their time to help in this study. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS
* Department of Medical Imaging, Royal Liverpool Children's Hospital, Eaton Road, Liverpool, L12 2AP, United Kingdom Laurence J Abernethy * Department of Child Health, University of
Liverpool, Institute of Child Health, Royal Liverpool Children's Hospital (Alder Hey), Liverpool, L12 2AP, UK Richard W I Cooke & Lynda Foulder-Hughes Authors * Laurence J Abernethy
View author publications You can also search for this author inPubMed Google Scholar * Richard W I Cooke View author publications You can also search for this author inPubMed Google Scholar
* Lynda Foulder-Hughes View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Laurence J Abernethy. RIGHTS AND
PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Abernethy, L., Cooke, R. & Foulder-Hughes, L. Caudate and Hippocampal Volumes, Intelligence, and Motor
Impairment in 7-Year-Old Children Who Were Born Preterm. _Pediatr Res_ 55, 884–893 (2004). https://doi.org/10.1203/01.PDR.0000117843.21534.49 Download citation * Received: 17 July 2002 *
Accepted: 21 August 2003 * Issue Date: 01 May 2004 * DOI: https://doi.org/10.1203/01.PDR.0000117843.21534.49 SHARE THIS ARTICLE Anyone you share the following link with will be able to read
this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative