A crispr/cas9 functional screen identifies rare tumor suppressors
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT An enormous amount of tumor sequencing data has been generated through large scale sequencing efforts. The functional consequences of the majority of mutations identified by such
projects remain an open, unexplored question. This problem is particularly complicated in the case of rare mutations where frequency of occurrence alone or prediction of functional
consequences are insufficient to distinguish driver from passenger or bystander mutations. We combine genome editing technology with a powerful mouse cancer model to uncover previously
unsuspected rare oncogenic mutations in Burkitt’s lymphoma. We identify two candidate tumor suppressors whose loss cooperate with MYC over-expression to accelerate lymphomagenesis. Our
results highlight the utility of _in vivo_ CRISPR/Cas9 screens combined with powerful mouse models to identify and validate rare oncogenic modifier events from tumor mutational data. SIMILAR
CONTENT BEING VIEWED BY OTHERS INTEGRATED CROSS-STUDY DATASETS OF GENETIC DEPENDENCIES IN CANCER Article Open access 12 March 2021 A SYSTEMATIC GENOME-WIDE MAPPING OF ONCOGENIC MUTATION
SELECTION DURING CRISPR-CAS9 GENOME EDITING Article Open access 11 November 2021 COPY NUMBER LOSSES OF ONCOGENES AND GAINS OF TUMOR SUPPRESSOR GENES GENERATE COMMON DRIVER MUTATIONS Article
Open access 20 July 2024 INTRODUCTION The International Cancer Genome Consortium is a colossal tumor sequencing endeavor that has profiled over 10,000 tumors and uncovered ~10 million
mutations1. Mutation frequency, predicted functional impact, and pan-cancer analysis of mutated networks are powerful approaches by which to identify oncogenic drivers from this data in
order to support diagnostic and therapeutic efforts2,3,4,5,6. However, cancers exhibit extensive mutational heterogeneity and in many cases it appears that only a few frequently mutated
genes (among all tumor-associated mutations) are significant for initiation and progression. Indeed, the vast proportion of gene mutations within a tumor are thought to represent “passenger”
or “bystander” mutations. However, it is unclear whether among these rarer events reside infrequent oncogenic drivers and this currently constitutes an obstacle to a full understanding of
tumor biology. Burkitt’s lymphoma (BL) is a common B-cell lymphoma, predominantly arising in children, which is characterized by the hallmark Burkitt translocation t(8;14)(q24;q32) or its
variants t(2;8) and t(8:22) - all of which juxtapose the MYC oncogene with one of three immunoglobulin loci7. Recent whole genome, exome, and transcriptome sequencing data from 104 sporadic
BL patient samples and BL cell lines has defined the mutational landscape in this cancer8,9,10. Among these studies, Schmitz _et al_.8 undertook RNA sequencing of 28 sporadic BL samples and
13 cell lines and identified >5000 mutations, Love _et al_.9 identified 70 recurrently mutated genes from exome sequencing of 51 primary BL tumors and 8 BL cell lines and Richter _et
al_.10 sequenced four Burkitt’s lymphomas and identified 119 genes with potentially protein-altering mutations. Within this rich source of BL mutational data lie known oncogenic drivers
alongside a large number of infrequently mutated genes, leading to a characteristic “long tail” phenomenon when analyzing gene mutation counts in tumors (see below). The significance of this
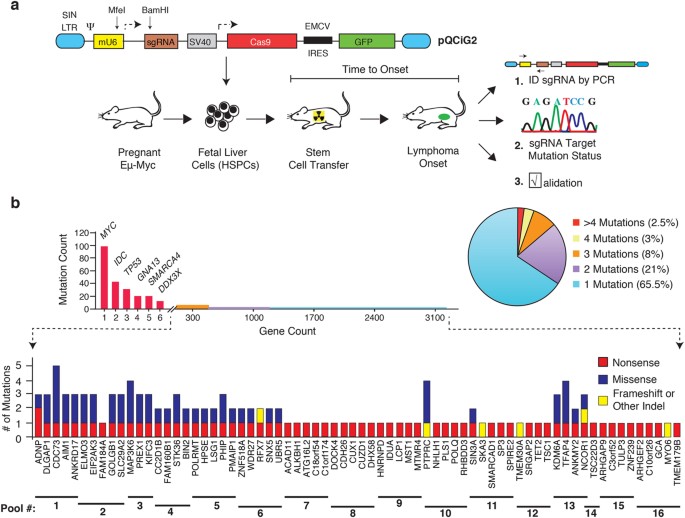
latter class of mutations in BL remains unknown and it is here that functional assays have much to offer. RESULTS COUPLING CRISPR/CAS9 AND THE E_Μ_-MYC MODEL TO IDENTIFY RARE MODIFIERS OF
TUMOR FORMATION IN BL To functionally screen for rare modifiers of tumor formation from BL sequencing data, we took advantage of an adoptive transfer strategy utilizing the E_μ_-Myc
genetically engineered mouse model (GEMM) (Fig. 1a). This GEMM is modeled after the defining Burkitt’s translocation and recapitulates typical genetic and pathological features of human
non-Hodgkin’s lymphomas11,12. It has been extremely useful for unraveling oncogene cooperation, defining pathway addictions, and elucidating drug response/genotype relationships _in vivo_ in
cancer13. From the large number of rarely mutated genes in BL, we focused on genes that had incurred nonsense or frameshift mutations and thus could easily be disrupted using CRISPR/Cas9
(Fig. 1b, Supplementary Figure 1a, and Supplementary Table S1)8,9,10. Perusal of the human BL mutation data identified 91 genes fulfilling this criteria, although in many cases, additional
missense mutations were noted in independent BL samples (Fig. 1b and Supplementary Table S1). Our screen focused on genes not known to be modifiers in this cancer type and that had not been
previously characterized in BL. A few well characterized tumor suppressors were retained (e.g. _Tsc1, TP53_) and served as positive controls in our assay14,15. In total, 75 sgRNAs targeting
the murine orthologs of genes infrequently mutated in BL were generated (Supplementary Table S1). We designed the sgRNAs to target their murine counterpart in the vicinity of the nonsense or
indel mutation that had been documented in the human BL data. Testing of 9 randomly chosen sgRNAs indicated that all displayed significant editing activity, as assessed by the T7EI cleavage
assay (Supplementary Figure 1b). One of the parameters that we wished to define before undertaking an _in vivo_ screen in the E_μ_-Myc model was to elucidate the sgRNA pool complexity that
would enable identification of “hits” following reconstitution of hematopoietic stem and progenitor cells (HSPCs) in transplanted recipients (Fig. 1a). To this end, we used a well
characterized p53-targeting sgRNA, sgp53-1 and an sgRNA targeting the neutral Rosa26 locus as positive and negative controls, respectively16,17. All sgRNAs were co-expressed with Cas9 from a
second generation “All-in-One” retroviral vector that also produced green fluorescent protein (GFP), enabling tracking of infected cells by flow cytometry (Fig. 1a)18. E_μ_-Myc HSPCs
transduced with undiluted sgp53-1, or with a 1:5 dilution of sgp53-1 in sgRosa26, produced tumors in recipients by ~25 days with complete penetrance (Supplementary Figure 2a). Mice receiving
HSPCs with sgp53-1 diluted 1:20 or 1:100 developed tumors with a slightly slower onset rate and with incomplete penetrance. In contrast, E_μ_-Myc HSPCs transduced with undiluted sgRosa26
produced tumors with a median onset rate of ~80 days (Supplementary Figure 2a). These results indicate that a functional sgRNA targeting a tumor suppressor gene could be reproducibly
enriched from pools containing 5 different sgRNAs. _IN VIVO_ SCREENING IDENTIFIES CANDIDATE SGRNAS CAPABLE OF PROMOTING LYMPHOMAGENESIS Based on the results of our dilution experiments, we
screened our candidate genes in pools maximally containing five sgRNAs (Fig. 1b and Supplementary Table S1). This yielded a total of 16 pools that were used to transduce at least three
independent HSPC populations and transplanted into five irradiated recipients. Four of the pools showed significantly increased tumor onset rates compared to mice having received HSPCs
infected with pQCiG2/sgRosa26 (Fig. 2a; p < 0.0001, (Log-Rank Mantel-Cox Test)). None of the recipients receiving HSPCs infected with the other pools developed lymphomas at rates that
were significantly different than those obtained with pQCiG2/sgRosa26 (Supplementary Figure 2b). Despite the presence of a GFP reporter within our transduction vector, we noted that not all
recovered tumors were GFP+, which we attributed to the absence of selective pressure to maintain expression from pQCiG2 following locus modification. To identify the tumor-promoting sgRNAs
in tumors arising from sgRNA pools 5, 11, 12, and 13, we isolated genomic DNA from all lymphomas, amplified the sgRNA encoding sequences by PCR, and sequenced the amplified products. Two of
five tumors from Pool 5 yielded PCR products that, when sequenced, revealed the presence of sgRNAs targeting only _Phip_ (data not shown). T7EI analysis of the _Phip_ locus in tumors
revealed the presence of mutations at the _Phip_ locus in those same two tumors (Fig. 2b, Top panel: T1 and T5). We have not further characterized the three remaining tumors (T2, T3, T4) to
determine the underlying oncogenic event since we failed to retrieve PCR products from these tumors and cannot exclude that these tumors arose independently of CRISPR-induced mutagenesis.
From Pool 11, 5/5 tumors revealed the presence of only _Sp3_-targeting sgRNA and T7EI analysis of these 5 tumors confirmed modification at the endogenous _Sp3_ locus (Fig. 2b, Middle panel).
All tumors from Pool 12 harbored an sgRNA targeting _Tsc1_ and indeed all 5 tumors showed evidence of mutagenesis at this locus (Fig. 2b, bottom panel). _Tsc1_, as well as _Tsc2_, has been
previously shown to accelerate lymphomagenesis in the E_μ_-Myc model and was not further pursued15,19. All tumors from Pool 13 revealed the presence of an sgRNA targeting _Tfap4_ (data not
shown). _IN VIVO_ VALIDATION OF CANDIDATE TUMOR SUPPRESSORS We undertook to validate these results by repeating the HSPC adoptive transfer experiment using only the original sgRNA as well as
a second independent, non-overlapping sgRNA (Figs 3 and 4 and Supplementary Table S2). For _Sp3_ and _Phip_, both sgRNAs lead to increased lymphoma onset compared to the Rosa26 cohort (Figs
3b and 4b). For _Tfap4_, we were able to recapitulate accelerated tumorigenesis with the original sgRNA, but not with a second independent sgRNA (data not shown) and thus did not further
pursue _Tfap4_ characterization. Sequencing of cloned amplicons obtained from PCR amplification across the sgRNA targeted loci for _Sp3_ and _Phip_ from tumors obtained in the validation
experiment revealed indel mutations (Supplementary Figures 3 and 4). We also noted considerable sequence heterogeneity at the _Sp3_ or _Phip_ loci within any given lymphoma indicating that
the tumors that arose were polyclonal in nature and thus unlikely to be due to rare integration events that inactivated a tumor suppressor locus. Western blot analysis of tumors obtained
generated by CRISPR/Cas9 targeting of _Sp3_ and _Phip_ indicated significant reductions in levels of both proteins in all tumors analyzed (Figs 3c and 4c). We attribute the small levels of
residual protein to normal cells contaminating the tumor samples. SP3 AND PHIP DISPLAY TUMOR SUPPRESSIVE ACTIVITY _IN VIVO_ Both SP3 and PHIP have been reported to exhibit pro-oncogenic
activity in some contexts (see Discussion) and yet, in the E_μ_-Myc model we clearly identified these as tumor suppressors. Therefore, to strengthen our results and also exclude the
possibility that modification of the _Sp3_ or _Phip_ loci by CRISPR/Cas9 had led to the generation of gain-of-function truncation mutants, we targeted _Sp3_ and _Phip_ for knockdown using
two independently generated shRNAs. Our rationale was that if loss of function was truly responsible for tumor initiation in our Cas9-based experiments (Figs 3b and 4b), we should be able to
phenocopy this using shRNAs to reduce gene activity. In both cases, using two independent shRNAs, we observed significantly accelerated tumor onset as compared to a neutral control shRNA
targeting renilla luciferase (Figs 3d and 4d). As expected, the resulting tumors showed significant reductions in target protein expression levels (Figs 3e and 4e). As well, ectopic
expression of a PHIP C-terminal truncation mutant, lacking the same functional regions as predicted from the human mutation identified in BL, did not lead to accelerated tumorigenesis
following infection of E_μ_-Myc HSPCs (Supplementary Figure 5). In sum, our results demonstrate that both _Sp3_ and _Phip_ behave as tumor suppressors in E_μ_-Myc driven lymphomas.
DISCUSSION Our results provide a framework for identifying functionally relevant rare mutations in human tumor sequencing data. Our approach is complementary to bioinformatics initiatives
that score for mutation frequency and predicted gene function to identify modifiers of tumor formation among mutational data. Interestingly, perusal of the Catalogue of Somatic Mutations in
Cancer (COSMIC) database revealed that _PHIP_ and _SP3_ are found mutated in other human cancers, including a small fraction of hematopoietic and lymphoid cancers (Supplementary Figure 6).
Both pro-survival and pro-apoptotic properties have been attributed to _Sp3_, making it impossible to predict its roles in tumor initiation simply based on perusal of the literature. Sp3 has
been identified as a component of the transcriptional regulatory network activated by MYC overexpression20. As well, both MYC and SP3 are involved in upregulation of the telomerase
catalytic subunit, TERT21 a process whose dysregulation is known to drive B-cell leukemia22. SP family members SP1/3/4 have been implicated as non-oncogenic addiction events in pancreatic
cancer xenograft experiments23. Yet, reduced SP3 expression is associated with decreased apoptosis-related caspase activity24 and decreased oncogenicity25,26,27 whereas elevated levels in
LS174 modified colon carcinoma cells leads to increased apoptosis and prevents tumor formation in nude mice28. Similar conflicting data exists for _Phip._ Increased _Phip_ copy number is
correlated with ulceration in melanoma29 and increased PHIP expression is linked to increased likelihood of metastasis and poor prognosis in melanoma patients; whereas knockdown of _Phip_ in
mouse models prolongs survival and has been reported to protect against metastasis30. In the E_μ_-Myc model, PHIP and SP3 demonstrated clear _in vivo_ tumor suppressor activity (Figs 3 and
4). This may reflect context-dependency of tumor-suppressor activity as exemplified by eIF5A, which acts as a promoter of oncogenesis in a liver cancer model31, but when knocked-down in the
Eμ-Myc system, acts as a potent tumor suppressor32. The possibility of context-dependent effects of PHIP and SP3 mutation is also supported by data summarized by the cBioPortal for cancer
genomics (http://www.cbioportal.org/) where 27.6% of cases in a breast cancer xenograft model show increased PHIP copy number, while 14.6% of DLBCL cases reported by the Cancer Genome Atlas
(TCGA) have deletions of PHIP33,34 Although less frequently reported as being altered in cancers than PHIP, differences in copy number variation of SP3 are also seen depending on the tissue
examined, with a majority of alterations in prostate samples reported by TCGA as deletions, while ovarian and breast cancers more frequently display amplifications33,34. CRISPR/Cas9 in
concert with available deep-sequencing data and an appropriate GEMM is thus a powerful approach to identify context-dependent lesions. CRISPR/Cas9 is well-suited for the type of _in vivo_
loss-of-function screens undertaken herein, but we note that our screen was not exhaustive. Expanding the number of sgRNAs used per gene, increasing the animal cohort size, and technological
improvements should improve the discovery rate and throughput of the screen. Related to the latter point - an obvious limitation of this screen is the fairly low complexity of sgRNA
screening pools used herein as compared to previously published shRNA screens32. One limitation is the large size of the pQCiG2 sgRNA/Cas9 retroviral delivery vector (~8 kbp) which leads to
reduced viral titers and subsequent lower infection efficiencies35. Hence the incorporation of Cas9 alleles into cancer GEMMs36 will allow the use of smaller sgRNA delivery vectors with
higher viral titers and hence, transduction efficiencies. Although BL and the E_μ_-Myc model share the same initiating genetic lesion (i.e. a translocation leading to elevated MYC
expression), the etiology of the murine and human diseases differ. As the translocation is present in the germline in the E_μ_-Myc model, transformation arises in pro- and pre-B cells in the
bone marrow at a time when the E_μ_ enhancer becomes activated and begins driving MYC expression. Human BL however arises as a consequence of a _Myc_ translocation occurring in more mature
B cells present in lymph node germinal centers. This may be a limitation of the E_μ_-Myc model and we may be under-estimating the number of oncogenic lesions in BL if any of these are B
cell-stage specific. Nonetheless the E_μ_-Myc model has proven itself as an excellent genetic system for identifying lesions that co-operate with MYC _in vivo_14. This model has correctly
reported on the ability of _p53_ suppression37 or _Tsc1 and Tsc2_ loss15 to accelerate tumorigenesis - genes mutated in human BL. Given that the Tsc1/2 complex regulates mTORC1 activity, and
that mTORC1 is a drug target for treatment of B-cell lymphomas38, these results highlight the potential of our approach for identifying therapeutic targets. In the era of personalized
medicine, the identification of rare alleles that restrict tumorigenesis has important therapeutic implications. If some of the identified genes are pro-oncogenic in certain settings and
tumor suppressive in others, defining context is critical to supporting correct clinical drug development. Also, understanding downstream networks perturbed by loss of PHIP or SP3 could lead
to identification of new therapeutic targets. Rare mutational events may dilute the response to therapeutics targeting the more frequent mutational events and a better understanding of
these events will enable better clinical stratification. If loss of PHIP or SP3 is also required for tumor maintenance, then this would support the rationale for biotherapeutic development,
such as approaches aiming to systemically deliver wild-type protein39. The priority placed on developing tailored therapeutics to rare mutations that can drive tumorigenesis will ultimate be
determined by their overall relevance to tumor biology. METHODS RETROVIRAL INFECTIONS, STEM CELL ISOLATION, AND ADOPTIVE TRANSFER Low passage Phoenix-Eco viral packaging cells were cultured
in complete DMEM (10% FBS, 1% Penicillin-Streptomycin, 1% L-Glutamine) at 37 °C in 5% CO2. Twenty-four hours prior to transfection, 3.5 × 106 cells were seeded in 10 mL DMEM in 10 cm tissue
culture plates. pQCiG2 constructs were pooled in equal molar ratios to a total of 10 _μ_g and co-transfected into Phoenix-Eco cells with 1 _μ_g pCL-eco replication-incompetent helper
vector40 using calcium phosphate. Twenty-four hours after transfection and twelve hours before the first virus harvest infection, plates were washed with PBS and refreshed with 5 mL complete
BCM (45% DMEM, 45% IMEM, 10% FBS, 1% Penicillin-Streptomycin, 1% L-Glutamine). Virus was collected 4 times, every 12 hrs starting from 12 hrs after BCM (B cell media) media change.
Hematopoietic stem and progenitor cells (HSPCs) were isolated from fetal livers at E13.5 and frozen until used. Cells were thawed 12 hrs before first infection in BCM supplemented with 1
ng/mL IL-3, 10 ng/mL IL-6, 100 ng/mL SCF (stem cell factor) and incubated at 37 °C in 5% CO2. Cultured HSPCs were infected four times at 12 h intervals with viral supernatant from
transfected Phoenix-Eco cells, supplemented with 1 ng/mL IL-3, 10 ng/mL IL-6, 100 ng/mL SCF and 4 μg/mL Polybrene, and spinoculated at 950 × g for 1 h at 37 °C. Transduction efficiency was
assessed by determining the GFP+ population by flow cytometry using a Guava 8HT flow cytometer (Millipore). For transplantations, 6–8 week old female C57BL/6 mice were placed on 0.125 mg/mL
ciprofloxacin + 2% sucrose two days before transplantation. Four hours before transplantation, mice were irradiated with 4 Gy of γ radiation. Approximately 6 × 105–8.2 × 105 cells were
transplanted into irradiated mice via intravenous tail-vein injection. Mice were maintained on antibiotics for 3 weeks post-transplantation. Mice were palpated twice a week to assess tumor
status until the experimental end point at day 120. When tumors arose, mice were sacrificed and the masses harvested. Lymphomas were gently macerated between the frosted ends of two
microscope slides and the resulting cell suspension was passed through a 40 _μ_m cell strainer to isolate single cells. These cells were then frozen in BCM + 20% FBS + 10% DMSO and stored in
liquid N2 until further used. All methods were performed in accordance with the relevant McGill guidelines and regulations, including licensing for use of biohazard material. In addition,
all animal studies were approved by the McGill University Faculty of Medicine Animal Care Committee. RECOVERY OF SGRNAS AND T7 ENDONUCLEASE I ASSAY (T7EI) Genomic DNA was prepared from
isolated tumor cells by lysing tumor cell pellets overnight in TNE buffer (10 mM Tris [pH 8.0], 100 mM NaCl, 25 mM EDTA [pH 8.0], 0.25% SDS, 125 _μ_g/mL Proteinase K, 125 _μ_g/mL RNase A) at
55 °C. Genomic DNA was deproteinized by extracting once with phenol, twice with phenol:chloroform: (50:50), and once with chloroform and recovered by ethanol precipitation using 0.3 M NaOAc
[pH 5.2]. PCR amplification of targeted loci was performed using Phusion High-Fidelity DNA polymerase (NEB) according to the manufacturer’s recommendations. Amplified DNA was purified using
BioBasic EZ-10 spin columns. The T7EI assay was then performed as previously described16 and the entire reaction was resolved on a 15% 1× TBE polyacrylamide gel (29:1
acrylamide:bisacrylamide) before staining with ethidium bromide. SEQUENCING OF MODIFIED LOCI Targeted loci were amplified from tumor genomic DNA using primers designed with Primer341 and
containing adaptor sequences (Supplementary Table S3). The amplified loci were then cloned into pSKII(+) and inserts sequenced via Sanger sequencing using the T7 sequencing primer. SMALL
HAIRPIN (SH) RNA DESIGN The Designer of siRNA (DSIR) algorithm with extended rules described by Fellman _et al_.42 was used to generate shRNAs targeting _Phip_ and S_p3_. Five shRNAs
targeting each gene were generated and cloned into the MLS retroviral backbone using unique _XhoI/EcoRI_ restriction sites. After validation _ex vivo_ in cell lines, the two most potent
shRNAs were chosen for use in HSPC adoptive transfer experiments. PHIP AND PHIP R1212Δ PLASMIDS The PHIP cDNA was kindly provided by Dr. Anne-Claude Gingras (The Lunenfeld-Tanenbaum Research
Institute, Toronto). From this cDNA, a truncation mutant was generated by excising the C-terminal region of PHIP using unique AgeI/XhoI sites and replacing it with an oligonucleotide
containing a premature stop codon to generate PHIP R1212Δ. For insertion into MLS, the proviral backbone was digested with _BglII_, repaired with Klenow, and digested with _XhoI_. PHIP and
PHIP R1212Δ were excised from the parental plasmid by digestion with _AscI_, klenow repaired, and digested with _XhoI_. Following gel purification, the PHIP cDNAs were ligated into MLS and
the integrity of the resulting clones verified by sequencing. ANTIBODY GENERATION AND WESTERN BLOTTING The DNA sequence encoding amino acids 661-913 of PHIP (Uniprot: Q8VDD9) was amplified
from the complete cDNA with PCR Primers 5′GAATTCGAAGCAGGTGTTAGTAATGCCAG3′ and 5′CTCGAGTCACTTTGGTGATGTTGGTCCATC3′. This product was then cloned into pSKII(+) before subcloning into pGEX6P1
using unique EcoRI/XhoI restriction sites, which allowed the in-frame addition of a GST tag to the N-terminus of the protein. The GST-fusion protein was then purified from BL21 _E. coli_
induced with 0.3 mM IPTG for 4 hours. Bacteria were lysed in 1 M NaCl, 50 mM Tris –HCl [pH 8.0], 1 mM EDTA [pH 8.0], 1 mM EDTA and protein was purified with Gluthatione Sepharose 4B
(Amersham) before eluting with 50 mM Tris [pH 7.5], 10 mM reduced Glutathione. Proteins were dialyzed and stored in 50 mM Tris [pH 8.0], 150 mM NaCl, 10 mM EDTA,1 mM DTT, and 20% Glycerol.
The GST tag was cleaved from the purified PHIP antigen using GST-3C protease followed by subsequent retrieval from the flow-through following passage through a Glutathione Sepharose column.
The resulting protein was used as antigen for subsequent immunizations. Protein extracts for immunoblotting were prepared by lysing tumor cell pellets in RIPA buffer (20 mM Tris-HCl [pH
7.5], 150 mM NaCl, 0.1% SDS, 1% NP40, 0.5% sodium deoxycholate, 1 mM β-glycerophosphate, 1 mM PMSF, 1 _μ_g/ml leupeptin, 10 _μ_g/ml aprotinin, and 2.5 _μ_M pepstatin A) on ice for 10
minutes, followed by sonication. Extracts were then boiled for 10 minutes at 95 °C in 1X Laemmli sample buffer and resolved on a 6% or 8% NuPAGE gel. Proteins were transferred to PVDF
membranes at 200 mA for 2 h. The primary antibodies used in this study were: α-PHIP (1:1000, Bethyl laboratories, A302-055A), α-PHIP-N (1:1000), α-SP3 (1:1000, Santa Cruz, sc-655), α-actin
(1:20000, Sigma, A5316), or α-eEF2 (1:1000, Cell Signaling, 2332). Secondary α–rabbit and α-mouse antibodies (Jackson Immunoresearch, 1:5000, 715-035-146/152) were used and the signal was
visualized using enhanced chemiluminescence (ECL) (Perkin Elmer). ADDITIONAL INFORMATION HOW TO CITE THIS ARTICLE: Katigbak, A. _et al_. A CRISPR/Cas9 Functional Screen Identifies Rare Tumor
Suppressors. _Sci. Rep._ 6, 38968; doi: 10.1038/srep38968 (2016). PUBLISHER'S NOTE: Springer Nature remains neutral with regard to jurisdictional claims in published maps and
institutional affiliations. REFERENCES * Ledford, H. End of cancer-genome project prompts rethink. Nature 517, 128–129 (2015). Article CAS ADS PubMed Google Scholar * Futreal, P. A. et
al. A census of human cancer genes. Nat Rev Cancer 4, 177–183 (2004). Article CAS PubMed PubMed Central Google Scholar * Pon, J. R. & Marra, M. A. Driver and passenger mutations in
cancer. Annu Rev Pathol 10, 25–50 (2015). Article CAS PubMed Google Scholar * Leiserson, M. D. et al. Pan-cancer network analysis identifies combinations of rare somatic mutations across
pathways and protein complexes. Nat Genet 47, 106–114 (2015). Article CAS PubMed Google Scholar * Niu, B. et al. Protein-structure-guided discovery of functional mutations across 19
cancer types. Nat Genet 48, 827–837 (2016). Article CAS PubMed PubMed Central Google Scholar * Alvarez, M. J. et al. Functional characterization of somatic mutations in cancer using
network-based inference of protein activity. Nat Genet 48, 838–847 (2016). Article CAS PubMed PubMed Central Google Scholar * Boerma, E. G., Siebert, R., Kluin, P. M. & Baudis, M.
Translocations involving 8q24 in Burkitt lymphoma and other malignant lymphomas: a historical review of cytogenetics in the light of todays knowledge. Leukemia 23, 225–234 (2009). Article
CAS PubMed Google Scholar * Schmitz, R. et al. Burkitt lymphoma pathogenesis and therapeutic targets from structural and functional genomics. Nature 490, 116–120 (2012). Article CAS ADS
PubMed PubMed Central Google Scholar * Love, C. et al. The genetic landscape of mutations in Burkitt lymphoma. Nat Genet 44, 1321–1325 (2012). Article CAS PubMed PubMed Central
Google Scholar * Richter, J. et al. Recurrent mutation of the ID3 gene in Burkitt lymphoma identified by integrated genome, exome and transcriptome sequencing. Nat Genet 44, 1316–1320
(2012). Article CAS PubMed Google Scholar * Adams, J. M. et al. The c-myc oncogene driven by immunoglobulin enhancers induces lymphoid malignancy in transgenic mice. Nature 318, 533–538
(1985). Article CAS ADS PubMed Google Scholar * Harris, A. W. et al. The E mu-myc transgenic mouse. A model for high-incidence spontaneous lymphoma and leukemia of early B cells. J Exp
Med 167, 353–371 (1988). Article CAS PubMed Google Scholar * Schmitt, C. A. & Lowe, S. W. Apoptosis and chemoresistance in transgenic cancer models. J Mol Med (Berl) 80, 137–146
(2002). Article CAS Google Scholar * Bric, A. et al. Functional identification of tumor-suppressor genes through an _in vivo_ RNA interference screen in a mouse lymphoma model. Cancer
Cell 16, 324–335 (2009). Article CAS PubMed PubMed Central Google Scholar * Mills, J. R. et al. mTORC1 promotes survival through translational control of Mcl-1. Proc Natl Acad Sci USA
105, 10853–10858 (2008). Article CAS ADS PubMed PubMed Central Google Scholar * Malina, A. et al. Repurposing CRISPR/Cas9 for _in situ_ functional assays. Genes Dev 27, 2602–2614
(2013). Article CAS PubMed PubMed Central Google Scholar * Cencic, R. et al. Protospacer adjacent motif (PAM)-distal sequences engage CRISPR Cas9 DNA target cleavage. PLoS One 9,
e109213; doi: 10.1371/journal.pone.0109213 (2014). Article CAS ADS PubMed PubMed Central Google Scholar * Malina, A. et al. Adapting CRISPR/Cas9 for functional genomics screens.
Methods Enzymol 546, 193–213 (2014). Article CAS PubMed Google Scholar * Robert, F. et al. Targeting protein synthesis in a Myc/mTOR-driven model of anorexia-cachexia syndrome delays its
onset and prolongs survival. Cancer Res 72, 747–756 (2012). Article CAS PubMed Google Scholar * Reymann, S. & Boriak, J. Transcription profiling of lung adenocarcinomas of
c-myc-transgenic mice: identification of the c-myc regulatory gene network. BMC Syst Biol 2, 46, doi: 10.1186/1752-0509-2-46 (2008). Article CAS PubMed PubMed Central Google Scholar *
Park, N. H., Guo, W., Kim, H. R., Kang, M. K. & Park, N. H. c-Myc and Sp1/3 are required for transactivation of hamster telomerase catalytic subunit gene promoter. Int J Oncol 19,
755–761 (2001). CAS PubMed Google Scholar * Nagel, I. et al. Deregulation of the telomerase reverse transcriptase (TERT) gene by chromosomal translocations in B-cell malignancies. Blood
116, 1317–1320 (2010). Article CAS PubMed Google Scholar * Hedrick, E., Cheng, Y., Jin, U. H., Kim, K. & Safe, S. Specificity protein (Sp) transcription factors Sp1, Sp3 and Sp4 are
non-oncogene addiction genes in cancer cells. Oncotarget 7, 22245–22256 (2016). Article PubMed PubMed Central Google Scholar * Ban, K. & Kozar, R. A. Glutamine protects against
apoptosis via downregulation of Sp3 in intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol 299, G1344–1353 (2010). Article CAS PubMed PubMed Central Google Scholar *
Yang, H. et al. Overexpression of histone deacetylases in cancer cells is controlled by interplay of transcription factors and epigenetic modulators. FASEB J 28, 4265–4279 (2014). Article
CAS PubMed PubMed Central Google Scholar * Kajita, Y. et al. The transcription factor Sp3 regulates the expression of a metastasis-related marker of sarcoma, actin filament-associated
protein 1-like 1 (AFAP1L1). PLoS One 8, e49709; doi: 10.1371/journal.pone.0049709 (2013). Article CAS ADS PubMed PubMed Central Google Scholar * Huang, Y. et al. Transcriptional
regulation of BNIP3 by Sp3 in prostate cancer. Prostate 75, 1556–1567 (2015). Article CAS PubMed Google Scholar * Essafi-Benkhadir, K. et al. Dual role of Sp3 transcription factor as an
inducer of apoptosis and a marker of tumour aggressiveness. PloS one 4, e4478; doi: 10.1371/journal.pone.0004478 (2009). Article CAS ADS PubMed PubMed Central Google Scholar *
Bezrookove, V. et al. Prognostic impact of PHIP copy number in melanoma: linkage to ulceration. The Journal of investigative dermatology 134, 783–790 (2014). Article CAS PubMed Google
Scholar * De Semir, D. et al. Pleckstrin homology domain-interacting protein (PHIP) as a marker and mediator of melanoma metastasis. Proc Natl Acad Sci USA 109, 7067–7072 (2012). Article
CAS ADS PubMed PubMed Central Google Scholar * Zender, L. et al. An oncogenomics-based _in vivo_ RNAi screen identifies tumor suppressors in liver cancer. Cell 135, 852–864 (2008).
Article CAS PubMed PubMed Central Google Scholar * Scuoppo, C. et al. A tumour suppressor network relying on the polyamine-hypusine axis. Nature 487, 244–248 (2012). Article CAS ADS
PubMed PubMed Central Google Scholar * Cerami, E. et al. The cBio Cancer Genomics Portal: An Open Platform for Exploring Multidimensional Cancer Genomics Data. Cancer Discovery 2, 401–404
(2012). Article PubMed Google Scholar * Gao, J. et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal 6, pl1; doi:
10.1126/scisignal.2004088 (2013). * Gelinas, C. & Temin, H. M. Nondefective spleen necrosis virus-derived vectors define the upper size limit for packaging reticuloendotheliosis viruses.
Proc Natl Acad Sci USA 83, 9211–9215 (1986). Article CAS ADS PubMed PubMed Central Google Scholar * Platt, R. J. et al. CRISPR-Cas9 knockin mice for genome editing and cancer
modeling. Cell 159, 440–455 (2014). Article CAS PubMed PubMed Central Google Scholar * Hemann, M. T. et al. Evasion of the p53 tumour surveillance network by tumour-derived MYC mutants.
Nature 436, 807–811 (2005). Article CAS ADS PubMed PubMed Central Google Scholar * Lee, J. S., Vo, T. T. & Fruman, D. A. Targeting mTOR for the treatment of B cell malignancies.
Br J Clin Pharmacol 82, 1213–1228 (2016). Article CAS PubMed PubMed Central Google Scholar * Sharma, R. et al. _In vivo_ genome editing of the albumin locus as a platform for protein
replacement therapy. Blood 126, 1777–1784 (2015). Article CAS PubMed PubMed Central Google Scholar * Naviaux, R. K., Costanzi, E., Haas, M. & Verma, I. M. The pCL vector system:
rapid production of helper-free, high-titer, recombinant retroviruses. J Virol 70, 5701–5705 (1996). CAS PubMed PubMed Central Google Scholar * Untergasser, A. et al. Primer3—new
capabilities and interfaces. Nucleic Acids Res 40, e115; doi: 10.1093/nar/gks596 (2012). Article CAS PubMed PubMed Central Google Scholar * Fellmann, C. et al. Functional identification
of optimized RNAi triggers using a massively parallel sensor assay. Mol Cell 41, 733–746 (2011). Article CAS PubMed PubMed Central Google Scholar * Kennett, S. B., Udvadia, A. J. &
Horowitz, J. M. Sp3 encodes multiple proteins that differ in their capacity to stimulate or repress transcription. Nucleic Acids Res 25, 3110–3117 (1997). Article CAS PubMed PubMed
Central Google Scholar Download references ACKNOWLEDGEMENTS A.K. is supported by a Lymphoma Canada Research Foundation Fellowship. This research was supported by a Canadian Cancer Society
Research Institute (CCSRI) grant (#702778) to J.P. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Biochemistry, McIntyre Medical Sciences Building, McGill University, Canada
Alexandra Katigbak, Regina Cencic, Francis Robert, Patrick Sénécha & Jerry Pelletier * Institute for Cancer Genetics, Columbia University Medical Center, 1130 St Nicholas, 10032, Ave,
NY, USA Claudio Scuoppo * The Rosalind and Morris Goodman Cancer Research Center, Canada Jerry Pelletier * Dept of Oncology, 546 Pine Ave. West, Montreal, H2W 1S6, Québec, Canada Jerry
Pelletier Authors * Alexandra Katigbak View author publications You can also search for this author inPubMed Google Scholar * Regina Cencic View author publications You can also search for
this author inPubMed Google Scholar * Francis Robert View author publications You can also search for this author inPubMed Google Scholar * Patrick Sénécha View author publications You can
also search for this author inPubMed Google Scholar * Claudio Scuoppo View author publications You can also search for this author inPubMed Google Scholar * Jerry Pelletier View author
publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS J.P. and A.K. conceived and designed the studies. A.K. and C.S. designed the sgRNA and shRNAs. A.K.,
R.C., F.R. and P.S. performed the E_μ_-Myc HSC screen with the Cas9/sgRNA library. C.S. provided expertise in the E_μ_-Myc HSC screen. P.S. provided technical assistance. J.P. and A.K.
analyzed the data. J.P. and A.K. wrote the manuscript. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing financial interests. ELECTRONIC SUPPLEMENTARY MATERIAL
SUPPLEMENTARY FIGURES SUPPLEMENTARY TABLE S1 RIGHTS AND PERMISSIONS This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party
material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons
license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ Reprints
and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Katigbak, A., Cencic, R., Robert, F. _et al._ A CRISPR/Cas9 Functional Screen Identifies Rare Tumor Suppressors. _Sci Rep_ 6, 38968
(2016). https://doi.org/10.1038/srep38968 Download citation * Received: 12 October 2016 * Accepted: 15 November 2016 * Published: 16 December 2016 * DOI: https://doi.org/10.1038/srep38968
SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to
clipboard Provided by the Springer Nature SharedIt content-sharing initiative