Solvent and intermediate phase as boosters for the perovskite transformation and solar cell performance
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT High power conversion efficiency and device stabilization are two major challenges for CH3NH3PbI3 (MAPbI3) perovskite solar cells to be commercialized. Herein, we demonstrate a
diffusion-engineered perovskite synthesis method using MAI/ethanol dipping, and compared it to the conventional synthesis method from MAI/iso-propanol. Diffusion of MAI/C2H5OH into the PbCl2
film was observed to be more favorable than that of MAI/C3H7OH. Facile perovskite conversion from ethanol and highly-crystalline MAPbI3 with minimized impurities boosted the efficiency from
5.86% to 9.51%. Additionally, we further identified the intermediates and thereby the reaction mechanisms of PbCl2 converting into MAPbI3. Through straightforward engineering to enhance the
surface morphology as well as the crystallinity of the perovskite with even faster conversion, an initial power conversion efficiency of 11.23% was obtained, in addition to superior
stability after 30 days under an ambient condition. SIMILAR CONTENT BEING VIEWED BY OTHERS SCALABLE FABRICATION OF WIDE-BANDGAP PEROVSKITES USING GREEN SOLVENTS FOR TANDEM SOLAR CELLS
Article 15 November 2024 NON-FULLERENE ELECTRON-TRANSPORTING MATERIALS FOR HIGH-PERFORMANCE AND STABLE PEROVSKITE SOLAR CELLS Article 04 March 2025 INTERMEDIATE-PHASE ENGINEERING VIA
DIMETHYLAMMONIUM CATION ADDITIVE FOR STABLE PEROVSKITE SOLAR CELLS Article 01 December 2022 INTRODUCTION The feasible challenges in solar cell commercialization are enhancement in power
conversion efficiency (PCE) and cost reduction to support the world-wide electricity consumption1,2,3,4,5. Alternatively, organometallic perovskite solar cells were first demonstrated by
Miyasaka’s group in 2009 with a PCE of 3.8%6, and an enormous growth has been achieved over the last 6 years with the highest efficiency of 22.10%7. Perovskite (CH3NH3PbI3) solar cells are
settled as the most attractive topic in photovoltaic research areas due to the low fabrication cost and high efficiencies, followed by inherent advantages of the perovskite material which
include an appropriate and direct bandgap, small exciton binding energy, balanced ambipolar charge transport properties, etc.8,9,10,11,12. Furthermore, the synthesis of CH3NH3PbI3 (MAPbI3)
goes through a simple process, by mixing PbI2 and MAI precursors6. In 2012, the superior performance via MAPbI3 synthesis with PbCl2 and MAI precursors was introduced by Snaith’s group, and
property analyses were carried out by many groups13,14,15,16. Since then, researchers widely studied the chlorine effect, and concluded that chlorine enhances the morphology of perovskite
films16,17,18,19,20. Even though the chlorine effect is suggested by many research groups, understanding the mechanisms on the synthesis is still required to be elucidated. Architectural
challenges are widely studied due to the ambipolar behavior21,22,23 of perovskites. Among them, the highest efficiency of 22.10%7 has been achieved with mesoporous structure, and mesoporous
layer allows the additional light trapping effect24,25. In the mesoscopic structure, there are two major MAPbI3 deposition methods. The one-step solution deposition generally uses a mixture
solution of PbI2 and MAI16, and the sequential deposition is carried out by pre-depositing the PbI2 film, followed by dipping it into an MAI-dissolved iso-propanol solution to form the
MAPbI3 film26. Among them, the one-step solution deposition is highly beneficial in that this process is quite simple and time-saving. However, the sequential deposition is reported with a
higher PCE than that of the one-step deposition27,28,29 due to the enhanced pore filling through the mesoporous TiO2 (mp-TiO2). Although the sequential deposition guarantees a high PCE, a
comparative disadvantage in the sequential deposition is that it is a long-time process, since it goes through multiple steps to fabricate the perovskite film26. In this article, we have
demonstrated a straightforward diffusion-controlled synthesis approach by replacing the conventional MAI-dissolved iso-propanol solution with a MAI-dissolved ethanol solution, which enhanced
the crystallinity, boosted the perovskite transformation, and minimized impurities. Moreover, we have detected intermediate phases when the PbCl2 precursor transforms into MAPbI3, and
engineered the MAPbI3 deposition procedure by artificially mixing those intermediates as deposition precursors. This novel approach allowed superior surface morphology and crystallinity with
enhanced conversion kinetics of MAPbI3, yielding an initial PCE of 11.23% and notable stability exhibiting 10.14% PCE after 30 days under ambient conditions. RESULTS ETHANOL CONVERSION
Sequential deposition is one of the most preferable methods for perovskite fabrication due to the high PCE. One major problem, however, is the long fabrication time by multiple fabrication
steps26. To reduce the fabrication time in the sequential deposition process, boosting the formation kinetics of MAPbI3 using MAI with PbCl2 precursors is required. Therefore, a low viscous
solvent and larger concentration of MAI are necessary for effective diffusion of MAI into the PbCl2 layer. In general, conventional dipping solution uses 10 mg mL−1 of MAI in iso-propanol26,
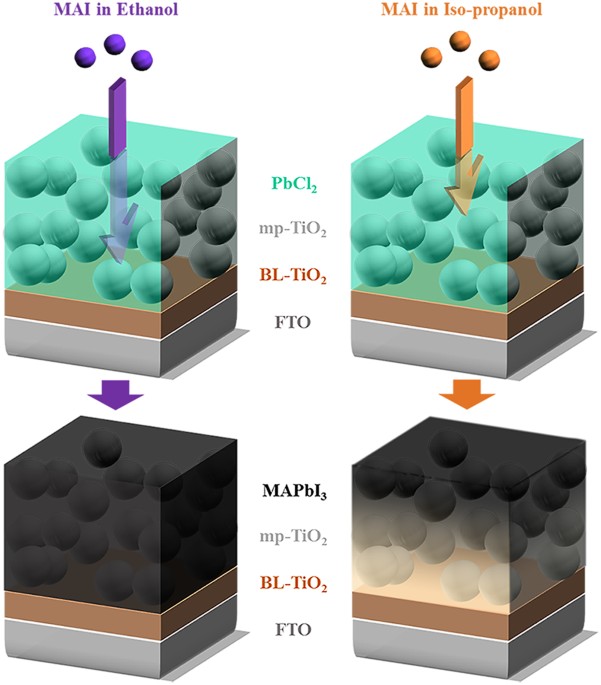
and a high concentration of MAI in solvent reduces both cuboid sizes and PCEs30. Thus, finding an alternative solvent is necessary for the viscosity and diffusion aspects. Figure 1
schematically illustrates the movements of ionized MAI into the PbCl2 film with ethanol (20 mg mL−1) and iso-propanol (10 mg mL−1), where the conversion kinetics of PbCl2 into MAPbI3 for
each solvent is quite different even with the optical images (Supplementary Fig. 1, fast conversion kinetics with MAI/ethanol). The extent of the reaction was easily estimated by color
changes (_E__g_ of MAPbI3 ≈ 1.55 eV). However, less viscous methanol was not effective due to the dissolution of MAPbI3 (Supplementary Fig. 2)26. The fabricated perovskite film with the same
concentration (20 mg mL−1) for the iso-propanol solution results in rather small cuboid sizes (~80 nm) with a low PCE of 2.08% in the solar cell performance, as shown in Supplementary Fig.
3. The cuboid size of MAPbI3 with an ethanol solution is also distinguishable from that with iso-propanol, as shown in the scanning electron microscopy (SEM) (Fig. 2a,b). The PbCl2-deposited
film and cuboid-size distributions are plotted in Fig. 2c, and the synthesized perovskite with twice-large cuboid sizes (~1180 nm) through an ethanol conversion is expected to produce
higher carrier mobilities31. Figure 3 illustrates qualitative analysis of the perovskite formation with ethanol or iso-propano. The energy disperse x-ray spectroscopy (EDS) was conducted to
identify the chlorine concentration (Fig. 3a). MAPbI3 converted from an ethanol solution contains lower Cl than that of iso-propanol-synthesized MAPbI3 since unreacted PbCl2 or
partially-reacted MAPbCl3 remains in the film. Still, ethanol-based MAPbI3 shows some chlorine content, and we believe that this is caused by MACl which is a co-product during the MAPbI3
synthesis16,17,18. Actually, there is a possibility that chlorine is doped in the perovskite structure (MAPbI3-xClx) as reported by several groups14,15,16. However, the small quantity of
chlorine in the perovskite structure is difficult to be evaluated, and the remaining chlorine may form other products17,19. Cross-sectional SEM image of MAPbI3 perovskite solar cell by
ethanol exhibits uniform film structures, as shown in Fig. 3b. For further understanding of impurities, X-ray diffraction (XRD) scans were compared, and an ethanol-based MAPbI3 shows clear
(110), (220) and (330) peaks (Fig. 3c). On the contrary, the conversion with iso-propanol produced impurity peaks of PbI2 and MAPbCl3 (Fig. 3c), indicating incomplete reaction. To explain
the effect of ethanol on the crystallinity, we have additionally confirmed that the longer dipping time increases the crystallinity of MAPbI3 (Supplementary Fig. 4) eVen when the reaction
was completed. Together with the optical observation in Supplementary Fig. 1, it can be said that MAI/ethanol-converted MAPbI3 completes the reaction faster with better crystallinity,
compared to that of iso-propanol for the same dipping time. Furthermore, the light absorption from the synthesized perovskite is clearly different between ethanol and iso-propanol (Fig. 3d).
The enhanced absorption at approximately 800 nm by ethanol is due to the superior purity of MAPbI3 (_E__g_ ≈ 1.55 eV), while partially-reacted MAPbI3 by iso-propanol contains high-bandgap
impurities, such as PbI2 (_E__g_ ≈ 2.36 eV)32 and MAPbCl3 (_E__g_ ≈ 3.17 eV)33. Therefore, the overall PCE is greatly improved from 5.86% to 9.51% (Fig. 3e) with much better stability (Fig.
3f), and both methods performed high reproducibility (Supplementary Fig. 5 and Supplementary Table 1). After 30 days, the PCE decreased from 9.51% to 8.53% and 5.86% to 3.75%, respectively,
for the ethanol and iso-propanol solution (Table 1). The improved crystallinity and enlarged grain of MAPbI3 by ethanol surely prevents possible air penetration through various grain
boundaries, leading to stability enhancement. The half-lifetime of the MAPbI3 perovskite solar cell (degradation details in Supplementary Fig. 6) was estimated to be ~150 and ~40 days,
respectively, for ethanol and iso-propanol. To identify the reaction mechanisms, intermediate phases during the perovskite formation were investigated by the concentration variations of MAI
in ethanol. With a low concentration of MAI/ethanol (Fig. 4a), PbCl2 partially reacts into PbI2 (5 mg mL−1). The chlorine in PbCl2 ion-exchanges with iodine in MAI to form PbI2, and the
dissociated MA+ and Cl− from outer PbCl2 intercalate into the inner PbCl2 layer, transforming to the MAPbCl3 phase (15 mg mL−1 of MAI/ethanol). The intermediate PbI2 reacts with MAI directly
to form MAPbI3 by intercalating MAI in the layered PbI2, while MAPbCl3 will ion-exchange with I− and reconstructs to the final MAPbI3, as schemed in Fig. 4b (20 mg mL−1 of MAI/ethanol). The
whole reaction occurs through the following steps: The PbI2 phase converts into MAPbI3 earlier than the formation of MAPbCl3, as shown by the X-ray diffraction of MAPbI3 vs. MAPbCl3 phases
for MAI concentrations of 10 and 15 mg mL−1 (Fig. 4a). We have also compared the perovskite formation from MAPbCl3 (MAPbCl3 + 3 MAI → MAPbI3 + 3 MACl) with PbI2 (PbI2 + MAI → MAPbI3)
(respectively, in the middle and right of Fig. 4a), and found that both have resulted in no intermediate phases. Synthesizing fully-converted MAPbI3 from PbCl2 requires both intercalation
and reconstruction steps, while the idea of reconstruction from MAPbCl3 to MAPbI3 was investigated in the previous report34. Therefore, we intuitively conclude that the recrystallization of
intermediates both inside and on top of the mp-TiO2 film can enhance the coverage morphology, nanostructures, and crystallinity16,17,18 of MAPbI3 by multiple crystal-alignment steps.
Moreover, ethanol conversion increases the kinetics of the reaction steps, and produces improved MAPbI3 film, compared to the conversion with iso-propanol. REACTION MECHANISM ENGINEERING To
understand the phase-formation paths of MAPbI3 from PbCl2, we came up with an idea to further optimize MAPbI3 by utilizing the identified intermediate phases. While the direct conversion of
MAPbCl3 to MAPbI3 can drastically reduce the reaction time, the repetition of crystallization from PbCl2 to MAPbI3 will enhance the surface morphology. Even though PbI2 appears during the
transformation of the PbCl2 precursor to MAPbI3, and increases the reaction kinetics, the MAPbCl3 precursor is more reactive than the PbI2 precursor to synthesize MAPbI3 (middle and right
graphs in Fig. 4a) where the MAPbCl3 precursor is likely to transform with lower MAI concentration than that of the PbI2 precursor. Also, the chlorine-based precursor should be preferred
considering the positive effects of chlorine on the MAPbI3 perovskite solar cells16,17,18,19,20. Therefore, a straightforward direction is rendered by mixing PbCl2 and MAPbCl3 in several
different ratios of 3:0 (reaction 1), 2:1 (reaction 2), 1:2 (reaction 3), and 0:3 (reaction 4), to optimize the reaction time with smooth surface morphology (Table 2 and Fig. 5). As
expected, we observed the morphology changes by synthesizing MAPbI3 films from precursors with different ratios of PbCl2 and MAPbCl3 through SEM, as shown in Fig. 6a. Perovskite films
synthesized by reaction 2 exhibits clearly improved coverage with ~2320-nm-sized cuboids. Furthermore, addition of MAPbCl3 (reaction 3 and 4) deteriorates the coverage, but increases the
cuboid size. When a larger amount of the MAPbCl3 precursor is added through reactions, the crystallinity of MAPbI3 is enhanced significantly, as shown by XRD (Fig. 6b,c). The enhanced
crystallinity can be explained by the extent of reaction, and the facilely-transformed perovskite is likely to have high crystallinity even with the same dipping time (Supplementary Fig. 4).
These observations are also consistent with the optical variations during the MAPbI3 formation (Supplementary Fig. 7). It should be noted that the coverages for the reactions 1, 2, 3, and 4
are different. However, conversions at ~10 s are distinct between reactions 1 (PbCl2 precursor) and 4 (MAPbCl3 precursor). The light absorption in Fig. 6d indicates that the absorption is
more influenced by the coverage rather than the crystallinity and cuboid size of MAPbI3. The maximum coverage in reaction 2 reached the highest absorption, and the minimum coverage with
reaction 4 yielded the lowest absorption. As a material perspective, MAPbI3 synthesis by reaction 4 is supposed to show excellent properties due to the high crystallinity and cuboid sizes,
as plotted in Fig. 7a. Moreover, EDS was additionally measured to identify the comparative chlorine contents with iodine, which is plotted in Fig. 7b for each reaction, indicating that
reaction 1 obtained the highest, and reaction 4 occupied the lowest concentration of chlorine. This chlorine tendency suggests that a co-product of MACl is minimized through the addition of
MAPbCl3. (It should be noted that the EDS technique may not reflect the accurate chlorine concentration due to the coverage difference of each reaction.) It is possible that the detected
chlorine through EDS is from the MACl phase or other products16,17,18. Moreover, excessive chlorine may lead to impurities, and deteriorate the device performance. Therefore, high
crystallinity and low impurity of MAPbI3 are highly beneficial in the carrier mobilities, but recombination of carriers arising from poor coverage31 is another factor that we should be aware
of. To understand the effects of crystallinity and coverage on the photovoltaic performance and stability, _J-V_ curves are measured for 30 days under ambient conditions (Supplementary Fig.
6, and Table 3). In Fig. 7c, _J-V_ curves were shown for the performance of the solar cells from each reaction, and the highly covered perovskite film from reaction 2 achieved the highest
PCE of 11.23%. The lowest PCE of 4.03% was obtained by reaction 4, and these results indicate that the initial PCE is highly dependent on the perovskite coverage, which plays crucial roles
in the carrier recombination. In contrast, MAPbI3 synthesized by reaction 4 was distinctively stable after 30 days. The stability is well correlated with the crystallinity and grain size,
apparent from the normalized PCE in Fig. 7d, confirming the reduced decomposition behavior from the low-defect perovskite. All of the solar cells with different experimental conditions
performed high reproducibility (Supplementary Fig. 5 and Supplementary Table 1). From the reactions 1, 2, 3, and 4, the half-lifetimes of MAPbI3 perovskite solar cells (degradation details
in Supplementary Fig. 6) are estimated to be ~150, ~160, ~270, and ~300 days. DISCUSSION Fast conversion can have positive influences on the device performance and stability by producing
highly-crystalline MAPbI3 perovskite. Therefore, we have controlled the diffusion reaction of MAI and PbCl2 with ethanol to boost the perovskite transformations, leading to phase-pure and
highly-crystalline perovskite films. Since PbCl2 goes through several intermediate phases during the formation of MAPbI3, we utilized the intermediates by mixing them with a conventional
PbCl2 precursor to boost the conversion kinetics and performance of the resulting solar cell. Thereby, the optimized crystallinity and coverage yielded a PCE of 11.23% with PbCl2: MAPbCl3 =
2:1 (reaction 2). Although the precursor with 100% MAPbCl3 (reaction 4) resulted in the fastest transformations of MAPbI3 and the most stable solar cell performance, poor coverage lowered
the PCE of the device. Therefore, compact coverage of the perovskite with super-sized cuboids expects to achieve further enhanced performance and stability of the MAPbI3 perovskite solar
cell. At this point, investigation of hysteresis still remains as a future work. METHODS PEROVSKITE SOLAR CELL FABRICATION Fluorine-doped tin oxide substrate (FTO, TEC 8: Pilkington) was
cleaned by sonication in Mucasol (Aldrich), ethanol (DEAJUNG), and DI water for 30 min sequentially. 50 nm of compact the TiO2 blocking layer was deposited by spin-coating the mixture
solution of 0.15 mM titanium diisopropoxide bis(acetylacetonate) (Aldrich) and 1-butanol (75.0 wt. % in iso-propanol, Aldrich) in 2500 rpm for 20 s followed by heating at 125 °C for 5 min in
an air oven. The same step was repeated with 0.3 mM concentration and the substrate was annealed at 500 °C for 30 min. After the TiO2 blocking layer was ready, TiO2 pastes (ENB Korea) with
20 nm-sized nanoparticles were mixed with terpineol (Aldrich) in 1:2 ratio, followed by spin-coating at 4000 rpm for 30 s, yielding a ~350 nm thickness of the mp-TiO2 layer. For the
perovskite synthesis, MAI was first synthesized by following literature method26. 1.5 M PbCl2 (Aldrich) was diluted in dimethyl sulfoxide (DMSO, Aldrich), and then MACl (Aldrich) was added
with different concentrations (0, 0.5, 1.0, and 1.5 M) to synthesize the reaction 1, 2, 3, and 4 precursors where the reaction 1, 2, and 3 precursors contain both MAPbCl3 and PbCl2 phases,
whereas the reaction 4 precursor contains only MAPbCl3 (MAPbCl3 forms by 1:1 molar stoichiometric ratios of MACl and PbCl2). After the preparation of precursor mixture solutions, the
solution was preheated at 100 °C and the substrate was preheated at 150 °C, then the solution was spin-coated at 2000 rpm for 5 s, followed by 6000 rpm for 5 s. The film was annealed at 150
°C for 30 min, and cooled down in an ambient condition. After the film was cooled down, it was dipped into 20 mg mL−1 of MAI in an anhydrous ethanol solution (Daejung) for 20 min under
ambient conditions (25 °C and 55% humidity) and annealed at 100 °C for 30 min. The hole transport solution was prepared by mixing 72.3 mg mL−1 of spiro-OMeTAD (Merck) in chlorobenzene
(Aldrich) with 28.8 μL of _tert_-butylpyridine (Aldrich) and a 17.5 μL solution of 520 mg of lithium _bis_(trifluoromethylsyfonyl)imide salt (Aldrich) in 1 mL acetonitrile (Aldrich) was
spin-coated at 3000 rpm for 45 s. Finally, 100 nm thickness of an Au electrode was then thermally evaporated. DEVICE CHARACTERIZATIONS The morphologies of MAPbI3 perovskite films were
analyzed using scanning electron microscope (Normal-SEM, JSM-6360: Hitachi). The chlorine compositions and distribution were examined using energy-dispersive X-ray spectroscopy (EDS,
ISIS-300: Oxford Instruments). The phases of the synthesized samples were characterized by X-ray diffraction (XRD, D8 Advance: Bruker). The photocurrent-voltage (_J–V_) curves of MAPbI3
perovskite solar cells were obtained with a potentiostat (CHI 608C: CH Instrumental Inc.) under AM 1.5 illumination at 100 mW cm−2 (K3000: McScience) with an active cell area of 0.09 cm2.
The field-emission scanning electron microscope (FE-SEM, Merlin-Compact: Carl Zeiss) was used to observe the plan and cross-sectional views. The absorption spectra of the MAPbI3-deposited
films were recorded on a UV-Vis spectrophotometer (Lambda 20: Perkin Elmer). Stability was measured every 5 days, and stored at 25 °C with 55% of humidity under dark conditions. ADDITIONAL
INFORMATION HOW TO CITE THIS ARTICLE: Kim, J. _et al._ Solvent and Intermediate Phase as Boosters for the Perovskite Transformation and Solar Cell Performance. _Sci. Rep._ 6, 25648; doi:
10.1038/srep25648 (2016). REFERENCES * Shin, B. et al. Thin film solar cell with 8.4% power conversion efficiency using an earth‐abundant Cu2ZnSnS4 absorber. _Prog. Photovolt.: Res. Appl._
21, 72–76 (2013). Article CAS Google Scholar * Choi, H. et al. The role of ZnO-coating-layer thickness on the recombination in CdS quantum-dot-sensitized solar cells. _Nano Energy_ 2,
1218–1224 (2013). Article CAS Google Scholar * Kim, D. et al. Backcontact CdSe/CdTE windowless solar cells. _Sol. Energ. Mat. Sol. C._ 109, 246–253 (2013). Article CAS Google Scholar *
Kim, J. et al. The role of a TiCl4 treatment on the performance of CdS quantum-dot-sensitized solar cells. _J. Power Sources_ 220, 108–113 (2012). Article CAS ADS Google Scholar * Choi,
H. et al. The construction of tandem dye-sensitized solar cells from chemically-derived nanoporous photoelectrodes. _J. Power Sources_ 274, 937–942 (2015). Article CAS ADS Google Scholar
* Kojima, A., Teshima, K., Shirai, Y. & Miyasaka, T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. _J. Am. Chem. Soc._ 131, 6050–6051 (2009).
Article CAS Google Scholar * National Renewable Energy Laboratory (NREL), Best Research-Cell Efficiencies, http://www.nrel.gov/ncpv/images/efficiency_chart.jpg (Accessed: 30th March,
2016). * Kazim, S., Nazerruddin M. K., Gratzel, M. & Ahmad, S. Perovskite as Light Harvester: A Game Charger in Photovoltaics. _Angew. Chem. Int. Ed._ 53, 2812–2824 (2014). Article CAS
Google Scholar * McGehee, M. D. Materials science: fast-track solar cells. _Nature_ 501, 323–325 (2013). Article CAS ADS Google Scholar * Hodes, G. Perovskite-based solar cells.
_Science_ 342, 317–318 (2013). Article CAS ADS Google Scholar * Ponseca, C. S. et al. Organometal halide perovskite solar cell materials rationalized: ultrafast charge generation, high
and microsecond-long balanced mobilities, and slow recombination. _J. Am. Chem. Soc._ 136, 5189–5192 (2014). Article CAS Google Scholar * Frost, J. M. et al. Atomistic origins of
high-performance in hybrid halide perovskite solar cells. _Nano Lett._ 14, 2584–2590 (2014). Article CAS ADS Google Scholar * Lee, M. M., Teuscher, J., Miyasaka, T., Murakami, T. N.
& Snaith, H. J. Efficient hybrid solar cells based on meso-superstructured organometal halide perovskites. _Science_ 338, 643–647 (2012). Article CAS ADS Google Scholar * Colella, S.
et al. MAPbI3−xClx mixed halide perovskite for hybrid solar cells: the role of chloride as dopant on the transport and structural properties. _Chem. Mater._ 25, 4613–4618 (2013). Article
CAS Google Scholar * Wehrenfennig, C., Eperon, G. E., Johnston, M. B., Snaith, H. J. & Herz, L. M. High charge carrier mobilities and lifetimes in organolead trihalide perovskites.
_Adv. Mater._ 26, 1584–1589 (2014). Article CAS Google Scholar * Williams, S. T. et al. Role of chloride in the morphological evolution of organo-lead halide perovskite thin films. _ACS
Nano_ 8, 10640–10654 (2014). Article CAS Google Scholar * Unger, E. L. et al. Chloride in lead chloride-derived organo-metal halides for perovskite-absorber solar cells. _Chem. Mater._
26, 7158–7165 (2014). Article CAS Google Scholar * Deepa, M., Ramos, J. F., Shivaprasad M. S. & Ahmad, S. Unravelling the Role of Monovalent Halides in Mixed-Halide Organic-Inorganic
Perovskite. _ChemPhysChem._ 17, 913–920 (2016). Article CAS Google Scholar * Yin, W.-J., Chen H., Shi, T., Wei, S.-H. & Yan, Y. Origin of high electronic quality in structurally
disordered CH3NH3PbI3 and the passivation effect of Cl and O at grain boundaries. _Adv. Electron. Mater._ 1, 1500044 (2015). Article Google Scholar * Mosconi, E., Ronca, E. & Angelis,
F. D. First-principles investigation of the TiO2/organohalide perovskites interface: the role of interfacial chlorine. _J. Phys. Chem. Lett._ 5, 2619–2625 (2014). Article CAS Google
Scholar * Chen, Q. et al. Planar heterojunction perovskite solar cells via vapor-assisted solution process. _J. Am. Chem. Soc._ 136, 622–625 (2013). Article Google Scholar * Eperon, G.
E., Burlakov, V. M., Docampo, P., Goriely, A. & Snaith, H. J. Morphological control for high performance, solution-processed planar heterojunction perovskite solar cells. _Adv. Funct.
Mater._ 24, 151–157 (2014). Article CAS Google Scholar * Liu, M., Johnston, M. B. & Snaith, H. J. Efficient planar heterojunction perovskite solar cells by vapour deposition. _Nature_
501, 395–398 (2013). Article CAS ADS Google Scholar * Mihi, A. Zhang, C. & Braun, P. V. Transfer of preformed three-dimensional photonic crystals onto dye-sensitized solar cells.
_Angew. Chem. Int. Ed._ 50, 5712–5715 (2011). Article CAS Google Scholar * Grandidier J., Callahan, D. M., Munday, J. N. & Atwater, H. A. Light absorption enhancement in thin-film
solar cells using whispering gallery modes in dielectric nanospheres. _Adv. Mater._ 23, 1272–1276 (2011). Article CAS Google Scholar * Burschka, J. et al. Sequential deposition route to
high performance perovskite-sensitized solar cells. _Nature_ 499, 316–320 (2013). Article CAS ADS Google Scholar * Im, J.-H., Kim, H.-S. & Park, N.-G. Morphology-photovoltaic
property correlation in perovskite solar cells: one-step versus two-step deposition of CH3NH3PbI3 . _APL Mater._ 2, 081510 (2014). Article ADS Google Scholar * Ma, Y. et al. A highly
efficient mesoscopic solar cell based on CH3NH3PbI3−xClx fabricated via sequential solution deposition. _Chem. Commun._ 50, 12458–12461 (2014). Article CAS Google Scholar * Yantara, N. et
al. Loading of mesoporous titania films by CH3NH3PbI3 perovskite, single step vs. sequential deposition. _Chem. Commun._ 51, 4603–4606 (2015). Article CAS Google Scholar * Im, J.-H.,
Jang, I.-H., Pellet, N., Gratzel, M. & Park, N.-G. Growth of CH3NH3PbI3 cuboids with controlled size for high-efficiency perovskite solar cells. _Nat. Nanotechnol._ 9, 927–932 (2014).
Article CAS ADS Google Scholar * Nie, W. et al. High-efficiency solution-processed perovskite solar cells with millimeter-scale grains. _Science_ 347, 522–525 (2015). Article CAS ADS
Google Scholar * Supasai, T., Rujisamphan, N., Ullrich, K., Chemseddine, A. & Dittrich, T. Formation of a passivating CH3NH3PbI3/PbI2 interface during moderate heating of CH3NH3PbI3
layers. _Appl. Phys. Lett._ 103, 183906–1 (2013). Article ADS Google Scholar * Comin, R. et al. Structural, optical, and electronic studies of wide-bandgap lead halide perovskites. _J.
Mater. Chem. C_ 3, 8839–8843 (2015). Article CAS Google Scholar * Zhou, H. et al. Interface engineering of highly efficient perovskite solar cells. _Science_ 345, 542–546 (2014). Article
CAS ADS Google Scholar Download references ACKNOWLEDGEMENTS This work was supported by the National Research Foundation of Korea (NRF): 2013R1A1A2065793, 2010-0029065 and
2015K2A1A2070386. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Materials Science and Engineering, WCU Hybrid Materials Program, Research Institute of Advanced Materials, Seoul
National University, 08826, Seoul, Korea Jinhyun Kim, Taehyun Hwang, Sangheon Lee, Byungho Lee, Jaewon Kim, Gil Su Jang & Byungwoo Park * Nano Mechanical Systems Research Division,
Department of Nano Mechanics, Korea Institute of Machinery & Materials (KIMM), 305-343, Daejeon, Korea Seunghoon Nam Authors * Jinhyun Kim View author publications You can also search
for this author inPubMed Google Scholar * Taehyun Hwang View author publications You can also search for this author inPubMed Google Scholar * Sangheon Lee View author publications You can
also search for this author inPubMed Google Scholar * Byungho Lee View author publications You can also search for this author inPubMed Google Scholar * Jaewon Kim View author publications
You can also search for this author inPubMed Google Scholar * Gil Su Jang View author publications You can also search for this author inPubMed Google Scholar * Seunghoon Nam View author
publications You can also search for this author inPubMed Google Scholar * Byungwoo Park View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS
J.K. fabricated the solar cell devices, designed the whole experiments, and participated in writing the paper. T.H. supported the optimization process on the solar cell fabrication, and
participated in writing the paper. S.L. carried out the electrochemical analyses, and participated in writing the paper. B.L. supported the optimization process of MAPbI3 perovskite
synthesis, and participated in writing the paper with vital comments. J.K. analyzed electrochemical properties of the MAPbI3 perovskite, and participated in writing the paper. G.S.J.
supported the optimization steps in thermal evaporation of an Au electrode. S.N. helped writing the paper, and gave constructive scientific comments. B.P. advised on the overall experiments
with vital comments, and finalized the manuscript. CORRESPONDING AUTHOR Correspondence to Byungwoo Park. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing financial
interests. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION (PDF 568 KB) RIGHTS AND PERMISSIONS This work is licensed under a Creative Commons Attribution 4.0 International License. The
images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included
under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit
http://creativecommons.org/licenses/by/4.0/ Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Kim, J., Hwang, T., Lee, S. _et al._ Solvent and Intermediate Phase as Boosters for
the Perovskite Transformation and Solar Cell Performance. _Sci Rep_ 6, 25648 (2016). https://doi.org/10.1038/srep25648 Download citation * Received: 14 December 2015 * Accepted: 20 April
2016 * Published: 09 May 2016 * DOI: https://doi.org/10.1038/srep25648 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link
Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative