Mcl-1 is a key regulator of the ovarian reserve
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT A majority of ovarian follicles are lost to natural death, but the disruption of factors involved in maintenance of the oocyte pool results in a further untimely follicular
depletion known as premature ovarian failure. The anti-apoptotic B-cell lymphoma 2 (Bcl-2) family member myeloid cell leukemia-1 (MCL-1) has a pro-survival role in various cell types;
however, its contribution to oocyte survival is unconfirmed. We present a phenotypic characterization of oocytes deficient in _Mcl-1_, and establish its role in maintenance of the primordial
follicle (PMF) pool, growing oocyte survival and oocyte quality. _Mcl-1_ depletion resulted in the premature exhaustion of the ovarian reserve, characterized by early PMF loss because of
activation of apoptosis. The increasingly diminished surviving cohort of growing oocytes displayed elevated markers of autophagy and mitochondrial dysfunction. _Mcl-1-_deficient ovulated
oocytes demonstrated an increased susceptibility to cellular fragmentation with activation of the apoptotic cascade. Concomitant deletion of the pro-apoptotic Bcl-2 member Bcl-2-associated X
protein (_Bax_) rescued the PMF phenotype and ovulated oocyte death, but did not prevent the mitochondrial dysfunction associated with _Mcl-1_ deficiency and could not rescue long-term
breeding performance. We thus recognize MCL-1 as the essential survival factor required for conservation of the postnatal PMF pool, growing follicle survival and effective oocyte
mitochondrial function. SIMILAR CONTENT BEING VIEWED BY OTHERS EPAS1 EXPRESSION CONTRIBUTES TO MAINTENANCE OF THE PRIMORDIAL FOLLICLE POOL IN THE MOUSE OVARY Article Open access 16 April
2024 _EXOCYST COMPLEX COMPONENT 1_ (_EXOC1)_ LOSS IN DORMANT OOCYTE DISRUPTS C-KIT AND GROWTH DIFFERENTIATION FACTOR (GDF9) SUBCELLULAR LOCALIZATION AND CAUSES FEMALE INFERTILITY IN MICE
Article Open access 20 January 2025 BNC1 DEFICIENCY-TRIGGERED FERROPTOSIS THROUGH THE NF2-YAP PATHWAY INDUCES PRIMARY OVARIAN INSUFFICIENCY Article Open access 05 October 2022 MAIN Estimates
of the human primordial follicle (PMF) reservoir, the size of which dictates the extent of the ovarian reserve, indicates the presence of at least half a million oocytes per ovary at
birth.1, 2 The essential decision that PMFs face is either long-term arrest with a possibility of recruitment toward the growing pool, or death. Even upon recruitment to the growing pool,
intricately orchestrated crosstalk of survival signals between ovarian somatic cells and oocytes facilitate the ovulation of a single oocyte in human in each cycle. Hence, the default fate
for millions of ovarian germ cells is death, as only a small fraction survive till ovulation.3 Insufficient endowment during fetal development or excessive oocyte loss during postnatal life
further limits the ovarian reserve and can result in an untimely exhaustion of the follicle pool leading to premature ovarian failure (POF); a syndrome that affects around 1% of all women,
with a higher prevalence (up to 30%) in families with heritable traits of this condition.4, 5 Mechanisms responsible for maintenance of the follicular reserve are poorly understood, however,
biological assessments and mathematical modeling reveal that progressive loss of follicles with age is non-linear and accelerates, especially after 38 years.6, 7 With a declining ovarian
reserve, poor oocyte quality is an additional factor that contributes to the reduced fertility associated with increased maternal age. Oocytes and resulting embryos of older mothers have
increased rates of aneuploidies likely due to defects in chromosomal cohesion and meiotic spindle stability, decreased DNA repair capacity, altered gene expression, impaired mitochondrial
function and elevated cellular redox, all contributing to increased rates of cell death.8, 9, 10 The marked decline of oocyte number in mammalian ovaries has been attributed to oocyte loss
via stage-specific modes of death. As yet, perinatal PMF loss in mice most frequently engages apoptotic cell death,11, 12 whereas within the postnatal ovary, oocytes in growing follicles
undergo atresia, a less 'molecularly' defined death, carrying hallmarks of both apoptosis and autophagy.13, 14, 15 It is thus surprising that no member of the anti-apoptotic B-cell
lymphoma 2 (Bcl-2) family has been identified with a definitive role in governing oocyte survival and the maintenance of the ovarian reserve. _Bcl-2l2_/Bcl-w and _Bcl-2-l10_/Diva deficiency
had no apparent impact on the ovarian reserve, and although ablation of _Bcl-2_ led to a loss of one-third of the adult PMF pool, the growing follicle pool was not significantly impacted
and these animals did not undergo POF.16, 17, 18, 19 Conditional Bcl-x _(Bcl-2l1)_ inactivation led to increased primordial germ cell apoptosis in the embryo,20 but postnatal inactivation of
_Bcl-x_ in oocytes did not compromise the ovarian reserve in young females.21 _Bcl2a1a_/_Bfl-1_/_A1_ was low to undetectable in fully grown germinal vesicle (GV) or ovulated murine
oocytes,22 however, the impact of _Bfl-1_ deficiency on the ovarian reserve has not yet been analyzed to the best of our knowledge. Consequently, either various anti-apoptotic Bcl-2 members
have overlapping roles in governing postnatal oocyte survival and maintenance of the adult ovarian reserve in mice, or the anti-apoptotic Bcl-2 member that regulates this decision has yet to
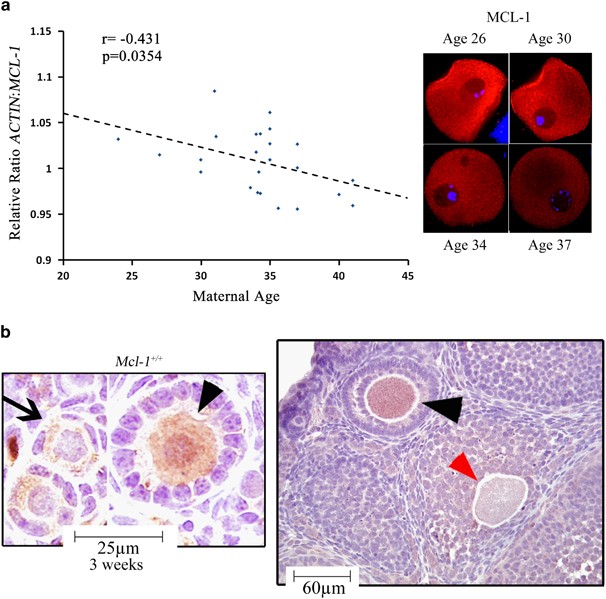
be identified. RESULTS MYELOID CELL LEUKEMIA-1 (MCL-1) IN OOCYTES IS REDUCED IN ASSOCIATION WITH AGING AND ACTIVATION OF FOLLICLE ATRESIA With the majority (99%) of all oocytes undergoing
some form of programmed cell death (PCD),3, 13, 14, 15, 23 and only a few Bcl-2 family members identified with roles in regulation of germ cell fate,16, 17, 18, 19, 20, 21 we set to
investigate which members of the anti-apoptotic Bcl-2 gene family decline with maternal age in human GV stage oocytes. A reduction in age-dependent oocyte quality has been previously
associated with activation of cell death or molecular pathways that regulate this fate.8, 10 From the analyzed targets (_BCL-2, BCL-2L1, BCL-2L10_, _BCL-2L11_ and _MCL-1_), only the _MCL-1_
transcript significantly declined in an age-associated manner, with a decrease in MCL-1 immunoreactivity in human GV oocytes (Figure 1a). Changes in _MCL-1_ transcript associated with
differences in clinical diagnoses, stimulation protocol or resultant outcome proved nonsignificant (Supplementary Table S2). MCL-1 has been shown to be expressed by fetal human and neonatal
mouse oocytes,16, 24 and abundant transcript levels were detected in ovulated human oocytes.25 We assessed the expression of MCL-1 in murine ovaries and before pubertal onset cytoplasmic
immunoreactivity of MCL-1 in oocytes increased with activation of follicle growth (from PMF to primary follicle (PF)), remained robust in the fully grown oocytes of preantral (PA) follicles
and virtually disappeared from oocytes undergoing atresia (Figure 1b). This pattern indicated that MCL-1 could be an essential regulator of oocyte survival. OOCYTE-SPECIFIC _MCL-1_
DEFICIENCY REDUCES OVARIAN FOLLICLE POOL, OVULATION AND FERTILITY To determine the functional requirement for _Mcl-1_ in oogenesis, we created a mouse model with oocyte-specific excision of
_Mcl-1_ using zona pellucida 3 (Zp3)-Cre. We confirmed spatial and temporal activity of the Cre transgene by introduction into two reporter lines, tdTomato and lacZ/alkaline phosphatase
reporter (Z/AP). Initial activation of Cre excision in oocytes was observed as early as embryonic day 17.5, with a large proportion of oocytes showing excision by postnatal day 3 (PN3;
Supplementary Figure S1A). The selectivity of excision was also confirmed in the adult Z/AP reporter line, with excision in virtually all growing oocytes (Supplementary Figure S1B). We next
combined the Zp3-Cre allele with floxed _Mcl-1_ (_Mcl-1__f/f_). Effective excision was confirmed by lack of MCL-1 immunoreactivity in oocytes of _Mcl-1__f/−_: Zp3-Cre (Mcl-1 conditional
knockout (_Mcl-1_cKO)) females (Supplementary Figure S1C). To determine the impact of _Mcl-1_ oocyte-specific deletion on fertility, _Mcl-1_cKO and control females were bred to wild-type
males of proven fertility for a period of 6 months. The cumulative breeding performance of _Mcl-1_cKO dams was markedly reduced with an average of two litters during this period, and each
with less than half of the pup number obtained from control females (Figure 2a, Supplementary Figure S2A). In addition, _Mcl-1_cKO females did not deliver any live litters beyond 4 months of
age, whereas all control females of the various genotypes were able to breed beyond 1 year of age (Supplementary Figure S2A). _Mcl-1_ excision was confirmed in all genotyped _Mcl-1_cKO
offspring (_n_=31). To establish whether the reduction in breeding performance was due to an overall reduction in the ovulatory capacity, females were primed with external gonadotropins and
their ovulatory response quantitated. We used females of various age groups (at 6 and 3 months, as well as 3 weeks) as these represented mature females, at a young reproductive age and at
pubertal onset, respectively. In _Mcl-1_cKO females, very poor ovulatory capacity was observed at 3 months and ovulation was virtually absent by 6 months (Figure 2b, Supplementary Figure
S2B). Histological examination revealed a severe depletion in PMF, PF and secondary follicles (SF) and a drastic reduction in ovary size already evident at 3 months of age (Figure 2c,
Supplementary Figure S2D). Furthermore, a significant reduction of PMFs and PFs was also observed in heterozygote females (Supplementary Figure S2C). Interestingly, at the onset of puberty
(3 weeks), females of all genotypes ovulated comparable numbers of oocytes (Supplementary Figure S2E), yet histomorphometric analyses revealed a sharp reduction in PMFs and a significant
decrease in growing follicle number (Figure 2d). The loss of growing oocytes may be partially due to an increased rate of atresia, as a significantly higher proportion of late-stage atretic
follicles was observed (Figure 2f). The loss of the resting PMF pool was the most marked outcome we have observed. At PN7, only about half of the PMFs were present with no change in the PF
or SF population (Figure 2e). This, combined with terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) analyses of ovaries at PN1 revealing that _Mcl-_1 deficiency resulted
in a doubling of the number of apoptotic (TUNEL positive) oocytes compared with controls (Figure 2g, Supplementary Figure S2F), led us to conclude that _Mcl-1_ is required for postnatal
maintenance of the ovarian reserve. _MCL-1_ DEFICIENCY LEADS TO ELEVATED ACTIVATION OF AUTOPHAGY IN ANTRAL FOLLICLE POPULATION Based on our observations that _Mcl-1_ ablation resulted in
mildly elevated atresia rates, and that MCL-1 expression was absent from oocytes undergoing atresia, we next assessed markers of PCD in the growing follicle pool. As PMF oocytes of
_Mcl-1_cKO ovaries die via an apparent apoptotic cell death, we set to investigate whether antral GV oocytes also activate this death pathway. Relative to controls, _Mcl-1_cKO oocytes
exhibited an increase in Bcl-2-associated X protein (BAX)-NT, indicative of a conformational change in the N-terminus of BAX and potentiating oligomerization; however, downstream hallmarks
of mitochondrial engagement (e.g., cytochrome C or apoptosis-inducing factor (AIF) release) showed no significant change, with no increase in pan-caspase activity (Supplementary Figures
S3A–D). This data effectively demonstrates that despite BAX activation, there is no subsequent instigation of the apoptotic cascade in antral _Mcl-1_cKO GV oocytes. As follicle atresia
exhibits hallmarks of both autophagic (PCD type 2) and apoptotic (PCD type 1) cell death13 and Mcl-1 has been previously linked to inhibition of both pathways,26, 27, 28 we turned to the
assessment of markers of autophagic activation in GV oocytes. Induction or phosphorylation of Beclin-1/BECN-1 has been shown to initiate autophagosome formation,29 and MCL-1 binding and
inhibition of Beclin-1 prevented autophagy.27 _Mcl-1_cKO oocytes displayed an increase in size and number of Beclin-1 foci, indicating an increase in Beclin-1-associated vesicle formation
(Figure 3a,Supplementary Figure S3F). In addition, microtubule-associated protein 1 light chain (MAP1LC3A), involved in autophagosome membrane elongation,30 was elevated in _Mcl-1_cKO GV
oocytes (Supplementary Figure S3E). We next examined markers of late autolysosomes. Lysosome-associated membrane proteins 1 and 2 (LAMP-1, LAMP-2) are an essential part of autophagosome
maturation, autolysosome formation and lysosomal fusion.31 _Mcl-1_cKO GV oocytes showed an increased number of foci positive for both Beclin-1 and LAMP-2 (Figure 3a). Colocalization analyses
performed on 3D rendered oocyte sections confirmed a significant increase in Beclin-1/LAMP-2 colocalization in _Mcl-1_cKO GV oocytes. These colocalization analyses also demonstrated that
oocytes possessing just one _Mcl-1_ allele (_Mcl-1__f/+_: Zp3-Cre), display an intermediary phenotype (Supplementary Figure S3F). The amplified abundance of autolysosome structures in
_Mcl-1_cKO oocytes was further validated by transmission electron microscopy (Figure 3b). The elevation in number of Beclin-1-positive foci, LC-3 immunoreactivity and Beclin-1/LAMP-2
colocalized structures, indicates an augmentation of autophagosome and autolysosome formation, triggered by _Mcl-1_ deficiency. OVULATED _MCL-1_-DEFICIENT OOCYTES DISPLAY MITOCHONDRIAL
DYSFUNCTION AND CHROMOSOMAL ABNORMALITIES Despite the marked loss of the ovarian pool at a young age, _Mcl-1_cKO females do ovulate and breed, albeit at reduced efficiencies. As oocyte
quality and reproductive competence have been suggested to rely on effective mitochondrial metabolism,32, 33, 34 and Mcl-1 has been implicated in regulation of mitochondrial function in
somatic cells,28, 35, 36 we assessed these parameters in _Mcl-1_cKO ovulated oocytes. Using a mitochondrial membrane potential sensitive dye, we observed two distinct patterns of DePsipher
fluorescence (Figure 4a). Control oocytes displayed a strong centrally localized inactive mitochondrial pool (green) with enrichment of polarized mitochondria (red) in the cortical region
(pattern 1). Conversely, many _Mcl-1_cKO oocytes exhibited a weak diffuse distribution of inactive mitochondria (green) and extremely few peripherally active polarized mitochondria (red)
(pattern 2). Moreover, the number of peripheral polarized mitochondrial foci was significantly decreased in _Mcl-1_cKO oocytes compared with controls (Figure 4a,Supplementary Figure S4A). A
decline in the respiring mitochondrial pool, evidenced by decreased MitoTracker Red intensity was also observed in _Mcl-1_cKO metaphase II (MII) oocytes (Figure 4b,Supplementary Figure S4C).
This decrease was significant despite a marked elevation in the total mitochondrial pool demonstrated by increased MitoTracker Green (Supplementary Figure S4B), a dye that binds
mitochondrial membranes irrespective of their activity. In measuring mitochondrial output, _Mcl-1_cKO MII oocytes presented with a sharp reduction in levels of the TCA cycle substrates
fumarate and malate (Figure 4c), but showed no apparent difference in total ATP level or citrate (Supplementary Figure S4D), when compared with control, implying no change in available
energy. In addition, total reactive oxygen species (ROS) and mitochondrial-derived superoxides were markedly increased (Figure 4d, Supplementary Figures S4E and 4F). High ROS levels can be
indicative of defective antioxidant machinery or an inhibition/block in the electron transport chain,37, 38 and elevated ROS and impaired oxidative phosphorylation have been linked with
defective development and meiotic spindle defects.33, 39, 40, 41 Microtubule staining of the meiotic spindle apparatus revealed increased chromosomal misalignments in _Mcl-1_cKO oocytes
compared with controls with misaligned chromosome(s) not attached to spindle (Figure 4e, Supplementary Figure S4G). _MCL-1_-DEFICIENT OVULATED OOCYTE POOL DISPLAYS ELEVATED MARKERS OF
APOPTOSIS AND INCREASED FRAGMENTATION RATES Defective mitochondrial bioenergetics are expected to impair oocyte survival, particularly after ovulation, when cumulus cells stop directly
providing oocytes with nutrients.42 Thus, we decided to investigate whether the viability of ovulated oocytes was compromised because of _Mcl-1_ deficiency. _In vitro_ culture revealed an
inability of _Mcl-1_cKO MII oocytes to sustain meiotic arrest over 24 h and an increased propensity to undergo cellular fragmentation (Figure 5a). As cellular fragmentation of oocytes is
thought to occur via apoptosis,43 we explored whether hallmarks of activation of the apoptotic pathway could be detected. Indeed, ovulated oocytes displayed an increase in BAX-NT (Figure 5b,
Supplementary Figure S5A) with no change in total BAX levels (Supplementary Figure S5A), accompanied by an increase in mitochondrial cytochrome C release (Figure 5c, Supplementary Figure
S5B) and caspase activity (Figure 5d, Supplementary Figure S5C). Thus, _Mcl-1_ deficiency in ovulated MII oocytes leads to an activation of the apoptotic cascade, and an increased proclivity
for these compromised oocytes to fragment. CONCURRENT DELETION OF _BAX_ RESCUES OVARIAN FOLLICLE LOSS AND INCREASED FRAGMENTATION RATE BUT DOES NOT IMPACT MITOCHONDRIAL DYSFUNCTION OR
CHROMOSOME ABNORMALITIES Deletion of the pro-apoptotic Bcl-2 family member _Bax_ rescues the primordial germ cell loss induced by ablation of the anti-apoptotic _Bcl-x_.20 By itself, Bax
deficiency has been linked to increased primordial germ cell survival,44 resulting in an increase in follicular endowment45 and extended ovarian function to advanced chronological age.46 In
order to evaluate whether Bax deficiency would also rescue loss of the ovarian reserve in _Mcl-1_cKO females, we crossed a _Bax_-deficient line to our _Mcl-1_cKO mouse line. Ovaries from 3
month _Bax__−/−_(Bax knockout (_Bax_KO)), _Mcl-1__f/−_: _Bax__−/−_: Zp3-Cre (double knockout with conditional Mcl-1 and total Bax deletion (_Mcl-1_c_/Bax_DKO)), _Mcl-1_cKO females and
controls were used for histomorphometric ovarian analyses. Bax deficiency alone substantially increased the cohort of PMFs; and the _Mcl-1_c/_Bax_DKO ovarian reserve resembled _Bax_KO
ovaries in all follicle numbers, implying a rescue of the _Mcl-1_-deficient oocyte phenotype (Figure 6a, Supplementary Figure S6A). Moreover, _Mcl-1_c/_Bax_DKO females displayed restored
ovulation rates (Figure 6b, Supplementary Figure S6B), and a resiliency of MII oocytes against cellular fragmentation during _in vitro_ culture, akin to wild-type controls and _Bax_KO
(Figure 6c, Supplementary Figure S6C). However, despite a very robust rescue to the ovarian follicle pool, Bax deficiency was not able to restore mitochondrial functionality (Figure 6d,
Supplementary Figure S6D) or correct the choromosomal misalignments (Figure 6e) in ovulated oocytes, indicating that, in oocytes, _Mcl-1_ regulates additional aspects of cellular and
mitochondrial physiology independent of Bax or cell death. Indeed, breeding trials of older (4- to 8-month old) _Bax_KO or _Mcl-1_c/_Bax_DKO mice revealed a longer breeding expectancy, but
not an increase in the number of pups, as litter size remained <2. Although no _Mcl-1c_KO mice (_n_=8) produced litters after 4 months of age, two out of three _Mcl-1_c/_Bax_DKO females
produced at least one litter during the 3-month breeding trial (Supplementary Table S1). DISCUSSION Limited ovarian phenotypes caused by deletion of anti-apoptotic Bcl-2 members16, 17, 18,
19, 21 has led to the postulation that they are either uninvolved or redundant in postnatal oocyte survival. Mcl-1 has a well-documented cell survival role for numerous somatic cell
types;36, 47, 48 however, its necessity for long-term germ cell viability has not been established. We present evidence of Mcl-1 as the first pro-survival Bcl-2 member required for life-time
maintenance of the ovarian reserve and stage-specific inhibition of varying modes of oocyte cell death. As demonstrated above, cytoplasmic MCL-1 expression increases during the transition
from PMF to PF, and continues to accumulate with sustained follicle growth; but oocytes in atretic follicles lack MCL-1 indicating that downregulation of MCL-1 could precede initiation of
oocyte atresia. Mcl-1 appears to mediate PMF survival by antagonizing Bax action, as _Mcl-1_ deficiency-induced PMF loss by apoptotic oocyte death, was restored by concurrent Bax ablation.
Those _Mcl-1-_deficient oocytes that escape the early PMF demise and begin to grow, exhibit increased markers of cellular autophagy without apoptotic activation, resulting in only mildly
elevated rates of atresia. This lack of apoptotic activation may in part be due to additional anti-apoptotic Bcl-2 members neutralizing activated BAX in the absence of MCL-1, or via
additional undetermined mechanisms. We also show that the ability to initiate the apoptotic cascade does not occur till ovulation, as meiotic completion is essential for apoptotic
activation.49, 50 We postulate that _Mcl-1_-deficient GV oocytes activate the autophagic machinery in response to mitochondrial dysfunction accompanied by disruption of metabolic machinery,
however, we cannot exclude the possibility that Mcl-1 can directly regulate autophagy. Deletion of Mcl-1 has been recently linked to elevated mitochondrial dysfunction and activation of
either autophagy or apoptosis in a stress- and cell-specific manner in cardiomyocytes, cortical neurons and MEFs.28, 35, 36 When evaluated, these phenotypes were accompanied by disruption of
mitochondrial membrane potential, defective ATP output and production of superoxides. Mitochondrial bioenergetic output and functionality have long been considered essential factors in
mediating oocyte quality and reproductive competence.32, 33 _Mcl-1_cKO oocytes display enhanced mitochondrial dysfunction; with elevated superoxides, impaired mitochondrial activity and
reduced fumarate and malate, associated with chromosome abnormalities and elevated MII oocyte fragmentation; the last phenotype alone being mitigated by concurrent Bax deficiency. These
mitochondrial phenotypes may be regulated by a newly identified matrix-localized isoform of MCL-1, associated with prevention of defective complex II enzymatic activity and disrupted
supercomplex assembly.35 Confirmation of a role for this isoform in oocytes is yet to be determined. As oocyte-cumulus cell contact is required for the continuance of meiotic arrest, oocyte
growth51, 52 and regulation of metabolite supply to the oocyte,53, 54, 55, 56 it is conceivable that maintained granulosa/cumulus cell support permits the _Mcl-1_-deficient immature oocytes
to prolong their demise and, in conjunction with activation of autophagy, compensates for associated metabolic deficiencies. Subtle changes in ATP levels57, 58 and relief of MCL-1 inhibition
of Beclin,27, 28 could be sufficient for autophagic activation in _Mcl-1_-deficient oocytes. Additional studies are required to elucidate whether the maintenance of mitochondrial
dysfunction with concomitant Bax deficiency may be due to a BAX-independent function of MCL-1, an additional role of BAX in mitochondrial function59 or some combination of both. As breeding
was prolonged but not fully rescued by Bax deficiency, it indicates that although oocyte death could be prevented by Bax ablation, additional roles of Mcl-1, independent of Bax, are present,
and may also govern embryo development.60 It would also be very informative to determine if depletion of Bok, another multi-channel pro-apoptotic member of Bcl-2 gene family, could rescue
the developmental competence of _Mcl-1-_deficient oocytes. It should also be noted that in keeping with previous data,46 _Bax_-deficient females do display variable breeding performance.
Fertility and reproductive proficiency has been well established to rely on the maintenance of the ovarian reserve in addition to preservation of oocyte quality. We observed a correlation
between maternal age and _MCL-1_ expression levels in human oocytes, and our animal model demonstrates that oocyte-specific ablation of _Mcl-1_ results in the accumulation of defects
associated with compromised oocyte quality.6, 7, 9, 10, 61 Furthermore, we establish that Mcl-1 has the defining role in mediation of oocyte survival via protection of the postnatal PMF
pool, in addition to the growing and ovulated oocyte pool. Thus, investigation into modulation of Mcl-1 expression by oocytes could prove informative in understanding what factors may
contribute to POF and age-related oocyte loss. MATERIALS AND METHODS ANIMALS _Mcl-1__tm3Sjk_ (_Mcl-1__fl_)47 mice carrying the floxed allele were obtained from the breeding colony of Dr.
Korsmeyer and were intercrossed to mice carrying the Tg(Zp3-Cre)3Mrt (Zp3-Cre) transgene62 and to _Bax__tm1Sjk_ mice.63 All mice were housed with free access to food and water and maintained
on a 12- h:12- h light–dark cycle. All mouse experiments were performed in accordance with the Canadian Council on Animal Care (CCAC) guidelines for Use of Animals in Research and
Laboratory Animal Care, under protocols approved by animal care committees of the Toronto Centre for Phenogenomics (TCP). To assess timing of excision, Zp3-Cre mice62 were also crossed to
reporter lines Tg(CAG-Bgeo/ALPP)1Lbe (Z/AP)64 and Gt(ROSA)26Sortm9(CAG-tdTomato)Hze (tdTomato).65 Animals were genotyped for possession of either _Mcl-1_+ or _Mcl-1__f_ alleles using primers
5'-CTGAGAGTTGTACCGGACAA-3' (7MCL1) and 5'-GCAGTACAGGTTCAAGCCGATG-3' (6MCL1), and for _Mcl-1__null_ (_Mcl-1__−_) allele using primers 7MCL1 and
5'-ACGCTCTTTAAGTGTTTGGCC-3' (2MCL1). Presence of Zp3-Cre transgene was assessed using 5'-TGATGAGGTTCGCAAGAACC-3' (CREF) and 5'-CCATGAGTGAACGAACCTGG-3' (CRER)
and genotyping for _Bax_+ _and Bax__−_ alleles utilized 5'-GAGCTGATCAGAACCATCATG-3' (BAX-EX5-F), 5'-GTTGACCAGAGTGGCGTAGG-3' (BAX-LN5-R) and
5'-CCGCTTCCATTGCTCAGCGG-3' (BAX-NEO). COLLECTION OF OOCYTES AND BREEDING Immature (GV) oocytes were collected from antral follicles 46–48 h after pregnant mare’s serum gonadotropin
(PMSG; NHPP, Torrance, CA, USA or ProSpec, Rehovot, Israel (HOR-272)) priming and manually stripped of cumulus cells using narrow glass pipettes. For mature ovulated oocytes (MII), human
chorionic gonadotropin (Sigma, Oakville, ON, Canada) priming was performed 44–48 h after PMSG and oocytes collected 14–16 h later; with cumulus removed by incubation in Hyaluronidase
(Sigma). MII oocyte fragmentation rates were performed by MII culture in HTF media (Life Global, Guilford, CT, USA) supplemented with 0.1% BSA (Sigma) for 24 h. For breeding rates, dams at
4–5 weeks were mated to young wild-type males for a 6-month breeding trial and total litter number and size recorded. For _Mcl-1c/Bax_DKO or _Bax_KO breedings, dams at 4–8 months of age were
bred to wild-type (_Mcl-1__f/+_) males for 2–3 months and litters were recorded. Immature human oocytes, obtained from patients undergoing IVF treatment, were donated to research after
obtaining patient consent approved by the Research Ethics Board at Mount Sinai Hospital, Toronto. Details of gene expression studies are described below. REAL-TIME PCR WITH HUMAN OOCYTES
Obtained single human oocytes at GV stage free of cumulus cells were loaded into guanidine iso-thiocyanate solution and total nucleic acids were precipitated using glycogen as a carrier.22
Upon treatment with DNase (Sigma-Aldrich, St. Louis, MO, USA), reverse transcription was performed on the whole sample using Revert Aid Kit (Fermentas, Invitrogen Life Technologies,
Burlington, ON, Canada) and oligo-dT primers. cDNA product was used in a real-time PCR reaction using LightCycler 480 SYBR Green I Master (Roche Applied Science, Indianapolis, IN, USA) and
LightCycler 480 (Roche, Mannheim, Germany). The amplification profile included a pre-incubation step at 95 °C for 5 min, followed by denaturation at 95 °C, annealing at 62 °C and extension
at 72 °C. The target gene concentration for the oocyte samples was extrapolated utilizing the standard curve for each target and the data were expressed as relative ratio to CP value of
_β-ACTIN_. Primer sequences for all genes analyzed (Supplementary Table S3). HISTOLOGICAL OVARIAN ANALYSES Ovaries from _Mcl-1__f/−_: Zp3-Cre (_Mcl-1_cKO), _Mcl-1__f/−_, _Mcl-1__f/f_,
_Mcl-1_+/+, _Mcl-1_+/+: Zp3-Cre, _Mcl-1__f/+_: Zp3-Cre, _Bax__−/−_, _Mcl-1__f/+_: _Bax__−/−_: Zp3-Cre and _Mcl-1__f/−_: _Bax__−/−_: Zp3-Cre females were collected at varying timepoints (6
months, 3 months, 3 weeks, PN14 or PN7) and fixed in Dietrichs (4% formalin, 28% EtOH, 0.34N Glacial Acetic Acid (Sigma)) or 10% formalin (Fisher, Ottawa, ON, Canada) and following standard
dehydration protocols were embedded in paraffin wax and sectioned (5 _μ_m) using a LEICA (Concord, ON, Canada) RM2255 Microtome and then mounted on Superfrost plus slides. Sections fixed in
Dietrichs were rehydrated and stained with a picric acid/methyl blue stain, allowing for better resolution for histomorphometric analyses. Every third section was counted for PN7 and PN14
ovaries, every fifth section counted for 3 weeks ovaries, and every 10th section for 3 months and 6 months. Oocytes with visible nuclei from primordial, primary, secondary and antral
follicles were quantitated and recorded and multiplied by associated factor (x3, x5 and x10) to gain an approximately full representation of the ovary. Atretic follicles were counted in
sections from 3-week ovaries and are shown as a proportion of the total post-secondary growing follicles. Ovaries from embryonic day 17.5 and PN3 tdTomato: Zp3-Cre animals were removed from
animals and washed in mHTF. Entire tissue samples were viewed under LEICA DMI60003 Spinning Disc Confocal or LEICA MZ 165A Stereomicroscope using TRITC-Red laser (561 excitation, 620
emission), and imaged. Cryosections of ovaries from Z/AP: Zp3-Cre mice were postfixed in 0.2% glutaraldehyde and endogenous alkaline phosphatase (AP) activity was inactivated by heating for
30 min in PBS at 70 °C and then rinsed in PBS. Sections were then washed in buffer (100 mM Tris-HCL pH 9.5, 100 mM NaCl, 10 mM MgCl2) for 10 min and stained with NBT/BCIP stain, washed in
PTM (0.1%Tween20, 2 mM MgCl2, in PBS) and counterstained with Nuclear Fast Red. Sections fixed in 10% formalin were rehydrated and used for immunohistochemical staining protocols. Sections
were submitted to antigen retrieval at ~95 °C for 10 min in sodium citrate buffer (10 mM tri-sodium citrate (Sigma) pH 6.0 with HCl), washed and blocked in 10% normal horse serum (NHS) for 1
h before overnight incubation in primary antibody (in 10% NHS) at 4 °C. Primary antibodies utilized include Mcl-1 (Rockland Immunochemicals, Limerick, PA, USA, 600-401-394S). Sections were
then washed in PBS and incubated with secondary protocols from ABC Vectastain kit (PK-4001; Vector Labs, Burlington, ON, Canada) and then visualized using diamino-benzidine (DAB) (Sigma)
substrate. After time-sensitive stain development, sections were counterstained in hematoxylin (Sigma) for identification of cell nuclei. TUNEL ANALYSES For detection of apoptotic cells,
sections of PN1 ovaries were fixed in formalin, rehydrated and incubated in Proteinase K followed by permeabilization with 0.1% Triton-X. Slides were incubated in Reaction Mix (4 _μ_M
biotin16-dUTP (Roche), 1.5 _μ_M dATP, 1X NEB4 buffer, 4 U/ul TdT enzyme (Roche) for 1.5 h at 37 °C and incorporated nucleotides were detected with streptavidin ABC Vectastain (Vector Labs)
and visualized with DAB. MITOCHONDRIAL ANALYSES – LIVE CELL STAINS MII ovulated oocytes of _Mcl-1cKO_ and controls were collected and subjected to a number of assays for determination of
mitochondrial function. Total and respiring mitochondria were stained using Mitotracker fluorescent dyes (MitoTracker Green FM (M7154), MitoTracker Red 580 (M22425); Molecular Probes,
Invitrogen LifeTechnologies) added to HTF in 100 nM concentration for 30 min, then washed in mHTF and imaged. For total cellular levels of ROS, oocytes were incubated in 10 _μ_M 2′,
7′-dichlorofluorescein diacetate (DCFDA; Molecular Probes) or 5 _μ_M MitoSox (Molecular Probes) in HTF for 15 min, washed in mHTF and imaged. The performance of fluorescent probes utilized
for detection of ROS, and various mitochondrial markers was validated (Supplementary Figures S5D and E), using MII oocytes cultured in the presence of inducers of apoptosis or inhibitors of
the electron transport chain. Induction of apoptosis in ovulated oocytes was done as previously described66 with 200 nM doxorubicin (DXR; Alexis, Enzo LifeSciences, Farmingdale, NY, USA) for
20 h followed by incubation with MitoTracker Green, MitoTracker Red, DCFDA or MitoSox. For disruption of mitochondrial function and inhibition of electron transport chain, MII oocytes were
incubated in 100 nM complex III inhibitor Antimycin (Sigma) or vehicle (ethanol) for 15 min followed by co-incubation with mitochondrial dyes. Death rates for MII oocytes with antimycin
treatment are included in Supplementary Figure S5E. For mitochondrial membrane potential, MII oocytes were incubated with 5 _μ_g/ml DePsipher (DePsipher, Trevigen (6300-100-K, Gaithersburg,
MD, USA)) in HTF for 90 min then washed and imaged. The mitochondrial fluorescent distribution of each oocyte was visually separated into pattern 1 – oocytes with strong central green
fluorescence distribution and peripheral scattering of polarized mitochondria (red); or pattern 2 – oocytes with diffuse distribution of green fluorescence and little to no peripheral
polarized mitochondria (red). To assess metabolic profile citrate, malate and fumarate levels, MII oocytes were first frozen on glass slides by dipping in cold isopentane. Oocytes were then
freeze–dried and processed for metabolic profile using protocol delineated in Chi _et al._67 ATP content in single oocytes was measured by Cell Titer GLO assay (Promega, Madison, WI, USA).
IMMUNOFLUORESCENCE STAINING GV or MII oocytes from _Mcl-1_cKO and control females were fixed in 10% formalin for 10 min and used for staining of markers of apoptosis and autophagy. Oocytes
were first transferred to cooling ~95 °C sodium citrate buffer for antigen retrieval for 10 min using pulled-glass pipettes. Oocytes were moved to three washes in 0.1% Triton-X in 10 mM PBS
and then blocked in 10% NHS in PBS. Following this step, oocytes were incubated in primary antibody overnight at 4 °C. Primary antibodies used include anti-Beclin-1 (Santa Cruz
Biotechnologies, Dallas, TX, USA, sc-11427), anti-LC-3 (MBL, EMD Millipore, Billerica, MA, USA, PM046), anti-Lamp1 (1D4B, Developmental Studies Hybridoma Bank, Iowa City, IA, USA),
anti-Lamp2 (ABL-93, Developmental Studies Hybridoma Bank), anti-Bax-NT (Upstate, EMD Millipore; 06-49), anti-BAX (Alexis, 210-003), anti-tubulin (Invitrogen LifeTechnologies, A11126),
anti-actin (Santa Cruz Biotechnologies, sc-1616), anti-AIF (Santa Cruz Biotechnologies, sc-9416), anti-Mcl-1 (Santa Cruz Biotechnologies, sc-819) and anti-Mcl-1 (Rockland Immunochemicals,
600-401-394S). After primary antibody incubation, oocytes were transferred to 0.1%TX washes and incubated in host-specific secondary antibody conjugated with Alexa Fluor dyes (Invitrogen
LifeTechnologies) and counterstained with blue fluorescent 4′,6-diamidino-2-phenylindole (DAPI, Sigma). Stained samples were mounted on slides in 50% glycerol for imaging. For negative
controls, oocytes were exposed to nonspecific IgG or secondary antibody alone. For evaluation of apoptotic induction, cytochrome c release, AIF release and pan-caspase activity assays were
performed on GV and MII oocytes with protocols modified from Carboxyfluorescein Multi-Caspase Activity Kit (Biomol, Enzo LifeSciences) and cytochrome c release kit (InnoCyte – Calbiochem,
EMD MIllipore). A portion of denuded GV and MII oocytes were permeabilized for 10 min with digitonin buffer. Both permeabilized and non-permeabilized oocytes were then fixed, and processed
through the staining. For AIF release, AIF (Santa Cruz Biotechnologies, sc-9416) was utilized instead of cytochrome c antibody. For pan-caspase activity, GV and MII oocytes were incubated in
FML-VAD-FMK stock dissolved in HTF medium for 2.5 h and washed and transferred to fixative as indicated in caspase activity kit. Oocytes were then washed and transferred to DAPI for 10–15
min, then mounted on Superfrost slides in 50% glycerol and imaged, as mentioned previously. The efficacy of these markers of apoptotic induction was verified (Supplementary Figure S5D),
using MII oocytes cultured in the presence of 200 nM DXR66 for 14 h (Bax, cytochrome c) or 20 h (pan-caspase). MII oocytes incubated with DXR for 24 h display death rates of 80%. IMAGING
Stained oocytes were imaged using LEICA DMI60003 Spinning Disc Confocal microscope with appropriate filters (objectives: 10X/0.40NA, 20X/0.70NA). Images were acquired and analyzed using
Volocity software (PerkinElmer, Waltham, MA, USA) with Z-stack images taken at 0.354 _μ_m increments across 10 _μ_m sections to the either side of the midpoint of the oocyte, and average
mean fluorescent intensity obtained from each sample was used for comparative purposes and expressed as random fluorescent units (RFUs). Colocalization data images were analyzed using Imaris
(Bitplane, Zurich, Switzerland) software. Histological ovarian sections were imaged with a LEICA/LEITX DMRXE microscope (objectives: 5X/0.12 NA, 10X/0.30 NA, 20X/0.50 NA, 40X/0.85 NA,
63X/0.75 NA, 100X/1.30 NA). Whole ovary images of TdTomato: Zp3-Cre mouse line and controls were acquired using the LEICA MZ16FA stereomicroscope (objectives: 4X/0.45 NA), with appropriate
filters. WESTERN BLOTS Two hundred GV oocytes from approximately 8–10 _Mcl-1_cKO and _Mcl-1_+/+ females were collected and lysed in RIPA buffer. Lysates were separated by SDS-PAGE and then
transferred on to a PVDF membrane. Blots were incubated with anti-Mcl-1 (Rockland Immunochemicals, 600-401-394S) or anti-actin (Santa Cruz Biotechnologies, sc-1616). Membranes were washed
and then incubated with specific HRP-conjugated donkey anti-rabbit (Santa Cruz Biotechnologies) or HRP-conjugated donkey anti-goat (Santa Cruz Biotechnologies) and detected using enhanced
chemiluminescence (Thermo Scientific, Burlington, ON, Canada) SuperSignal West Femto Chemiluminescent Substrate and then imaged on the VersaDoc 5000MP Imaging System (Bio-Rad, Mississauga,
ON, Canada). STATISTICAL ANALYSIS Data were analyzed using either one-way ANOVA with Holm–Sidak multiple comparisons test (Breeding performance, histomorphometric analyses, PCC, PCC-C); or
using non-parametric Kruskal–Wallis one-way ANOVA on ranks, followed by Dunns _post-hoc_ test for comparisons between groups, when normality failed or sample sizes were vastly different
(ovulation rates, active Bax (GV), beclin, LC-3, MitoTracker green and red, active Bax (MII), fragmentation rates, MCC-A, MCC-B, MitoSox, ROS, cytochrome c, caspase activity, chromosomal
misalignments). Statistical measures for two groups were performed using the unpaired _t_-test (histomorphometric analyses, atresia rates, metabolites), two-way ANOVA (DePsipher) or _χ_2
test (chromosome misalignments; Figure 6). Association maternal age and gene expression was done by Pearson correlation. All analysis was done using the Sigma Stat (Systat, San Jose, CA,
USA) or PRISM software package (Graph Pad, San Diego, CA, USA). In all cases, differences were considered significant if value reached _P_<0.05. ABBREVIATIONS * AIF: apoptosis-inducing
factor * Bax: Bcl-2-associated X protein * Bcl-2: B-cell lymphoma 2 * BaxKO: Bax knockout * GV: germinal vesicle * LAMP: lysosome-associated membrane protein * MAP1LC3A:
microtubule-associated protein 1 light chain * Mcl-1: myeloid cell leukemia-1 * Mcl-1cKO: Mcl-1 conditional knockout * Mcl-1c/BaxDKO: Double knockout with conditional Mcl-1 and total Bax
deletion * MII: metaphase II * PA: preantral * PCD: programmed cell death * PF: primary follicle * PMF: primordial follicle * PMSG: pregnant mare’s serum gonadotropin * PN: postnatal day *
POF: premature ovarian failure * ROS: reactive oxygen species * SF: secondary follicle * TUNEL: Terminal deoxynucleotidyl transferase dUTP nick end labeling * Z/AP: lacZ/alkaline phosphatase
reporter * ZP3: zona pellucida 3 REFERENCES * Forabosco A, Sforza C . Establishment of ovarian reserve: a quantitative morphometric study of the developing human ovary. _Fertil Steril_
2007; 88: 675–683. Article Google Scholar * Wallace WH, Kelsey TW . Human ovarian reserve from conception to the menopause. _PloS One_ 2010; 5: e8772. Article Google Scholar * Morita Y,
Tilly JL . Oocyte apoptosis: like sand through an hourglass. _Dev Biol_ 1999; 213: 1–17. Article CAS Google Scholar * Conway GS . Premature ovarian failure. _Curr Opin Obstet Gynecol_
1997; 9: 202–206. Article CAS Google Scholar * Goswami D, Conway GS . Premature ovarian failure. _Horm Res_ 2007; 68: 196–202. CAS PubMed Google Scholar * Faddy MJ, Gosden RG . A model
conforming the decline in follicle numbers to the age of menopause in women. _Hum Reprod_ 1996; 11: 1484–1486. Article CAS Google Scholar * Faddy MJ . Follicle dynamics during ovarian
ageing. _Mol Cell Endocrinol_ 2000; 163: 43–48. Article CAS Google Scholar * Hamatani T, Falco G, Carter MG, Akutsu H, Stagg CA, Sharov AA _et al_. Age-associated alteration of gene
expression patterns in mouse oocytes. _Hum Mol Genet_ 2004; 13: 2263–2278. Article CAS Google Scholar * Hunt PA, Hassold TJ . Human female meiosis: what makes a good egg go bad? _Trends
Genet_ 2008; 24: 86–93. Article CAS Google Scholar * Jurisicova A, Rogers I, Fasciani A, Casper RF, Varmuza S . Effect of maternal age and conditions of fertilization on programmed cell
death during murine preimplantation embryo development. _Mol Hum Reprod_ 1998; 4: 139–145. Article CAS Google Scholar * Ghafari F, Gutierrez CG, Hartshorne GM . Apoptosis in mouse fetal
and neonatal oocytes during meiotic prophase one. _BMC Dev Biol_ 2007; 7: 87. Article Google Scholar * Kerr JB, Myers M, Anderson RA . The dynamics of the primordial follicle reserve.
_Reproduction_ 2013; 146: R205–R215. Article CAS Google Scholar * Escobar ML, Echeverria OM, Ortiz R, Vazquez-Nin GH . Combined apoptosis and autophagy, the process that eliminates the
oocytes of atretic follicles in immature rats. _Apoptosis_ 2008; 13: 1253–1266. Article CAS Google Scholar * Lobascio AM, Klinger FG, Scaldaferri ML, Farini D, De Felici M . Analysis of
programmed cell death in mouse fetal oocytes. _Reproduction_ 2007; 134: 241–252. Article CAS Google Scholar * De Felici M, Lobascio AM, Klinger FG . Cell death in fetal oocytes: many
players for multiple pathways. _Autophagy_ 2008; 4: 240–242. Article CAS Google Scholar * Jones RL, Pepling ME . Role of the antiapoptotic proteins BCL2 and MCL1 in the neonatal mouse
ovary. _Biol Reprod_ 2013; 88: 46. PubMed Google Scholar * Print CG, Loveland KL, Gibson L, Meehan T, Stylianou A, Wreford N _et al_. Apoptosis regulator bcl-w is essential for
spermatogenesis but appears otherwise redundant. _Proc Natl Acad Sci USA_ 1998; 95: 12424–12431. Article CAS Google Scholar * Ratts VS, Flaws JA, Kolp R, Sorenson CM, Tilly JL . Ablation
of bcl-2 gene expression decreases the numbers of oocytes and primordial follicles established in the post-natal female mouse gonad. _Endocrinology_ 1995; 136: 3665–3668. Article CAS
Google Scholar * Russell HR, Lee Y, Miller HL, Zhao J, McKinnon PJ . Murine ovarian development is not affected by inactivation of the bcl-2 family member diva. _Mol Cell Biol_ 2002; 22:
6866–6870. Article CAS Google Scholar * Rucker EB 3rd, Dierisseau P, Wagner KU, Garrett L, Wynshaw-Boris A, Flaws JA _et al_. Bcl-x and Bax regulate mouse primordial germ cell survival
and apoptosis during embryogenesis. _Mol Endocrinol_ 2000; 14: 1038–1052. Article CAS Google Scholar * Riedlinger G, Okagaki R, Wagner KU, Rucker EB 3rd, Oka T, Miyoshi K _et al_. Bcl-x
is not required for maintenance of follicles and corpus luteum in the postnatal mouse ovary. _Biol Reprod_ 2002; 66: 438–444. Article CAS Google Scholar * Jurisicova A, Latham KE, Casper
RF, Varmuza SL . Expression and regulation of genes associated with cell death during murine preimplantation embryo development. _Mol Reprod Dev_ 1998; 51: 243–253. Article CAS Google
Scholar * Tilly JL . Commuting the death sentence: how oocytes strive to survive. _Nat Rev Mol Cell Biol_ 2001; 2: 838–848. Article CAS Google Scholar * Hartley PS, Bayne RA, Robinson
LL, Fulton N, Anderson RA . Developmental changes in expression of myeloid cell leukemia-1 in human germ cells during oogenesis and early folliculogenesis. _J Clin Eendocrinol Metab_ 2002;
87: 3417–3427. Article CAS Google Scholar * Jurisicova A, Acton BM . Deadly decisions: the role of genes regulating programmed cell death in human preimplantation embryo development.
_Reproduction_ 2004; 128: 281–291. Article CAS Google Scholar * Youle RJ, Strasser A . The BCL-2 protein family: opposing activities that mediate cell death. _Nat Rev Mol Cell Biol_ 2008;
9: 47–59. Article CAS Google Scholar * Erlich S, Mizrachy L, Segev O, Lindenboim L, Zmira O, Adi-Harel S _et al_. Differential interactions between Beclin 1 and Bcl-2 family members.
_Autophagy_ 2007; 3: 561–568. Article CAS Google Scholar * Germain M, Nguyen AP, Le Grand JN, Arbour N, Vanderluit JL, Park DS _et al_. MCL-1 is a stress sensor that regulates autophagy
in a developmentally regulated manner. _EMBO J_ 2011; 30: 395–407. Article CAS Google Scholar * Kang R, Zeh HJ, Lotze MT, Tang D . The Beclin 1 network regulates autophagy and apoptosis.
_Cell Death Differ_ 2011; 18: 571–580. Article CAS Google Scholar * Kirisako T, Baba M, Ishihara N, Miyazawa K, Ohsumi M, Yoshimori T _et al_. Formation process of autophagosome is traced
with Apg8/Aut7p in yeast. _J Cell Biol_ 1999; 147: 435–446. Article CAS Google Scholar * Huynh KK, Eskelinen EL, Scott CC, Malevanets A, Saftig P, Grinstein S . LAMP proteins are
required for fusion of lysosomes with phagosomes. _EMBO J_ 2007; 26: 313–324. Article CAS Google Scholar * Wai T, Ao A, Zhang X, Cyr D, Dufort D, Shoubridge EA . The role of mitochondrial
DNA copy number in mammalian fertility. _Biol Reprod_ 2010; 83: 52–62. Article CAS Google Scholar * Zeng HT, Ren Z, Yeung WS, Shu YM, Xu YW, Zhuang GL _et al_. Low mitochondrial DNA and
ATP contents contribute to the absence of birefringent spindle imaged with PolScope in _in vitro_ matured human oocytes. _Hum Reprod_ 2007; 22: 1681–1686. Article CAS Google Scholar *
Downs SM, Humpherson PG, Leese HJ . Pyruvate utilization by mouse oocytes is influenced by meiotic status and the cumulus oophorus. _Mol Reprod Dev_ 2002; 62: 113–123. Article CAS Google
Scholar * Perciavalle RM, Stewart DP, Koss B, Lynch J, Milasta S, Bathina M _et al_. Anti-apoptotic MCL-1 localizes to the mitochondrial matrix and couples mitochondrial fusion to
respiration. _Nat Cell Biol_ 2012; 14: 575–583. Article CAS Google Scholar * Wang X, Bathina M, Lynch J, Koss B, Calabrese C, Frase S _et al_. Deletion of MCL-1 causes lethal cardiac
failure and mitochondrial dysfunction. _Genes Dev_ 2013; 27: 1351–1364. Article CAS Google Scholar * Boveris A, Cadenas E, Stoppani AO . Role of ubiquinone in the mitochondrial generation
of hydrogen peroxide. _Biochem J_ 1976; 156: 435–444. Article CAS Google Scholar * Turrens JF, Alexandre A, Lehninger AL . Ubisemiquinone is the electron donor for superoxide formation
by complex III of heart mitochondria. _Arch Biochem Biophys_ 1985; 237: 408–414. Article CAS Google Scholar * Thouas GA, Trounson AO, Jones GM . Developmental effects of sublethal
mitochondrial injury in mouse oocytes. _Biol Reprod_ 2006; 74: 969–977. Article CAS Google Scholar * Thouas GA, Trounson AO, Wolvetang EJ, Jones GM . Mitochondrial dysfunction in mouse
oocytes results in preimplantation embryo arrest _in vitro_. _Biol Reprod_ 2004; 71: 1936–1942. Article CAS Google Scholar * Johnson MT, Freeman EA, Gardner DK, Hunt PA . Oxidative
metabolism of pyruvate is required for meiotic maturation of murine oocytes _in vivo_. _Biol Reprod_ 2007; 77: 2–8. Article CAS Google Scholar * Norris RP, Freudzon M, Mehlmann LM, Cowan
AE, Simon AM, Paul DL _et al_. Luteinizing hormone causes MAP kinase-dependent phosphorylation and closure of connexin 43 gap junctions in mouse ovarian follicles: one of two paths to
meiotic resumption. _Development_ 2008; 135: 3229–3238. Article CAS Google Scholar * Perez GI, Tao XJ, Tilly JL . Fragmentation and death (a.k.a. apoptosis) of ovulated oocytes. _Mol Hum
Reprod_ 1999; 5: 414–420. Article CAS Google Scholar * Stallock J, Molyneaux K, Schaible K, Knudson CM, Wylie C . The pro-apoptotic gene Bax is required for the death of ectopic
primordial germ cells during their migration in the mouse embryo. _Development_ 2003; 130: 6589–6597. Article CAS Google Scholar * Greenfeld CR, Pepling ME, Babus JK, Furth PA, Flaws JA .
BAX regulates follicular endowment in mice. _Reproduction_ 2007; 133: 865–876. Article CAS Google Scholar * Perez GI, Jurisicova A, Wise L, Lipina T, Kanisek M, Bechard A _et al_.
Absence of the proapoptotic Bax protein extends fertility and alleviates age-related health complications in female mice. _Proc Natl Acad Sci USA_ 2007; 104: 5229–5234. Article CAS Google
Scholar * Opferman JT, Letai A, Beard C, Sorcinelli MD, Ong CC, Korsmeyer SJ . Development and maintenance of B and T lymphocytes requires antiapoptotic MCL-1. _Nature_ 2003; 426: 671–676.
Article CAS Google Scholar * Opferman JT, Iwasaki H, Ong CC, Suh H, Mizuno S, Akashi K _et al_. Obligate role of anti-apoptotic MCL-1 in the survival of hematopoietic stem cells.
_Science_ 2005; 307: 1101–1104. Article CAS Google Scholar * Jurisicova A, Lee HJ, D'Estaing SG, Tilly J, Perez GI . Molecular requirements for doxorubicin-mediated death in murine
oocytes. _Cell Death Differ_ 2006; 13: 1466–1474. Article CAS Google Scholar * Lefevre B, Gougeon A, Nome F, Testart J . _In vivo_ changes in oocyte germinal vesicle related to follicular
quality and size at mid-follicular phase during stimulated cycles in the cynomolgus monkey. _Reprod Nutr Dev_ 1989; 29: 523–531. Article CAS Google Scholar * Conti M, Hsieh M, Zamah AM,
Oh JS . Novel signaling mechanisms in the ovary during oocyte maturation and ovulation. _Mol Cell Endocrinol_ 2012; 356: 65–73. Article CAS Google Scholar * Norris RP, Ratzan WJ, Freudzon
M, Mehlmann LM, Krall J, Movsesian MA _et al_. Cyclic GMP from the surrounding somatic cells regulates cyclic AMP and meiosis in the mouse oocyte. _Development_ 2009; 136: 1869–1878.
Article CAS Google Scholar * Colonna R, Mangia F . Mechanisms of amino acid uptake in cumulus-enclosed mouse oocytes. _Biol Reprod_ 1983; 28: 797–803. Article CAS Google Scholar *
Eppig JJ, Pendola FL, Wigglesworth K, Pendola JK . Mouse oocytes regulate metabolic cooperativity between granulosa cells and oocytes: amino acid transport. _Biol Reprod_ 2005; 73: 351–357.
Article CAS Google Scholar * Eppig JJ . Analysis of mouse oogenesis _in vitro_. Oocyte isolation and the utilization of exogenous energy sources by growing oocytes. _J Exp Zool_ 1976;
198: 375–382. Article CAS Google Scholar * Biggers JD, Whittingham DG, Donahue RP . The pattern of energy metabolism in the mouse oocyte and zygote. _Proc Natl Acad Sci USA_ 1967; 58:
560–567. Article CAS Google Scholar * Schutt F, Aretz S, Auffarth GU, Kopitz J . Moderately reduced ATP levels promote oxidative stress and debilitate autophagic and phagocytic capacities
in human RPE cells. _Invest Ophthalmol Vis Sci_ 2012; 53: 5354–5361. Article Google Scholar * Schellens JP, Meijer AJ . Energy depletion and autophagy. Cytochemical and biochemical
studies in isolated rat hepatocytes. _Histochem J_ 1991; 23: 460–466. Article CAS Google Scholar * Hoppins S, Edlich F, Cleland MM, Banerjee S, McCaffery JM, Youle RJ _et al_. The soluble
form of Bax regulates mitochondrial fusion via MFN2 homotypic complexes. _Mol Cell_ 2011; 41: 150–160. Article CAS Google Scholar * Rinkenberger JL, Horning S, Klocke B, Roth K,
Korsmeyer SJ . Mcl-1 deficiency results in peri-implantation embryonic lethality. _Genes Dev_ 2000; 14: 23–27. CAS PubMed PubMed Central Google Scholar * Tarin JJ, Perez-Albala S, Cano A
. Cellular and morphological traits of oocytes retrieved from aging mice after exogenous ovarian stimulation. _Biol Reprod_ 2001; 65: 141–150. Article CAS Google Scholar * Lewandoski M,
Wassarman KM, Martin GR . Zp3-cre, a transgenic mouse line for the activation or inactivation of loxP-flanked target genes specifically in the female germ line. _Curr Biol_ 1997; 7: 148–151.
Article CAS Google Scholar * Knudson CM, Tung KS, Tourtellotte WG, Brown GA, Korsmeyer SJ . Bax-deficient mice with lymphoid hyperplasia and male germ cell death. _Science_ 1995; 270:
96–99. Article CAS Google Scholar * Lobe CG, Koop KE, Kreppner W, Lomeli H, Gertsenstein M, Nagy A . Z/AP, a double reporter for cre-mediated recombination. _Dev Biol_ 1999; 208: 281–292.
Article CAS Google Scholar * Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H _et al_. A robust and high-throughput Cre reporting and characterization system for the whole
mouse brain. _Nat Neurosci_ 2010; 13: 133–140. Article CAS Google Scholar * Jurisicova A, Lee HJ, D'Estaing SG, Tilly J, Perez GI . Molecular requirements for doxorubicin-mediated
death in murine oocytes. _Cell Death Differ_ 2006; 13: 1466–1474. Article CAS Google Scholar * Chi MM, Hoehn A, Moley KH . Metabolic changes in the glucose-induced apoptotic blastocyst
suggest alterations in mitochondrial physiology. _Am J Physiol Endocrinol Metab_ 2002; 283: E226–E232. Article CAS Google Scholar Download references ACKNOWLEDGEMENTS We acknowledge S
Cao, A Borrego-Alvarez and T Yavorska for their technical assistance. We also express our thanks to the staff of the Reproductive Biology Unit at MSH as well as patients for donations of
immature oocytes. This work was supported by operating grant from Canadian Institutes of Health Research (FRN 123518) and Canada Research Chair CRC/CFI program (AJ). AUTHOR INFORMATION
AUTHORS AND AFFILIATIONS * Lunenfeld Tanenbaum Research Institute, Mount Sinai Hospital, 25 Orde Street, Toronto, M5T 3H7, Ontario, Canada S Omari, M Waters, T Naranian, K Kim, A L
Perumalsamy & A Jurisicova * Department of Physiology, University of Toronto, 1 King's College Circle, Toronto, M5S 1A8, Ontario, Canada S Omari, T Naranian, K Kim & A
Jurisicova * Department of Obstetrics and Gynecology, Washington University in St. Louis, 660S Euclid Avenue, St. Louis, 63110, MO, USA M Chi & K H Moley * Centre for Fertility and
Reproductive Health, Mount Sinai Hospital, 250 Dundas Street, Toronto, M5T 2Z5, Ontario, Canada E Greenblatt * Department of Obstetrics and Gynecology, University of Toronto, 92 College
Street, Toronto, M5G 1L4, Ontario, Canada E Greenblatt & A Jurisicova * Department of Biochemistry, St. Jude Children's Research Hospital, MS 340, Room D4063D, 262 Danny Thomas
Place, Memphis, 38105, TN, USA J T Opferman Authors * S Omari View author publications You can also search for this author inPubMed Google Scholar * M Waters View author publications You can
also search for this author inPubMed Google Scholar * T Naranian View author publications You can also search for this author inPubMed Google Scholar * K Kim View author publications You
can also search for this author inPubMed Google Scholar * A L Perumalsamy View author publications You can also search for this author inPubMed Google Scholar * M Chi View author
publications You can also search for this author inPubMed Google Scholar * E Greenblatt View author publications You can also search for this author inPubMed Google Scholar * K H Moley View
author publications You can also search for this author inPubMed Google Scholar * J T Opferman View author publications You can also search for this author inPubMed Google Scholar * A
Jurisicova View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to A Jurisicova. ETHICS DECLARATIONS COMPETING INTERESTS
The authors declare no conflict of interest. ADDITIONAL INFORMATION Edited by E Baehrecke Supplementary Information accompanies this paper on Cell Death and Disease website SUPPLEMENTARY
INFORMATION SUPPLEMENTARY TABLE 1 (DOC 43 KB) SUPPLEMENTARY TABLE 2 (DOC 58 KB) SUPPLEMENTARY TABLE 3 (DOC 29 KB) SUPPLEMENTARY FIGURE LEGENDS (DOC 61 KB) SUPPLEMENTARY FIGURE 1 (JPG 103 KB)
SUPPLEMENTARY FIGURE 2 (JPG 114 KB) SUPPLEMENTARY FIGURE 3 (JPG 161 KB) SUPPLEMENTARY FIGURE 4 (JPG 122 KB) SUPPLEMENTARY FIGURE 5 (JPG 130 KB) SUPPLEMENTARY FIGURE 6 (JPG 73 KB) RIGHTS AND
PERMISSIONS _Cell Death and Disease_ is an open-access journal published by _Nature Publishing Group_. This work is licensed under a Creative Commons Attribution 4.0 International License.
The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not
included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit
http://creativecommons.org/licenses/by/4.0/ Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Omari, S., Waters, M., Naranian, T. _et al._ Mcl-1 is a key regulator of the ovarian
reserve. _Cell Death Dis_ 6, e1755 (2015). https://doi.org/10.1038/cddis.2015.95 Download citation * Received: 14 November 2014 * Revised: 07 February 2015 * Accepted: 18 February 2015 *
Published: 07 May 2015 * Issue Date: May 2015 * DOI: https://doi.org/10.1038/cddis.2015.95 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get
shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative