Dna methylation signatures follow preformed chromatin compartments in cardiac myocytes
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Storage of chromatin in restricted nuclear space requires dense packing while ensuring DNA accessibility. Thus, different layers of chromatin organization and epigenetic control
mechanisms exist. Genome-wide chromatin interaction maps revealed large interaction domains (TADs) and higher order A and B compartments, reflecting active and inactive chromatin,
respectively. The mutual dependencies between chromatin organization and patterns of epigenetic marks, including DNA methylation, remain poorly understood. Here, we demonstrate that
establishment of A/B compartments precedes and defines DNA methylation signatures during differentiation and maturation of cardiac myocytes. Remarkably, dynamic CpG and non-CpG methylation
in cardiac myocytes is confined to A compartments. Furthermore, genetic ablation or reduction of DNA methylation in embryonic stem cells or cardiac myocytes, respectively, does not alter
genome-wide chromatin organization. Thus, DNA methylation appears to be established in preformed chromatin compartments and may be dispensable for the formation of higher order chromatin
organization. SIMILAR CONTENT BEING VIEWED BY OTHERS CTCF CHROMATIN RESIDENCE TIME CONTROLS THREE-DIMENSIONAL GENOME ORGANIZATION, GENE EXPRESSION AND DNA METHYLATION IN PLURIPOTENT CELLS
Article 29 July 2021 DNA SEQUENCE-DEPENDENT FORMATION OF HETEROCHROMATIN NANODOMAINS Article Open access 06 April 2022 THE IMPACT OF DNA METHYLATION ON CTCF-MEDIATED 3D GENOME ORGANIZATION
Article 18 March 2024 INTRODUCTION The development of chromosome conformation capture methods such as Hi-C provided insight into spatial chromatin organization1. Hi-C data identify different
layers of chromatin organization. Topologically associated domains (TADs)2, 3 are one of these layers. TADs represent self-interacting chromatin domains, often separated by genomic
insulators like CTCF and are stabilized by the cohesin complex4. TADs are thought to act as regulatory units of the genome5. A second layer is represented by spatially separated A and B
compartments consisting of single or multiple TADs6. The spatially segregated A and B compartments have been identified as active and inactive chromatin, respectively1. A compartments are
enriched for active histone modifications, including H3K27ac, H3K4me1/me3, H3K9me1, the polycomb mark H3K27me3 while B compartments contain the heterochromatin mark H3K9me37. Recent studies
suggested an association of DNA methylation with chromatin organization in differentiated cells8,9,10,11 and during early embryogenesis12. Previously, DNA methylation has been shown to be
crucial for cell development13. Especially, reduced CpG methylation at _cis_-regulatory sites is associated with transcription factor occupancy and establishment of cell-type-specific gene
expression during cell differentiation14. However, the chronology and dependency of DNA methylation and higher order chromatin organization remains unknown. To study the chronological order
of chromatin organization and DNA methylation, we analyze chromatin interactions (in situ Hi-C) and DNA methylation (WGBS) together with gene expression (RNA-seq) during differentiation and
maturation of mouse cardiac myocytes (CM), representing a terminally differentiated cell type. To clarify the significance of DNA methylation signatures for chromatin organization, we
analyzed embryonic stem (ES) cells lacking DNA methylation15, 16 and adult CM after embryonic ablation of the de novo DNA-methyltransferases 3A and 3B17, 18. These data show that CM-specific
A/B compartments are established during early CM differentiation. These compartments serve as a template for the establishment of partially methylated domains (PMDs) in B compartments and
active DNA methylation turnover in A compartments. Additionally, we demonstrate that DNA methylation is dispensable for the establishment of higher order chromatin architecture in ES cells.
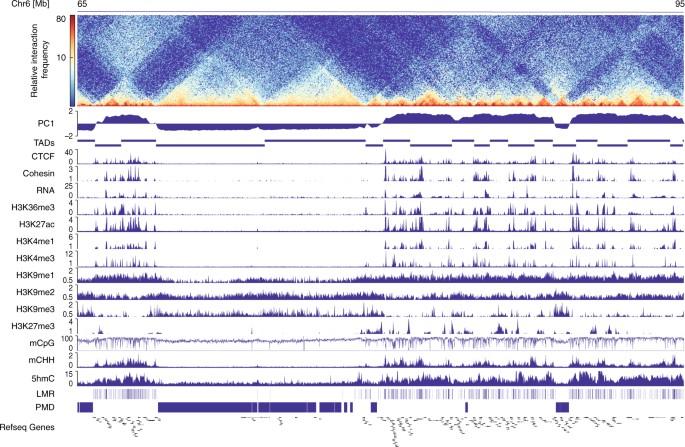
RESULTS CHROMATIN ARCHITECTURE AND EPIGENETIC PROFILING OF CM Applying in situ Hi-C analysis7 to pure FACS-sorted CM nuclei18,19,20 (Fig. 1; Supplementary Fig. 1, Supplementary Table 1), we
identified TADs and A/B compartments of CM. In adult CM, 44.8 % of the genome consists of A compartments (Supplementary Fig. 2a). As anticipated, A compartments of adult CM were enriched for
active histone modifications, including H3K27ac, H3K4me1/me3, H3K9me1 as well as CTCF and cohesin (Fig. 1; Supplementary Fig. 2a). In contrast, B compartments were decorated with the
heterochromatin mark H3K9me3 (Fig. 1; Supplementary Fig. 2a). Thus, CM recapitulate the previously described interplay between histone marks and chromatin organization7. For the analysis of
CpG methylation signatures, we segmented the genome of adult CM into low-methylated regions (LMRs), representing potential _cis_-regulatory elements such as enhancers (Supplementary Fig.
3)14, and large PMDs21 with seemingly disordered CpG methylation. Remarkably, LMRs were characteristic for A compartments and PMDs for B compartments, respectively (Fig. 1; Supplementary
Fig. 2a). PMDs have been previously associated with inactive chromatin in other differentiated cell types9, 11. Surprisingly, 5-hydroxymethylcytosine (5hmC) as well as non-CpG (mCHH)
methylation clearly demarcated A compartments (Fig. 1; Supplementary Fig. 2a). Thus, A and B compartments show distinct DNA modification signatures including CpG and non-CpG methylation. A/B
COMPARTMENTS PRECEDE MANIFESTATION OF DNA METHYLATION We next analyzed mouse ES cells, cardiac progenitor cells isolated from embryonic hearts22 (E9-11) and differentiated fetal (E14),
newborn (P1), and adult (10–12 weeks) CM to get insights into the dynamics of A/B compartment formation during in vivo CM differentiation and maturation (Supplementary Tables 1 and 2).
Compared with ES cells, cardiac progenitor cells and different stages of differentiated CM revealed 7.9% of compartments with dynamic A/B status (Supplementary Figs. 4a and 5a). A
compartments which are found in CMs but not in ES cells (CM-A) contain genes essential for CM function, such as myocardin _(Myocd)_ or the sodium–calcium-exchanger _Slc8a1_ (Supplementary
Fig. 5b, c) and display the cell-type-specific character of chromatin compartments. To get insight into the regulatory landscape of A/B compartments in CM, we identified enhancers positive
for H3K27ac and H3K4me1 using ChromHMM23 (Supplementary Fig. 3a). CM-A showed a significantly higher number of enhancers containing motifs characteristic for key cardiac transcription
factors, including GATA, MEF2, T-box, and Nkx2, as compared to A compartments shared between ES cells and adult CM (common A; Supplementary Fig. 5e). This suggests that establishment of
cell-type-specific compartments like CM-A coincides with recruitment of specific sets of transcription factors. Visual inspection of a locus with highly dynamic A/B status, containing the
laminin subunit alpha2 (_Lama2_) and triadin (_Trdn_), as well as genome-wide analysis (Fig. 2a; Supplementary Fig. 4a) imply that compartment switches gradually establish during
differentiation. These changes in chromatin organization coincide with the manifestation of DNA methylation signatures and gene expression (Fig. 2a; Supplementary Fig. 4b–d). To analyze the
chronology of chromatin organization and DNA methylation, we concentrated on genomic regions showing A/B compartment switches in ES vs. cardiac progenitor cells and CM (Fig. 2b, c). In
regions that underwent A to B switches before the CM progenitor stage, PMDs were first apparent in fetal CM (Fig. 2b). Conversely, compartments switching from B to A in ES vs. CM progenitors
gained LMRs and gene expression mainly in fetal and newborn CM (Fig. 2c). We next asked, to which extent genome-wide establishment of A/B compartments and CpG methylation differ during
differentiation and maturation. To compare the patterning of A/B compartments between different samples we applied principal component analysis (Fig. 2d; Supplementary Fig. 6). Remarkably,
A/B compartment data build a trajectory spanning from ES cells to fetal CM. In contrast, fetal, newborn, and adult CM cluster tightly together, indicating stable A/B patterning in fetal,
newborn, and adult CM (Fig. 2d, left panel; Supplementary Fig. 6). Thus, manifestation of A/B compartments occurs predominantly during differentiation. In contrast, CpG methylation patterns
are established gradually during CM differentiation and maturation until the adult stage (Fig. 2d; Supplementary Fig. 6). Taken together our findings clearly show that A/B compartments are
mainly established in pluripotent ES cells and multipotent progenitors, whereas CpG methylation is shaped in a continuous process until final maturation to adult CM. Distinct CpG methylation
changes take also place in A/B compartments that are common in ES cells and CM (Fig. 2e). Global analysis of CpG methylation status in common A and B compartments demonstrated that B
compartments in undifferentiated ES and progenitor cells are hypermethylated as compared to A compartments. Remarkably, A compartments in differentiated CM showed higher CpG methylation
values as compared to B compartments (Fig. 2e). This indicates that hypermethylation of inactive chromatin is restricted to pluripotent and multipotent cells. DNMT3 AFFECTS CPG METHYLATION
AND NON-CPG METHYLATION IN A COMPARTMENTS Given these specific changes in DNA methylation, we asked whether modulation of DNA methylation impacts the establishment of A/B patterns during CM
maturation (Fig. 3). Since ablation of the maintenance DNA methyltransferase DNMT1 leads to embryonic lethality24, we first characterized DNA methylation in CM with deletion of the de novo
methyltransferases 3a and b (CM-DKO, _Dnmt3a_ _−/−_ _/Dnmt3b_ _−/−_)17, 19. As expected, we observed a marked CpG hypomethylation upon deletion of DNMT3A/B (Fig. 3a; Supplementary Fig. 7).
To our surprise, differentially methylated sites (DMR) were predominantly located in A compartments (Fig. 3a). This observation also held true in pluripotent human ES cells with ablation of
DNMT3A and/or DNMT3B enzymes25, where hypomethylation took place almost exclusively within A compartments (Supplementary Fig. 8). In good agreement with this observation, enzymes modifying
DNA methylation such as TET1, DNMT3A, and B were found to be enriched in A compartments of mouse ES cells (Supplementary Fig. 2b). In order to comprehensively assess DNA methylation, we also
analyzed non-CpG methylation (mCHH) during CM maturation. Similarly to the dynamic changes of CpG methylation, deposition of non-CpG methylation was mainly restricted to A compartments
(Fig. 3a). Yet, non-CpG methylation was only detected in adult CM, which are mostly postmitotic (Fig. 3a). Establishment of non-CpG methylation during maturation has previously been detected
in neurons26, 27. Non-CpG methylation in adult CM is significantly enriched in fully methylated regions (FMR) (Fig. 3c), which mainly correspond to actively transcribed regions marked by
H3K36me3 (Supplementary Fig. 2). Ablation of DNMT3A/B prevented the establishment of mCHH in adult CMs (Fig. 3a, d). These findings let us hypothesize that chromatin organization restricts
DNA accessibility for DNA modifying enzymes to A compartments and thus serves as a barrier for DNA modification dynamics. DNA METHYLATION IS DISPENSABLE FOR CHROMATIN ARCHITECTURE Next, we
asked whether modification of DNA methylation affects chromatin structure. Remarkably, ablation of DNMT3A/B in CM had no major impact on A/B patterning, as indicated by highly correlating
PC1 values (_r_ 2 = 0.96) (Fig. 3a; Supplementary Fig. 9). To test whether DNA methylation is entirely dispensable for the establishment of TADs and A/B structures, we generated Hi-C maps of
wild-type (WT) mouse ES cells and ES cells lacking all three DNA-methyltransferases, DNMT1, DNMT3A, and DNMT3B (ESC-TKO15, Fig. 4a). ESC-TKO are phenotypically not affected by the resulting
loss of DNA methylation15, 28. Lack of DNA methylation in ESC-TKO did not affect the patterning of A and B compartments (Fig. 4a, b; Supplementary Fig. 7). To confirm this finding, we
assessed a second independent ESC-TKO cell line (cell line 2)16. Again, Hi-C experiments did not reveal major changes in the A/B pattern of ESC-TKO compared to WT cells (Supplementary Fig.
10). Since CTCF binding is DNA methylation-sensitive14, 29, we tested if the absence of DNA methylation leads to establishment of de novo CTCF binding sites and alters chromatin topology.
Notably, Hi-C data of ES-TKOs showed no significant changes in TAD boundaries as compared to ES cells in both tested cell lines (Fig. 4c; Supplementary Fig. 10). Furthermore, analysis of
CTCF ChIP-seq data showed highly correlating binding patterns in ES and ES-TKO cells (Fig. 4d). DISCUSSION Here we report that CM-specific A/B compartments are mainly shaped during early
cardiac differentiation. This higher order spatial chromatin structure predefines regions of cell-type-specific DNA methylation signatures. These comprise particularly low-methylated regions
(LMRs) which represent _cis_-regulatory elements30 and PMDs21, 31. PMDs have been identified previously in various differentiated cell types31, 32. Our results show that PMDs establish
within preformed B compartments after cell lineage decision in CMs. However, future studies are necessary to unravel the underlying mechanisms and the biological function of PMDs. Our
results clearly show that LMR manifestation is confined to A compartments. These regions were enriched for DNA methylation modifying enzymes, including DNMT3-isoenzymes and TET-isoenzymes.
Ablation of the de novo methylation machinery in different cell types induced hypermethylation of A compartments and loss of non-CpG methylation in CM. On the other site
5-hydroxymethylation, which is established by TET-enzymes and indicates active removal of DNA methylation33 marks A compartments. These observations indicate that accessibility for
DNMT3-isoenzymes and TET-isoenzymes is higher in A compartments as compared to B compartments, which is in agreement with a recent publication reporting that A compartments show lower CpG
methylation and higher accessibility for DNAse during mammalian embryogenesis as compared to B compartments12. Recent studies revealed that the insulator protein CTCF is necessary for proper
formation of chromatin loops34, 35. A study by Nora et al.36 used auxin-inducible degradation of CTCF to show that CTCF is essential for the stability of TADs but not for A/B compartments.
DNA methylation-sensitive binding of CTCF29 let us speculate that proper CpG methylation is required for TAD formation. However, our results indicate that loss of DNA methylation in mouse ES
cells has no major impact on TAD insulation and does not alter the establishment of CTCF binding sites in pluripotent cells. Furthermore, a microscopic study found that the dispersed
chromatin fiber architecture typical for ES cells is not altered in ES-TKO37. These data support our finding that ablation of DNA methylation in pluripotent cells as well as of DNMT3A/B
enzymes in CMs has a negligible effect on higher order chromatin organization. In contrast, a targeted methylation of CTCF binding sites by dCas9-Dnmt3a leads to depletion of CTCF binding
and disruption of TADs8. Probably the genome engineering approach by Liu et al.8 overrides the physiological DNA demethylation induced by CTCF occupancy14, 38. In summary, our findings
support a model in which higher order chromatin conformation is a regulatory mechanism guiding cell-type-specific establishment of CpG methylation and non-CpG methylation signatures. METHODS
ANIMAL PROCEDURES Animal procedures were permitted by the responsible Committee on the Ethics of Animal Experiments (Regierungspräsidium Freiburg, Germany and Regierung von Oberbayern,
Munich, Germany) and they conformed to the Guide for the Care and Use of Laboratory Animals published by the National Academy of Sciences, 2011. Mice with CM-specific ablation of DNMT3A/B
expression were generated by crossing _Dnmt3a_ flox and _Dnmt3b_ flox mice with mice expressing cre recombinase under control of the cardiac _Mlc2a_ promoter17, 18. Cardiac progenitor cells
were isolated from mice expressing enhanced green fluorescent protein under control of an Nkx2.5 enhancer (Nkx2.5-enhancer-EGFP)22. For all experiments, we used WT mice of the C57BL/6 J
strain. Fetal, newborn, and adult hearts were retrieved at embryonic day 14, postnatal day 1, and 8–12 weeks after birth, respectively. ORIGIN OF EMBRYONIC STEM CELL LINES In this study, we
used two independent ES cell lines with genetic ablation of DNMT1, DNMT3a, and DNMT3b (TKO) and corresponding WT cell lines. WT and TKO cell line 1 was derived from HA36CB1/159-2 cells15. WT
and TKO cell line 2 consisted of HA36CB1 and DNMT TKO-133 cells16. SORTING OF CARDIOMYOCYTE NUCLEI All steps during the isolation, staining and sorting of CM nuclei18, 19 were performed at
≤4 °C. All buffers contained fresh protease inhibitor (cOmplete Protease Inhibitor Cocktail, Roche) and DTT (1 mM, dithiothreitol). Frozen mouse ventricles were thawed in 3 mL lysis buffer
(5 mM CaCl2, 3 mM MgAc, 2 mM EDTA, 0.5 mM EGTA, 10 mM Tris-HCl, pH 8) and were dissected using Miltenyi gentleMACS dissociator M tubes and the protocol ‘protein_01’. An aliquot of 3 mL of
lysis buffer supplemented with 0.4% Triton X-100 was added and the suspension was filtered using 40 µm cell strainer (BD Bioscience). After washing the filter with 2 mL lysis buffer, the
suspension was centrifuged (1000 × _g_, 5 min, 4 °C). The pellet was resuspended and overlayed on 1 M sucrose (3 mM MgAc, 10 mM Tris-HCl, pH8), then centrifuged (1000 × _g_, 5 min, 4 °C) and
the pellet resuspended in 500 µL staining buffer (PBS, 5% BSA, 0.2% Igepal CA-630). Nuclei of CMs were stained by rabbit anti-PCM-1 antibody (1:1000, HPA023374, Sigma-Aldrich) and/or mouse
anti-PLN antibody (1:1000, A010-14, Badrilla) for 30 min at room temperature (RT) and subsequently with an anti-rabbit secondary antibody conjugated to Alexa 568 (1:1000, A11011, Life
Technologies) and/or with anti-mouse secondary antibody conjugated to Alexa 488 (1:1000, A11029, Life Technologies) for 30 min at RT. Then, nuclei were incubated with Draq-7 (1:100, Cell
Signaling Technology) for 10 min at RT. CM nuclei were sorted using a S3 cell sorter (BioRad). FSC pulse width was used to exclude doublets from sorting. Sorted CM nuclei were processed
immediately. SORTING OF ADULT CARDIAC MYOCYTES FOR RNA-SEQ Adult mouse hearts were retrograde perfused with digestion buffer (Tyrode’s solution, 25 mM butanedione monoxime, 2 mM CaCl2, 0.8
mg/mL collagenase B (Roche, Mannheim, Germany), 0.4 mg/mL hyaluronidase (Sigma), 3 μg/mL trypsin (Sigma)) for 12 min. Single cell suspension were obtained after stopping of the enzymatic
digestion (5% FCS) and gentle dissection. After passing through a 100 µm filter CMs were sorted by FACS using a Bio-Rad S3. CMs were identified by high FSC signal and viable cells were
discriminated using DRAQ5 (Cell Signaling Technology)18. Gates were set to obtain viable CMs. SORTING OF FETAL CARDIAC MYOCYTES FOR WGBS EXPERIMENTS Fetal CMs (E14) were sorted after
enzymatic digestion18, 19. Therefore, fetal hearts were dissociated by several rounds of digestion with trypsin (Gibco) in Hank’s Balanced Salt Solution (HBSS, Life Technologies). Digestion
was stopped by resuspension in HBSS containing 4% FCS. Cells were permeabilized with 0.1% saponin (Sigma) and stained with antibodies against cardiac troponin I (1:1000, ab47003, Abcam) and
α-actinin (1:1000, At7811, Sigma) in combination with secondary Alexa 568 and 488 antibodies (1:1000, A11011, A11029, Life Technologies). CMs and non-CMs were visualized using the
cell-permeable nucleic acid stain Vybrant DyeCycle Ruby (1:200, V10309, Life Technologies). Sorting of CMs from cardiac cell suspensions was carried out using a BioRad S3 cell sorter. FSC
pulse width was used to exclude doublets from sorting. SORTING OF CARDIAC PROGENITOR CELLS Cardiac progenitor cells were isolated from embryonic hearts (E9-11) of Nkx2.5-enhancer-EGFP
mice22, 39. Embryos of the Nkx2.5-enhancer-EGFP mice were collected on E 9.5–11 from timed matings (a positive mating plug indicated E 0.5). For extraction of embryos, mice were anesthetized
with isoflurane (2-chloro-2-(difluoromethoxy)-1,1,1-trifluoroethane) and killed by cervical dislocation. Isolated embryos were cut and digested with a collagenase II (10,000 U/mL,
Worthington Biochemical Corporation, Lakewood, NJ) and DNase I (10,000 U/µL, Roche) mixture shaking for 1 h at 37 °C to obtain single cell suspension. For embryonic stages >E10
erythrocyte lysis (Red blood cell lysis solution, Miltenyi Biotec, Bergisch-Gladbach, Germany) was additionally performed. Dissociated embryos were washed with PBS and resuspended in PBS/0.5
% BSA/2 mM EDTA for flow cytometry. Dead cells were stained with propidium iodide solution (2 µg/mL, Sigma-Aldrich). GFP-positive cells indicating multipotent cardiac progenitor cells were
isolated using a FACS ARIATM Illu flow cytometer (BD Biosciences, San Jose, CA) and the BD FACSDiva software version 6.1.2 (BD Biosciences). FSC pulse width was used to exclude doublets from
sorting. For Hi-C isolated cells were directly flash-frozen in liquid nitrogen. For WGBS and RNA-seq cells were sorted into RLTplus Buffer (Qiagen) containing β-mercaptoethanol (10 µL/mL)
to extract DNA and total RNA. WHOLE-GENOME BISULFITE SEQUENCING Genomic DNA was extracted (AllPrep DNA/RNA Mini Kit, Qiagen) and DNA-seq libraries were prepared using the NEXTflex Methyl-Seq
Library Prep Kit for Illumina (Bioo) using 1–1.5 µg of DNA or in case of the cardiac progenitor cells using the Ovation Ultralow Methyl-Seq DNA Library System (Nugen) using 15–125 ng DNA
according to the manufacturer’s instructions. CHIP-SEQ ChIP-seq was performed from FACS-sorted CM nuclei18, 19. The following antibodies were used in this study: CTCF (diagenode,
C15410210-50, 4 µg/ChIP), Cohesin (anti-SMC1; biomol, A300-055A, 4 µg/ChIP), H3K9me1 (abcam, ab8896, 4 µg/ChIP), H3K9me2 (Cell Signaling Technology, #9753, 4 µg/ChIP), H3K9me3 (Diagenode,
C15410193, 4 µg/ChIP). For CTCF and Cohesin immunoprecipitation, the iDeal ChIP-seq kit for Transcription Factors (Diagenode) was used with 1 µg of chromatin and for H3K9me1/2/3 the ChIP-IT
High Sensitivity Kit (Active Motif) was used with 200 ng of chromatin according to manufacturer’s instructions. Sequencing libraries were prepared from the resulting DNA with the NEBNext
Ultra DNA Library Prep Kit for Illumina (NEB). To avoid over-amplification of the sequencing library, test qPCR-amplification was carried out to determine minimum cycles for final PCR.
RNA-SEQ Polyadenylated RNA was isolated from total RNA with magnetic beads (NEBNext Poly(A) mRNA Magnetic Isolation Module, NEB). Libraries were constructed using the NEBNext Ultra RNA
Library Prep Kit for Illumina (NEB) according to manufacturer´s instruction. IN SITU HI-C OF FACS-SORTED NUCLEI The in situ Hi-C method was adopted from Rao et al.7 At least 10,000
FACS-sorted cardiomyocyte nuclei (up to 230,000 nuclei per technical replicate) were centrifuged (1000 × _g_, 5 min, 4 °C), washed with cold PBS and resuspended in PBS. Nuclei then were
fixated using fresh PFA (1% final concentration) for 10 min at RT under constant rotation. Fixation was stopped with 2.5 M glycine (final concentration: 0.25 M). Nuclei were centrifuged,
washed with PBS (1000 × _g_, 5 min, 4 °C), and resuspended in 50 µL of 0.5% SDS. Nuclei permeabilization was carried out for 10 min at 62 °C under constant rotation. After adding of 145 µL
water and 25 µL 10% Triton × 100, the nuclei were incubated at 37 °C for 15 min. Then, 25 µL of restriction enzyme buffer (NEB buffer 2.1 respectively DpnII-buffer) and 400 U of restriction
enzyme (HindIII respectively DpnII, NEB) were added. Restriction was carried out at 37 °C over night under constant rotation. Both, HindIII and DpnII restricted samples were heat inactivated
at 62 °C for 10 min. Sticky-ends were filled in by incubation with 1.5 µL of 10 mM dCTP, dGTP, dTTP, and 37.5 µL 0.4 mM biotin-14-dATP (Life Technologies) and 50 U of Klenow-Pol (NEB) at 37
°C for 90 min. Proximity ligation was carried out at RT for 4 h after adding 663 µL water, 120 µL 10× T4 DNA ligase buffer, 100 µL Triton X-100, 12 µL 10 mg/mL BSA, and 2000 U of T4 DNA
ligase (NEB). Two DNA precipitation steps were performed after proteinase K decrosslinking (over night) including RNase A digestion (37 °C, 30 min) after first precipitation. The ligated DNA
was resuspended in 100 µL water and sheared using 30 cylces of Bioruptur (Diagenode, 30 s, on, 90 s off, ‘low’). In total, 200–600 bp fragments were size selected using Ampure Beads XP
(Beckman), biotin pull-down was carried out with 100 µL Dynabeads MyOne Streptavidin T1 (Thermo Fisher). Illumina sequencing adapters were ligated to streptavidin bead-bound DNA. To reduce
PCR duplicates, a 1:100 dilution of T1 beads (Invitrogen) containing the Hi-C library was taken to determine minimum number of cycles for final library amplification (KAPA SYBR FAST qPCR,
Kapabiosystems). The final amplification was performed in technical replicates using undiluted T1 beads and Phusion High-Fidelity DNA polymerase (NEB). PCR products of technical replicates
were pooled and cleaned twice using Ampure Beads XP (Beckman, 0.9× beads). HI-C OF MOUSE EMBRYONIC STEM CELLS AND PROGENITOR CELLS Mouse embryonic mutant (ES-TKO) and WT stem cells (ES-WT)
were cultured without feeders on 0.2% gelatine-coated dishes in DMEM with non-essential amino acids, supplemented with 15% fetal calf serum, 2 mM l-glutamine, LIF, and 0.001%
β-mercaptoethanol (37 °C, 7% CO2)15. A total of 106 ES cells or 104 FACS-sorted (see above) progenitor cells were used for in situ Hi-C7. Briefly, cells were detached and centrifuged (300 ×
_g_ for 5 min), then resuspended in 1xPBS (1 × 106 cells/mL). Fresh formaldehyde was added to a final concentration of 1% and incubated for 10 min at RT. The crosslinking reaction was
quenched by adding glycine (0.25 M final concentration). After subsequent washing, the pellets were centrifuged for 5 min at 300 × _g_ at 4 °C and flash-frozen in liquid nitrogen. Cells were
processed directly or stored at −80 °C. Cells were lysed with 300 µL cold lysis buffer (10 mM Tris-HCl pH 8.0, 10 mM NaCl, 0.2% Igepal CA630, freshly added protease inhibitor) for 15 min on
ice and then centrifuged at 2500 × _g_ for 5 min. Pelleted nuclei were washed with 500 µL lysis buffer, then permeabilized and processed as described above. SEQUENCING OF DNA LIBRARIES The
concentration of DNA libraries was determined by Qubit (Invitrogen) and the insert size using a Bioanalyzer (High Sensitivity, Agilent Technologies). Pooling of multiplexed sequencing
samples, clustering and sequencing were carried out as recommended by the manufacturer on Illumina HiSeq 2500 or Nextseq 500. All libraries were sequenced in paired-end mode. Previously
published RNA sequencing libraries, constructed using the identical methods applied in this study, were sequenced in paired-end mode to conform to the data generated in this study. ANALYSIS
OF HI-C DATA All tools used in this study (besides HOMER tools40) were implemented into the Galaxy platform9. We analyzed only autosomes. Mouse sequencing data were mapped to the reference
genome mm9, human ESC data to reference genome hg19. Raw reads were trimmed as paired-end reads using Trim Galore! (https://github.com/FelixKrueger/TrimGalore) with default parameters.
Trimmed reads were mapped as single-end reads to the reference genome mm9 with Bowtie241 using the local alignment mode (--local). Then, properly mapped reads were sorted by their read names
(-n) with SAMtools42. The HiCExplorer43 (version 1.7.2, https://github.com/maxplanck-ie/HiCExplorer) was used for processing of mapped reads, normalizing, analyzing, and visualization of
Hi-C data. Using mapped and sorted reads, _hicBuildMatrix_ (-bs 40000 –-minMappingQuality = 1) gave the HiC-matrix as output at given resolution. _hicCorrectMatrix_ was used to determine
thresholds for the following correction of the Hi-C matrix. Once set, the matrix was corrected using the command _hicCorrectMatrix correct_ (--filterThreshold –x.x y.y --perchr). TADs were
identified by _hicFIndTADs_. First, the TADscore was calculated using the command _hicFindTADs TAD_score_ (--minDepth 300000 --maxDepth 3000000 --step 300000 -p 50); then the TAD boundaries
and TAD domains were called by using _hicFindTADs find_TADs_ (--minBoundaryDistance 400000). For visualization of the HiC-matrix and the integration of epigenetic data _hicPlotTADs_ was
used. A/B patterns were identified at 40 kb resolution using HOMER tools40. A paired-end tag directory of all mapped reads (as described above) was created using the command
_makeTagDirectory_ (-removeSelfLigation -removePEbg -genome mm9 -restrictionSite NNNNNN (AAGCTT for HindIII digested and GATC for DpnII digested samples) –removeRestrictionEnds–fragLength
500). This directory was used for further analysis. _runHiCpca.pl_ (-res 40000 -cpu 50 -genome mm9) gave the A/B pattern of the given Hi-C experiment. The tool automatically creates a
background model for normalization of the Hi-C matrix. Differential A/B pattern were identified by comparing PC1 values per bin. As criteria we defined a different sign and a delta PC1 of 1.
For annotation of differential compartments at least two subsequent bins had to fulfill these criteria. In CM nuclei after ablation of DNMT3A/B enzymes, principle component 2 (PC2)
corresponds to the A/B pattern of chromosome 16 from Mb 65 to the end of the chromosome, similar to results described in the initial Hi-C study by Lieberman et al. for chromosome 4 and 5 in
a human lymphoblastoid cell line1. We therefore exclude this part of Chr 16 from our quantitative analysis, in particular for the calculation of differential A/B pattern and for the
correlation of A/B pattern. To determine the Pearson correlation of the A/B pattern of two or more HiC-matrices, the PC1 values (bedGraph-files) were used as input for _multiBigwigSummary_
(bin size = 40,000 bp, respectively, 500,000 bp for biological replicates). The resulted matrix was visualized using _plotCorrelation_ (default parameters). Both tools are part of
deepTools44. GENE ONTOLOGY ANALYSIS Genomic bins with cell-type-specific A/B pattern were intersected with Refseq-genes. The obtained genes were used as input for ClueGO v 2.1.6, a Cytoscape
plug-in for gene ontology analysis45 with following settings: Min GO level = 3, Max GO level = 4, GO fusion = true, Kappa score threshold = 0.4, statistical test used = enrichment
(right-sided hypergeometric test), correction method used = Bonferroni step down, number of genes = 10, min percentage = 10. ANALYSIS OF CHIP-SEQ AND 5HMC DATA ChIP-seq18, 19, 46 raw reads
were trimmed and mapped as PE-reads to the reference genome (mm9) using Bowtie2 with default parameters. PCR duplicates were removed by RmDup (part of SAMtools). For data visualization
(i.e., heatmaps) we used deepTools44. Peaks were called using MACS2 (--qvalue 0.05)47. ANALYSIS OF METHYLATION DATA Whole-genome bisulfite sequencing (WGBS) data were mapped using Bismark48
and DNA methylation data was called using MethylDackel (https://github.com/dpryan79/MethylDackel). Results for both strands were combined after calling of CpG methylation values.
Differentially methylated regions were calculated using Methtools (https://github.com/bgruening/methtools)18. Regions displaying a mean CpG methylation delta ≥40% over five CpGs in two
compared datasets were selected as DMRs. Only CpGs with a minimal coverage of four in both data sets were included in differential methylation analysis. Additional exclusion criteria were a
minimal difference of 10% for each individual CpG and a maximal distance of 1 kb between adjacent CpGs. A CpG methylation guided genome segmentation30 was used to identify LMRs and PMDs.
PMDs < 100 kb were excluded, since they mostly represent partially demethylated genic regions of highly expressed genes in CMs. FMRs show an average CpG methylation of at least 85% and
were located between LMRs, PMDs and un-methylated regions. After calling of CHH methylation values results from 1 kb bins were combined to increase the coverage. ANALYSIS OF RNA-SEQ DATA
Quality and adapter trimming of sequencing reads was performed prior to mapping to remove low quality reads and adapter contaminations. RNA-seq data were mapped to the mouse genome (mm9)
using STAR49. PCR duplicates were removed using SAMtools42. Visualizations (i.e., heatmaps) were carried out with deepTools44. CHROMATIN STATE ANALYSIS AND MOTIF ANALYSIS ChromHMM23 was used
to learn a 10-state model predict chromatin states based on ChIP-seq data. Prediction of transcription factor-binding sites within strong enhancers were determined using HOMER tools40. The
motifs used in this study were derived from ChIP-seq data. REPLICATES All experiments supporting the main findings of the manuscript were independently replicated. If not stated otherwise,
main figures show merged data from all replicates. A detailed overview of biological replicates is given in Supplementary Table 1. DATA AVAILABILITY Hi-C data, DNA methylomes, ChIP-Seq and
RNA-seq data supporting the findings of this study have been deposited in the NCBI SRA databases under accession codes PRJNA378914 (Hi-C), PRJNA229470 (WGBS and ChIP-seq), and PRJNA229481
(RNA-seq). Previously published data used for this study are listed in Supplementary Table 2. Additional data that support the findings of this study are available from the corresponding
author. REFERENCES * Lieberman-Aiden, E. et al. Comprehensive mapping of long-range interactions reveals folding principles of the human genome. _Science_ 326, 289–293 (2009). Article ADS
CAS PubMed PubMed Central Google Scholar * Nora, E. P. et al. Spatial partitioning of the regulatory landscape of the X-inactivation centre. _Nature_ 485, 381–385 (2012). Article ADS
CAS PubMed PubMed Central Google Scholar * Dixon, J. R. et al. Topological domains in mammalian genomes identified by analysis of chromatin interactions. _Nature_ 485, 376–380 (2012).
Article ADS CAS PubMed PubMed Central Google Scholar * Haarhuis, J. H. I. et al. The cohesin release factor WAPL restricts chromatin loop extension. _Cell_ 169, 693–707.e614 (2017).
Article CAS PubMed PubMed Central Google Scholar * Dixon, J. R., Gorkin, D. U. & Ren, B. Chromatin domains: the unit of chromosome organization. _Mol. Cell_ 62, 668–680 (2016).
Article CAS PubMed PubMed Central Google Scholar * Wang, S. et al. Spatial organization of chromatin domains and compartments in single chromosomes. _Science_ 353, 598–602 (2016).
Article ADS CAS PubMed PubMed Central Google Scholar * Rao, S. S. et al. A 3D map of the human genome at kilobase resolution reveals principles of chromatin looping. _Cell_ 159,
1665–1680 (2014). Article CAS PubMed PubMed Central Google Scholar * Liu, X. S. et al. Editing DNA methylation in the mammalian genome. _Cell_ 167, 233–247 e217 (2016). Article CAS
PubMed PubMed Central Google Scholar * Fortin, J. P. & Hansen, K. D. Reconstructing A/B compartments as revealed by Hi-C using long-range correlations in epigenetic data. _Genome
Biol._ 16, 180 (2015). Article PubMed PubMed Central CAS Google Scholar * Keown, C. L. et al. Allele-specific non-CG DNA methylation marks domains of active chromatin in female mouse
brain. _Proc. Natl Acad. Sci. USA_ 114, E2882–E2890 (2017). Article CAS PubMed PubMed Central Google Scholar * Berman, B. P. et al. Regions of focal DNA hypermethylation and long-range
hypomethylation in colorectal cancer coincide with nuclear lamina-associated domains. _Nat. Genet._ 44, 40–46 (2011). Article PubMed PubMed Central CAS Google Scholar * Ke, Y. et al. 3D
chromatin structures of mature gametes and structural reprogramming during mammalian embryogenesis. _Cell_ 170, 367–381 e320 (2017). Article CAS PubMed Google Scholar * Smith, Z. D.
& Meissner, A. DNA methylation: roles in mammalian development. _Nat. Rev. Genet._ 14, 204–220 (2013). Article CAS PubMed Google Scholar * Stadler, M. B. et al. DNA-binding factors
shape the mouse methylome at distal regulatory regions. _Nature_ 480, 490–495 (2011). ADS CAS PubMed Google Scholar * Domcke, S. et al. Competition between DNA methylation and
transcription factors determines binding of NRF1. _Nature_ 528, 575–579 (2015). Article ADS CAS PubMed Google Scholar * Tsumura, A. et al. Maintenance of self-renewal ability of mouse
embryonic stem cells in the absence of DNA methyltransferases Dnmt1, Dnmt3a and Dnmt3b. _Genes Cells_ 11, 805–814 (2006). Article CAS PubMed Google Scholar * Nührenberg, T. G. et al.
Cardiac myocyte de novo DNA methyltransferases 3a/3b are dispensable for cardiac function and remodeling after chronic pressure overload in mice. _PLoS ONE_ 10, e0131019 (2015). Article
PubMed PubMed Central CAS Google Scholar * Gilsbach, R. et al. Dynamic DNA methylation orchestrates cardiomyocyte development, maturation and disease. _Nat. Commun._ 5, 5288 (2014).
Article CAS PubMed PubMed Central Google Scholar * Preissl, S. et al. Deciphering the epigenetic code of cardiac myocyte transcription. _Circ. Res._ 117, 413–423 (2015). Article CAS
PubMed Google Scholar * Bergmann, O. et al. Identification of cardiomyocyte nuclei and assessment of ploidy for the analysis of cell turnover. _Exp. Cell Res._ 317, 188–194 (2011). Article
CAS PubMed Google Scholar * Gaidatzis, D. et al. DNA sequence explains seemingly disordered methylation levels in partially methylated domains of Mammalian genomes. _PLoS Genet._ 10,
e1004143 (2014). Article PubMed PubMed Central CAS Google Scholar * Wu, S. M. et al. Developmental origin of a bipotential myocardial and smooth muscle cell precursor in the mammalian
heart. _Cell_ 127, 1137–1150 (2006). Article CAS PubMed Google Scholar * Ernst, J. & Kellis, M. ChromHMM: automating chromatin-state discovery and characterization. _Nat. Methods_ 9,
215–216 (2012). Article CAS PubMed PubMed Central Google Scholar * Lei, H. et al. De novo DNA cytosine methyltransferase activities in mouse embryonic stem cells. _Development_ 122,
3195–3205 (1996). CAS PubMed Google Scholar * Liao, J. et al. Targeted disruption of DNMT1, DNMT3A and DNMT3B in human embryonic stem cells. _Nat. Genet._ 47, 469–478 (2015). Article CAS
PubMed PubMed Central Google Scholar * Guo, J. U. et al. Distribution, recognition and regulation of non-CpG methylation in the adult mammalian brain. _Nat. Neurosci._ 17, 215–222
(2014). Article CAS PubMed Google Scholar * Lister, R. et al. Global epigenomic reconfiguration during mammalian brain development. _Science_ 341, 1237905 (2013). Article PubMed PubMed
Central CAS Google Scholar * Baubec, T. et al. Genomic profiling of DNA methyltransferases reveals a role for DNMT3B in genic methylation. _Nature_ 520, 243–247 (2015). Article ADS CAS
PubMed Google Scholar * Wang, H. et al. Widespread plasticity in CTCF occupancy linked to DNA methylation. _Genome Res._ 22, 1680–1688 (2012). Article CAS PubMed PubMed Central
Google Scholar * Burger, L., Gaidatzis, D., Schübeler, D. & Stadler, M. B. Identification of active regulatory regions from DNA methylation data. _Nucleic Acids Res._ 41, e155 (2013).
Article PubMed PubMed Central CAS Google Scholar * Lister, R. et al. Human DNA methylomes at base resolution show widespread epigenomic differences. _Nature_ 462, 315–322 (2009).
Article ADS CAS PubMed PubMed Central Google Scholar * Lister, R. et al. Hotspots of aberrant epigenomic reprogramming in human induced pluripotent stem cells. _Nature_ 471, 68–73
(2011). Article ADS CAS PubMed PubMed Central Google Scholar * Tahiliani, M. et al. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1.
_Science_ 324, 930–935 (2009). Article ADS CAS PubMed PubMed Central Google Scholar * Sanborn, A. L. et al. Chromatin extrusion explains key features of loop and domain formation in
wild-type and engineered genomes. _Proc. Natl Acad. Sci. USA_ 112, E6456–6465 (2015). Article CAS PubMed PubMed Central Google Scholar * Fudenberg, G. et al. Formation of chromosomal
domains by loop extrusion. _Cell Rep._ 15, 2038–2049 (2016). Article CAS PubMed PubMed Central Google Scholar * Nora, E. P. et al. Targeted degradation of CTCF decouples local
insulation of chromosome domains from genomic compartmentalization. _Cell_ 169, 930–944 e922 (2017). Article CAS PubMed Google Scholar * Hassan-Zadeh, V., Rugg-Gunn, P. &
Bazett-Jones, D. P. DNA methylation is dispensable for changes in global chromatin architecture but required for chromocentre formation in early stem cell differentiation. _Chromosoma_ 126,
605–614 (2017). Article CAS Google Scholar * Feldmann, A. et al. Transcription factor occupancy can mediate active turnover of DNA methylation at regulatory regions. _PLoS Genet._ 9,
e1003994 (2013). Article PubMed PubMed Central CAS Google Scholar * Doppler, S. A. et al. Myeloid zinc finger 1 (Mzf1) differentially modulates murine cardiogenesis by interacting with
an Nkx2.5 cardiac enhancer. _PLoS ONE_ 9, e113775 (2014). Article ADS PubMed PubMed Central CAS Google Scholar * Heinz, S. et al. Simple combinations of lineage-determining
transcription factors prime cis-regulatory elements required for macrophage and B cell identities. _Mol. Cell_ 38, 576–589 (2010). Article CAS PubMed PubMed Central Google Scholar *
Langmead, B. & Salzberg, S. L. Fast gapped-read alignment with Bowtie 2. _Nat. Methods_ 9, 357–359 (2012). Article CAS PubMed PubMed Central Google Scholar * Li, H. et al. The
sequence alignment/map format and SAMtools. _Bioinformatics_ 25, 2078–2079 (2009). Article PubMed PubMed Central CAS Google Scholar * Ramirez, F. et al. High-resolution TADs reveal DNA
sequences underlying genome organization in flies. Preprint at _bioRxiv_ https://doi.org/10.1101/115063 (2017). * Ramirez, F., Dundar, F., Diehl, S., Gruning, B. A. & Manke, T.
deepTools: a flexible platform for exploring deep-sequencing data. _Nucleic Acids Res._ 42, W187–191 (2014). Article CAS PubMed PubMed Central Google Scholar * Bindea, G. et al. ClueGO:
a Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. _Bioinformatics_ 25, 1091–1093 (2009). Article CAS PubMed PubMed Central Google
Scholar * Kranzhöfer, D. K. et al. 5′-Hydroxymethylcytosine precedes loss of CpG methylation in enhancers and genes undergoing activation in cardiomyocyte maturation. _PLoS ONE_ 11,
e0166575 (2016). Article PubMed PubMed Central CAS Google Scholar * Zhang, Y. et al. Model-based analysis of ChIP-Seq (MACS). _Genome Biol._ 9, R137 (2008). Article PubMed PubMed
Central CAS Google Scholar * Krueger, F. & Andrews, S. R. Bismark: a flexible aligner and methylation caller for Bisulfite-Seq applications. _Bioinformatics_ 27, 1571–1572 (2011).
Article CAS PubMed PubMed Central Google Scholar * Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. _Bioinformatics_ 29, 15–21 (2013). Article CAS PubMed Google Scholar
Download references ACKNOWLEDGEMENTS We thank Claudia Domisch for excellent technical support. We thank Sebastian Arnold, Dirk Schübeler and Fidel Ramirez for helpful discussions and advice.
We acknowledge help from Nils Hammann for isolation of CM from DKO. We thank the Deep Sequencing Facilities at the MPI of Immunobiology and Epigenetics (Freiburg) and EMBL (Heidelberg) for
sequencing. We acknowledge the support of the Freiburg Galaxy Team: Prof. Rolf Backofen, Bioinformatics, University of Freiburg, Germany funded by Collaborative Research Centre 992 Medical
Epigenetics (DFG grant SFB 992/2 2016) and German Federal Ministry of Education and Research (BMBF grant 031 A538A RBC (de.NBI)). We are grateful for mouse ES cells and ES-TKO cells from
Dirk Schübeler. This study was supported by the Deutsche Forschungsgemeinschaft SFB 992 project B03, DFG projects GI 747/2-1, PR 1668/1-1 and HE 2073/5-1, the BIOSS Centre for Biological
Signalling Studies, the Innovationsfonds Baden-Württemberg and DZHK B 15-005. AUTHOR INFORMATION Author notes * Sebastian Preissl Present address: Ludwig Institute for Cancer Research,
Gilman Drive 9500, La Jolla, CA, 92093, USA AUTHORS AND AFFILIATIONS * Institute of Experimental and Clinical Pharmacology and Toxicology, Faculty of Medicine, University of Freiburg,
Albertstrasse 25, 79104, Freiburg, Germany Stephan Nothjunge, Thomas G. Nührenberg, Sebastian Preissl, Martin Schwaderer, Carolin Rommel, Lutz Hein & Ralf Gilsbach * Hermann Staudinger
Graduate School, University of Freiburg, Albertstrasse 25, 79104, Freiburg, Germany Stephan Nothjunge & Martin Schwaderer * University Heart Center Freiburg-Bad Krozingen, Department for
Cardiology und Angiology II, Südring 15, 79189, Bad Krozingen, Germany Thomas G. Nührenberg * Bioinformatics Group, Department of Computer Science, University of Freiburg,
Georges-Köhler-Allee 106, 79110, Freiburg, Germany Björn A. Grüning * Department of Cardiovascular Surgery, Division of Experimental Surgery, German Heart Center, Lazarettstraße 36, 80636,
München, Germany Stefanie A. Doppler & Markus Krane * Faculty of Biology, University of Freiburg, Schänzlestrasse 1, 79104, Freiburg, Germany Carolin Rommel * DZHK (German Center for
Cardiovascular Research)-Partner Site Munich Heart Alliance, Biedersteiner Strasse 29, 80802, München, Germany Markus Krane * BIOSS Centre for Biological Signaling Studies, University of
Freiburg, Schänzlestrasse 1, 79104, Freiburg, Germany Lutz Hein Authors * Stephan Nothjunge View author publications You can also search for this author inPubMed Google Scholar * Thomas G.
Nührenberg View author publications You can also search for this author inPubMed Google Scholar * Björn A. Grüning View author publications You can also search for this author inPubMed
Google Scholar * Stefanie A. Doppler View author publications You can also search for this author inPubMed Google Scholar * Sebastian Preissl View author publications You can also search for
this author inPubMed Google Scholar * Martin Schwaderer View author publications You can also search for this author inPubMed Google Scholar * Carolin Rommel View author publications You
can also search for this author inPubMed Google Scholar * Markus Krane View author publications You can also search for this author inPubMed Google Scholar * Lutz Hein View author
publications You can also search for this author inPubMed Google Scholar * Ralf Gilsbach View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS
R.G. designed the project. S.N. and R.G. performed Hi-C and ChIP-seq experiments. S.P., R.G., and T.G.N. performed WGBS-seq. S.P., R.G., T.G.N., and S.N. performed RNA-seq. M.S. performed
ChromHMM annotation. C.R. performed CTCF ChIP-seq experiments. S.A.D. isolated cardiac progenitor cells. R.G. and S.N. analyzed and visualized the data. B.A.G. developed bioinformatic tools.
S.N., L.H., and R.G. wrote the manuscript. T.G.N., S.A.D., and M.K. edited the manuscript. All authors discussed the results and commented on the manuscript. CORRESPONDING AUTHOR
Correspondence to Ralf Gilsbach. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing financial interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE: Springer Nature
remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. ELECTRONIC SUPPLEMENTARY MATERIAL SUPPLEMENTARY INFORMATION PEER REVIEW FILE RIGHTS AND
PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any
medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The
images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not
included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly
from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Nothjunge, S.,
Nührenberg, T.G., Grüning, B.A. _et al._ DNA methylation signatures follow preformed chromatin compartments in cardiac myocytes. _Nat Commun_ 8, 1667 (2017).
https://doi.org/10.1038/s41467-017-01724-9 Download citation * Received: 15 June 2017 * Accepted: 10 October 2017 * Published: 21 November 2017 * DOI:
https://doi.org/10.1038/s41467-017-01724-9 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative