Sphingomyelin synthase 2 promotes an aggressive breast cancer phenotype by disrupting the homoeostasis of ceramide and sphingomyelin
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Breast cancer is the most common type of carcinoma in women worldwide, but the mechanisms underlying tumour development and progression remain unclear. Sphingomyelin synthase 2
(SGMS2) is a crucial regulator involved in ceramide (Cer) and sphingomyelin (SM) homoeostasis that is mostly studied for its role in lipid metabolism. Our primary study indicated that high
SGMS2 expression is associated with breast cancer metastasis. Gain- and loss-of-function assays in vitro and in vivo revealed that SGMS2 promotes cancer cell proliferation by suppressing
apoptosis through a Cer-associated pathway and promotes cancer cell invasiveness by enhancing epithelial-to-mesenchymal transition (EMT) initiation through the TGF-β/Smad signalling pathway.
Further study determined that SGMS2 activated the TGF-β/Smad signalling pathway primarily by increasing TGF-β1 secretion, which was likely associated with aberrant expression of SM. Thus,
our findings indicate that SGMS2-mediated activation of the TGF-β/Smad signalling pathway is important in breast cancer progression, which provides new insight into the mechanisms underlying
breast cancer metastasis and suggests a possible anticancer therapy for breast cancer. SIMILAR CONTENT BEING VIEWED BY OTHERS ALTERNATIVE SPLICING OF _CERAMIDE SYNTHASE 2_ ALTERS LEVELS OF
SPECIFIC CERAMIDES AND MODULATES CANCER CELL PROLIFERATION AND MIGRATION IN LUMINAL B BREAST CANCER SUBTYPE Article Open access 10 February 2021 CLINICAL RELEVANCE OF CERK AND SPHK1 IN
BREAST CANCER AND THEIR ASSOCIATION WITH METASTASIS AND DRUG RESISTANCE Article Open access 29 October 2022 ECHS1, AN INTERACTING PROTEIN OF LASP1, INDUCES SPHINGOLIPID-METABOLISM IMBALANCE
TO PROMOTE COLORECTAL CANCER PROGRESSION BY REGULATING CERAMIDE GLYCOSYLATION Article Open access 06 October 2021 BACKGROUND Breast cancer is the most common malignancy and one of the
leading causes of cancer-related death and reduced disability-adjusted life years for women1. Although numerous studies have determined that tumour metastasis is the most important reason
for the death of patients with breast cancer, the mechanism underlying tumour metastasis is still not clear2,3. Thus, improving our understanding of the molecular mechanisms underlying
breast cancer progression may help us develop effective methods to manage this disease. Sphingomyelin synthase (SGMS) is a transferase that regulates the synthesis of sphingomyelin (SM) from
ceramide (Cer)4. Although SGMS has three homologues, namely, SGMS1, SGMS2 and SGMS-related protein (SGMSr), only SGMS1 and SGMS2 promote SM synthesis, while SGMSr promotes synthesis of the
SM analogue ceramide phosphoethanolamine5. Cer plays a vital role in regulation of cell apoptosis6. A previous study determined that upregulating SGMS2 significantly decreased the expression
of Cer, which led to aberrant cell apoptosis activity, consequently promoting cell proliferation7. It is well-known that SM is the major component of various biological membranes; it
participates in regulation of membrane stability and cell secretion activity. Studies in many types of cancer have determined that SM promotes cancer development and progression by
regulating cell proliferation and migration potential5. Thus, we suppose that SGMS2 is quite important in promotion of an aggressive breast cancer cell type by regulating the expression of
Cer and SM. However, the mechanism by which SGMS2 promotes breast cancer development and progression remains unknown. Due to the heterogeneity of breast cancer, we generally characterise
several intrinsic molecular breast cancer subtypes according to the tumour gene-expression profile, such as luminal, basal-like, normal-like and triple-negative breast cancer8. Prognosis and
treatment differ between molecular subtypes9. Given this context, two distinct human breast cancer cell lineages were used in our research: non-invasive breast cancer cells (MCF-7)
corresponding to the epithelial subtype and invasive breast cancer cells (MDA-MB-231) corresponding to the mesenchymal subtype10. We investigated the role of SGMS2 in proliferation and
migration of breast cancer cells through both in vitro and in vivo studies and analysed the related signalling pathways that enhance the aggressive of breast cancer cells. MATERIALS AND
METHODS BREAST CANCER CELL LINES AND TUMOUR TISSUE SAMPLES The breast cancer cell lines MCF-7 and MDA-MB-231were obtained from the Cell Bank of the Chinese Academy of Science (Shanghai,
China) and maintained as the protocol required. All cells were authenticated by short-tandem repeat profiling after receipt and were propagated for less than 6 months after resuscitation.
The cells were grown in RPMI 1640 medium (Life Technologies Corporation; Grand Island, NY) supplemented with 10% foetal bovine serum (Life Technologies Corporation; Grand Island, NY). Fresh
primary breast cancer specimens and paired noncancerous breast tissue specimens were provided by the Department of General Surgery, Zhujiang Hospital of Southern Medical University in
Guangzhou, China. Each patient was diagnosed with primary invasive ductal carcinoma of the breast and received modified radical mastectomy in Zhujiang Hospital between Jan 2016 and March
2017. The pathological diagnosis was made by the Department of Pathology of Zhujiang Hospital. The study was approved by the Ethics Committee of Southern Medical University, and all aspects
of the study complied with the criteria of the Declaration of Helsinki. The Committee approved the collection of tissue without requiring informed consent, given that the data would be
analysed anonymously. RNA ISOLATION, REVERSE TRANSCRIPTION AND QUANTITATIVE REAL-TIME PCR Total RNA was extracted using Trizol (Invitrogen; Carlsbad, CA). To quantify the expression of
SGMS2, the total RNA was subjected to polyadenylation and reverse transcription (RT) using a ThermoScriptTM RT-PCR System (Invitrogen). Real-time PCR analysis was carried out using SYBR
Green PCR master mix (Applied Biosystems; Foster City, CA) on an ABI 7500HT system. GAPDH (for cell samples) and RPLP0 (for tumour tissue samples) snRNA were used as endogenous controls. All
samples were normalised to internal controls, and fold changes were calculated through relative quantification (2−ΔΔCT). The primers used are shown in Supplementary Table S1. WESTERN BLOT
ANALYSIS Protein expression was assessed by immunoblot analysis of cell and tissue lysates (20–60 μg) in RIPA buffer in the presence of rabbit antibodies against SGMS2, E-cadherin,
N-cadherin, β-catenin, vimentin, Snail, TGF-β1, Smad2 and phosphorylated-Smad2 (p-Smad2) and mouse antibodies against fibronectin and GAPDH (1:1000; Cell Signalling Technology; Danvers, MA).
IMMUNOFLUORESCENCE ASSAY Cells were cultured on coverslips overnight, fixed with 4% paraformaldehyde for 30 min, and then treated with 5% Triton X-100 (Sigma, USA) for 15 min. After being
blocked in 10% normal blocking serum (Sigma, USA) at room temperature for 15 min, the slides were incubated with rabbit antibody against E-cadherin (1:150) (Cell Signalling Technology; USA)
and rabbit antibody against Vimentin (1:100) (Proteintech, USA) at 4 °C overnight, followed by three washes with phosphate-buffered saline. Cover slips were incubated with a fluorescein
isothiocyanate-conjugated anti-rabbit or anti-mouse antibody or a Texas Red-conjugated anti-mouse or anti-rabbit antibody (1:200) (SantaCruz Biotech; USA) for 30 min at room temperature and
then stained with 6-diamidino-2-phenylindole (DAPI, Invitrogen). ENZYME-LINKED IMMUNOSORBENT ASSAY Cytoplasm and supernatants from MDA-MB-231 and MCF-7 cells were collected, and the cell
number was determined. The total Cer and SM levels in the cytoplasm were measured using a human ceramide and SM ELISA Kit (Enzyme-linked Biotechnology, Shanghai, China) according to the
manufacturer’s instructions. The total level of secretory TGF-β1 in the supernatant was measured using a human TGF-β1 ELISA Kit (Enzyme-linked Biotechnology, Shanghai, China) according to
the manufacturer’s protocol. The cytokine expression level (pg/ml) per 105 cells was analysed. TUMOUR GROWTH ASSAY Four-to-five-week-old male BALB/c nude mice were purchased from the
Laboratory Animal Services Centre at the Southern Medical University. Animal handling and experimental procedures were approved by the Animal Experimental Ethics Committee of Southern
Medical University. For the tumour growth assay, 1 × 107 SGMS2 overexpression cells were injected subcutaneously into the right back of nude mice, while the control cells were
correspondingly injected into the left back (_n_ = 5/group). To facilitate oestrogen-dependent tumour establishment, each mouse in the MCF7 cell groups received 17-estradiol (0.72 mg/
pellet, 60 day release; Innovative Research of America). Tumour volume was calculated using the following formula: _V_ = 0.5 × _D_ × _L_2, where _V_ represents volume, _D_ represents the
longitudinal diameter and _L_ represents the latitudinal diameter. STATISTICAL ANALYSIS Data were analysed using SPSS version 19.0 software (SPSS, Chicago, IL). Nonparametric test (Wilcoxon
and Mann–Whitney) was used to analyse clinical data. Student’s _t_ test and one-way ANOVA were carried out for comparisons between two groups. Paired-samples _t_ test was used to analyse
paired data. All statistical tests were two-sided, and statistical significance was established at _P_ < 0.05. For additional Materials and methods please refer to the Supplementary
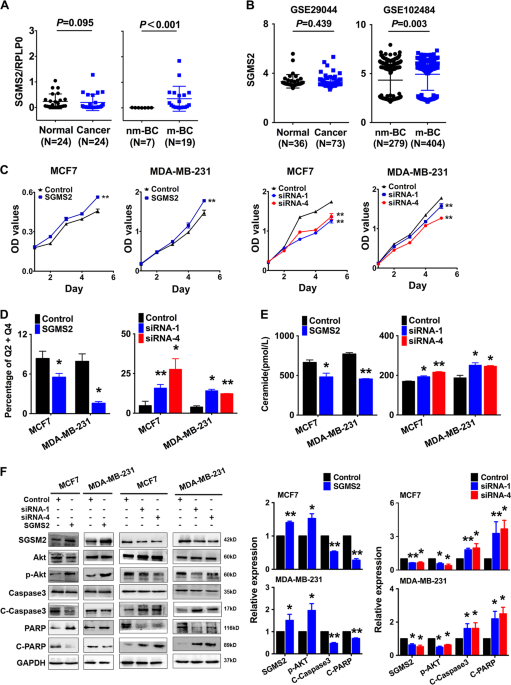
materials. RESULTS SGMS2 IS INCREASED IN BREAST CANCER METASTASIS SGMS2 expression was significantly higher in breast cancer tissues from patients with lymph node or distant metastasis
compared with non-metastasis samples (_P_ < 0.001, Fig. 1a, right). However, there was no significant difference in SGMS2 expression between breast cancer and adjacent normal tissue (_P_
_=_ 0.095, Fig. 1a left). The expression of SGMS2 was also investigated in Gene Expression Omnibus (GEO) GSE102484 (_P_ = 0.003, Fig. 1b right) and GSE29044 (_P_ = 0.439, Fig. 1b left)
datasets and the results were consistent with the above-described results. These findings suggest that the alteration of SGMS2 in breast cancer is associated with tumour metastasis but not
tumorigenesis. SGMS2 PROMOTES THE PROLIFERATION POTENTIAL OF BREAST CANCER CELLS IN VITRO BY DISRUPTING THE CER-ASSOCIATED APOPTOSIS PATHWAY Further studies were applied to determine the
effects of SGMS2 on the bioactivities of breast cancer cells. Real-time PCR (Fig. S1) and immunoblotting (Fig. 1f) assays were used to confirm the efficiency of _SGMS2_ ORF constructs and
anti-_SGMS2_ small interfering RNA oligonucleotides (siRNA) in MCF-7 and MDA-MB-231 cells. CCK-8 assays revealed that SGMS2 significantly promoted the proliferation of both MCF-7 and
MDA-MB-231 cells (_P_ < 0.05, Fig. 1c). In addition, annexin V staining showed that SGMS2 decreased the percentage of cells undergoing apoptosis in both cell lines, whereas anti-SGMS2
promoted cell apoptosis (_P_ < 0.05, Fig. 1d, Supplementary Fig. S2). It is well-known that SGMS2 is a key enzyme in the hemeostasis of Cer, which is tightly associated with cell
apoptosis5,11. Many studies have revealed that ceramide plays a pivotal role in cell apoptosis via the P-AKT → caspase-3 → PARP signalling pathway in several types of diseases, including
renal cell carcinoma12, hepatocellular carcinoma13,14 and retinopathy15. To determine the mechanism underlying the SGMS2-induced increase in cell proliferation, we investigated the
expression of Cer and activation of the Cer-associated apoptosis pathway. Enzyme-linked immunosorbent assays demonstrated that SGMS2 significantly decreased the expression of Cer, whereas
suppression of SGMS2 presented the opposite result (Fig. 1e). Immunoblot assays showed that overexpression of SGMS2 promoted Akt phosphorylation and decreased the cleavage of Caspase3 and
PARP. By contrast, suppression of SGMS2 inhibited Akt phosphorylation but promoted the cleavage of Caspase3 and PARP (Fig. 1f). SGMS2 PROMOTES MIGRATION/INVASION OF BREAST CANCER VIA EMT
TRIGGERED BY THE TGF-Β/SMAD SIGNALLING PATHWAY Transwell and tumour invasion assays revealed that SGMS2 significantly enhanced the migration and invasion potential of both MCF-7 and
MDA-MB-231 cells (_P_ < 0.05, Fig. 2a, b). Wound-healing assays showed that SGMS2 also promoted the motility potential of MCF-7 and MDA-MB-231 cells (_P_ < 0.05, Fig. 2c, and
Supplementary Fig. S3). Cell morphology assays showed that only MCF-7 cells significantly transformed into a more mesenchymal phenotype after transfection with _SGMS2_ ORF constructs. By
contrast, inhibiting _SGMS2_ expression only transformed MDA-MB-231 cells into a more epithelial phenotype (Fig. 3a). However, further immunoblotting assays determined that SGMS2 inhibited
the expression of epithelial markers (E-cadherin and β-catenin) and stimulated the expression of mesenchymal markers (Fibronectin, N-cadherin and Vimentin) in both cell lines. By contrast,
inhibition of SGMS2 expression stimulated the expression of epithelial markers but suppressed the expression of mesenchymal markers. (Fig. 3b). Additional immunofluorescence assays
determined that SGMS2 could facilitate epithelial-to-mesenchymal (EMT) transition in both of the breast cancer cell lines (Fig. 3c). It has been well-characterised that EMT is tightly
involved in activation of the TGF/Smad signalling pathway. To determine the mechanism underlying the role of SGMS2 in promoting EMT in breast cancer cells, we investigated the TGF-β/Smad
signalling pathway. Immunoblotting assay findings suggested that SGMS2 promoted Smad2 phosphorylation, whereas silencing of SGMS2 reduced the expression of phosphorylated Smad2 (Fig. 3b). In
addition, SGMS2 overexpression promoted the expression of Snail, a key trigger of epithelial-to-mesenchymal transition mediated by TGF-β/Smad signalling, while suppression of SGMS2
decreased Snail expression (Fig. 3b). SGMS2 EXERTS TUMOUR-PROMOTING EFFECTS PRIMARILY BY ENHANCING TGF-Β1 SECRETION AND ACTIVATING TGF-Β/SMAD SIGNALLING PATHWAY To determine the role of the
TGF-β/Smad signalling pathway in the tumour-promoting effects of SGMS2, we specifically disrupted the TGF-β/Smad signalling pathway using pirfenidone in SGMS2 transient overexpression breast
cancer cells. We found that blocking the TGF-β/Smad signalling pathway removed the SGMS2-mediated promotion of motility and the migration potential of MCF-7 and MDA-MB-231 cells (Fig. 4a,
b, and Supplementary Fig. S4). Immunoblotting assays showed that blocking the TGF-β/Smad signalling pathway reversed the SGMS2-mediated alteration in EMT signature (Fig. 4c). These findings
strongly suggest that the TGF-β/Smad signalling pathway plays a vital role in the SGMS2-induced invasiveness of breast cancer cells. To determine the mechanism underlying the activation of
TGF-β/Smad signalling mediated by SGMS2, we investigated the expression of TGF-β1. Real-time PCR analysis revealed that SGMS2 increased the number of TGF-β1 transcripts (Fig. 5a,
Supplementary Fig. S5A), whereas immunoblotting assays showed that SGMS2 reduced the expression of intracellular TGF-β1 (Fig. 5c, Supplementary Fig. S5B). Further enzyme-linked immunosorbent
assays (ELISAs) demonstrated that both transient and stable SGMS2 overexpression enhanced the secretion of TGF-β1, whereas silencing of SGMS2 reduced the expression of secretory TGF-β1
(Fig. 5b, Supplementary Fig. S5C). These findings suggest that SGMS2 significantly promotes the expression of extracellular TGF-β1 by enhancing its secretion. In addition, ELISA assays
demonstrated that SGMS2 upregulation increased SM expression, while SGMS2 suppression inhibited the expression of SM (Fig. 5d, Supplementary Fig. S5D). It is well-known that SM is involved
in regulation of cell secretory activity16,17. Thus, we supposed that SGMS2 promotes TGF-β1 secretion by upregulating the expression of SM. In conclusion, our hypothetical model showed that
aberrant upregulation of SGMS2 disrupts the hemeostasis of ceramide and SM, which activates the Cer-associated apoptosis pathway and TGF-β/Smad pathway, leading to BRC development and
progression (Fig. 5e). SGMS2 PROMOTES THE PROLIFERATION AND METASTASIS OF BREAST CANCER IN VIVO A lentivirus (LV)-based system (Genepharma, Shanghai, China) was used to investigate the
biological function of SGMS2 _in vivo_. Immunofluorescence assays determined the LV transfection efficiency (Fig. 6a), and real-time PCR assays confirmed a significant increase in SGMS2
transcripts in LV-SGMS2-transfected cells compared with LV-control-transfected cells (_P_ < 0.05, Supplementary Fig. S6A). For in vitro studies, stable overexpression of SGMS2 in MCF-7
and MDA-MB-231 cells led to an increased potential for cell proliferation and clone formation and disturbed the Cer-associated apoptosis pathway (Fig. 6b, Supplementary Figs. S6B, C, S7). In
addition, stable overexpression of SGMS2 promoted migration/invasion, motility and EMT in both MCF-7 and MDA-MB-231 cells after transduction with LV (Supplementary Figs. S8–S11). A
subcutaneous tumour mouse model was used to determine the effect of SGMS2 on the growth of breast cancer cells in vivo. We found that SGMS2 significantly promoted the proliferation of tumour
cells in vivo in both MCF-7 and MDA-MB-231 cell lines (_P_ < 0.05, Fig. 6c). To determine the effect of SGMS2 on homing capacity in vivo, 5 × 106 cancer cells were injected into nude
mice through the tail vein to observe the rate of nodule formation in the lungs and liver. Compared with controls, more and bigger tumour nodules were found in the lungs of mice that
received MDA-MB-231 cells overexpressing SGMS2 (_P_ < 0.05, Fig. 6d, e). Interestingly, metastatic liver lesions were found in 3 mice in the MDA-MB-231/LV-SGMS2 group, while no metastatic
nodules were found in mice in the MDA-MB-231/LV-control group (_P_ < 0.05, Fig. 6f). However, both the MCF-7/LV-SGMS2 and MCF-7/LV-control groups showed a complete absence of tumour
nodules in the lungs and liver (Supplementary Fig. S12). DISCUSSION Although many molecules are involved in the synthesis of SM from Cer5,18, SGMSs, especially SGMS2, remain the driving
pathway that accounts for this synthesis in mammals19,20. Many studies have demonstrated that aberrant downregulation of Cer promotes cancer cell proliferation by inhibiting cell
apoptosis21,22,23. In addition, studies have revealed that SM plays a vital role in abnormal activation of tumour-associated signalling pathways, including the mTOR24 and RAS-MAPK signalling
pathways25. Studies of human cervical carcinoma26 and leukaemia11,27 have even revealed that the effect of SGMS2 on cell proliferation and differentiation involves the homoeostasis of Cer
and SM. Although the role of SGMS2 in the development and progression of breast cancer is still unknown, the findings mentioned above strongly suggest that SGMS2 may act as a tumour promoter
in breast cancer. A previous study revealed that high-SGMS activity is positively associated with the incidence of haematological malignancies28. However, our study found no significant
difference in SGMS2 expression between breast cancer tissue and paired normal tissue, which suggests that aberrant expression of SGMS2, is unrelated to the incidence of breast cancer.
However, it was intriguing that the expression of SGMS2 was higher in patients with lymph node metastasis than in those without metastasis, which strongly suggests that SGMS2 is associated
with tumour metastasis in breast cancer patients. However, the role of SGMS2 in tumour metastasis and its underlying mechanism are still not clear. To further elucidate the exact role of
SGMS2 in breast cancer, we carried out gain- and loss-of-function studies in vitro and in vivo. SGMS2 expression was positively associated with cell viability and colony formation and
negatively associated with cell apoptosis through its inhibition of the Cer-related pathway, which was consistent with the results of studies in lymphocytes, hepatocytes, astrocytes and
fibroblasts29,30,31. In addition, our studies revealed that SGMS2 significantly promoted in vitro migration, motility and invasiveness of breast cancer cells through EMT. Notably, a
significant change in mesenchymal type was observed only in MCF-7 cells after transient and stable SGMS2 overexpression. However, a significant change in epithelial type was observed only in
MDA-MB-231 cells with decreased SGMS2 expression. Moreover, tumour-lung homing was only found in subjects receiving the MDA-MB-231 cell line, while subjects receiving the MCF-7 cell line
showed a complete absence of nodule formation in lungs. We considered that these different results between the MCF-7 and MDA-MB-231 cell lines may be attributed to their distinct
characteristics and gene-expression profiles10,32,33,34. The classic TGF-β/Smad signalling pathway is well-known to play a crucial role in EMT initiation by regulating downstream expression
of proteins, such as Snail/Slug, ZEB1/2 and Twist family proteins35,36,37. Our studies revealed that SGMS2 indeed activates the TGF-β/Smad signalling pathway and subsequently increases the
expression of its downstream protein Snail. In addition, we found that aggressive breast cancer cell phenotypes were recovered when the TGF-β/Smad signalling pathway was specifically
arrested in SGMS2 overexpression cells by pirfenidone, and the expression of EMT-related markers was also reversed. These findings strongly suggest that SGMS2 promotes an aggressive breast
cancer cell phenotype by activating the TGF-β/Smad signalling pathway. It is well-known that TGF-β1 plays an important role in activating the TGF-β/Smad signalling pathway38,39. To determine
the mechanism underlying the activation of TGF-β/Smad signalling pathway mediated by SGMS2, we investigated the expression and secretion of TGF-β1. Our studies found that SGMS2 indeed
increased the transcription and secretion of TGF-β1. However, we found significantly decreased expression of intracellular TGF-β1 with transient SGMS2 overexpression. Some studies have
revealed the potential role of SGMSs in cellular secretory activity40. SGMSs can promote the transport of vesicular stomatitis virus G protein and enhance the secretion of insulin in rat β
cells41. A recent study even determined that SGMSs regulate the defensive activity of neutrophils by promoting the release of an antifungal factor42.These findings strongly suggest that
SGMS2 promotes the expression of extracellular TGF-β1 primarily by enhancing its secretion level. SM is a dominant component of membranous vesicles, including early/late and recycling
endosomes and lysosomes43. Studies have confirmed that SM is broadly required for cell secretory activity40, thus suppression of SM significantly inhibits the secretion of influenza
haemagglutinin44. A recent study also revealed that a short form of the auxiliary TGF-β receptor endoglin selectively interacts with SM and is released into circulation via SM-mediated
exosomes45. Considering the effects of SGMS2 on cell secretory activity and SM homoeostasis, we propose that SGMS2 enhances the secretion of TGF-β1 by promoting the expression of SM.
CONCLUSION In summary, we found increased expression of SGMS2 in patients with metastatic breast cancer. The aberrant expression of SGMS2 disrupted the hemeostasis of Cer and SM. In turn,
suppression of Cer expression led to enhanced cell proliferation potential through inhibition of cell apoptosis. SGMS2 increased the expression of TGF-β1 by upregulating SM, which
subsequently activated the TGF-β/Smad signalling pathway and promoted EMT in breast cancer cells, thus increasing the migration and invasiveness of breast cancer cells. These findings may
help confirm the mechanisms underlying breast cancer development and progression and allow us to devise novel anticancer therapies. DATA AVAILABILITY All data and materials would be
available if required. REFERENCES * Fitzmaurice, C. et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and
Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: a systematic analysis for the Global Burden of Disease Study. _JAMA Oncol._ 3, 524–548 (2017). Article PubMed Google
Scholar * Marino, N. et al. Breast cancer metastasis: issues for the personalization of its prevention and treatment. _Am. J. Pathol._ 183, 1084–1095 (2013). Article CAS PubMed PubMed
Central Google Scholar * Hassan, M., Watari, H., AbuAlmaaty, A., Ohba, Y. & Sakuragi, N. Apoptosis and molecular targeting therapy in cancer. _Biomed. Res. Int._ 2014, 150845 (2014).
PubMed PubMed Central Google Scholar * Adada, M., Luberto, C. & Canals, D. Inhibitors of the sphingomyelin cycle: sphingomyelin synthases and sphingomyelinases. _Chem. Phys. Lipids_
197, 45–59 (2016). Article CAS PubMed Google Scholar * Taniguchi, M. & Okazaki, T. The role of sphingomyelin and sphingomyelin synthases in cell death, proliferation and
migration-from cell and animal models to human disorders. _Biochim. Biophys. Acta_ 1841, 692–703 (2014). Article CAS PubMed Google Scholar * Cheng Q. et al. The ceramide pathway is
involved in the survival, apoptosis and exosome functions of human multiple myeloma cells in vitro. _Acta Pharmacol. Sin._ 39, 561–56832 (2017). * Li, Y. et al. Sphingomyelin synthase 2
activity and liver steatosis: an effect of ceramide-mediated peroxisome proliferator-activated receptor gamma2 suppression. _Arterioscler. Thromb. Vasc. Biol._ 33, 1513–1520 (2013). Article
CAS PubMed PubMed Central Google Scholar * Prat, A. et al. Clinical implications of the intrinsic molecular subtypes of breast cancer. _Breast_ 24, S26–35 (2015). Article PubMed
Google Scholar * Morris, G. J. et al. Differences in breast carcinoma characteristics in newly diagnosed African–American and Caucasian patients: a single-institution compilation compared
with the National Cancer Institute’s Surveillance, Epidemiology, and End Results database. _Cancer_ 110, 876–884 (2007). Article PubMed Google Scholar * Hooshmand, S. et al.
Differentially expressed proteins in ER + MCF7 and ER-MDA-MB-231 human breast cancer cells by RhoGDI-α silencing and overexpression. _Asian Pac. J. Cancer Prev._ 15, 3311–3317 (2014).
Article PubMed Google Scholar * Watanabe, M. et al. Increase of nuclear ceramide through caspase-3-dependent regulation of the “sphingomyelin cycle” in Fas-induced apoptosis. _Cancer
Res._ 64, 1000–1007 (2004). Article CAS PubMed Google Scholar * Boojar, M. M. A., Boojar, M. M. A., Golmohammad, S. & Bahrehbar, I. Data on cell survival, apoptosis, ceramide
metabolism and oxidative stress in A-494 renal cell carcinoma cell line treated with hesperetin and hesperetin-7-O-acetate. _Data Brief_ 20, 596–601 (2018). Article PubMed PubMed Central
Google Scholar * Lin, M. et al. Exosomal neutral sphingomyelinase 1 suppresses hepatocellular carcinoma via decreasing the ratio of sphingomyelin/ceramide. _FEBS J._ 285, 3835–3848 (2018).
Article CAS PubMed Google Scholar * Zhu, Q. et al. Dihydroceramide-desaturase-1-mediated caspase 9 activation through ceramide plays a pivotal role in palmitic acid-induced HepG2 cell
apoptosis. _Apoptosis_ 21, 1033–1044 (2016). Article CAS PubMed Google Scholar * Prado Spalm, F. H. et al. Ceramide induces the death of retina photoreceptors through activation of
parthanatos. _Mol. Neurobiol._ (2018). epub aheand of print. * Chen, J. et al. Structure/activity relationship of thapsigargin inhibition on the purified Golgi/secretory pathway
Ca2+/Mn2+-transport ATPase (SPCA1a). _J. Biol. Chem._ 292, 6938–6951 (2017). Article CAS PubMed PubMed Central Google Scholar * Kavishwar, A. & Moore, A. Sphingomyelin patches on
pancreatic beta-cells are indicative of insulin secretory capacity. _J. Histochem. Cytochem._ 61, 910–919 (2013). Article CAS PubMed PubMed Central Google Scholar * Bielawski, J. et al.
Sphingolipid analysis by high performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS). _Adv. Exp. Med. Biol._ 688, 46–59 (2010). Article CAS PubMed Google Scholar * van
den Hill, A., van Heusden, G. P. & Wirtz, K. W. The synthesis of sphingomyelin in the Morris hepatomas 7777 and 5123D is restricted to the plasma membrane. _Biochim. Biophys. Acta_ 833,
354–357 (1985). Article PubMed Google Scholar * Ziulkoski, A. L., Zimmer, A. R. & Guma, F. C. De novo synthesis and recycling pathways of sphingomyelin in rat Sertoli cells. _Biochem.
Biophys. Res. Commun._ 281, 971–975 (2001). Article CAS PubMed Google Scholar * Li, F. & Zhang, N. Ceramide: therapeutic potential in combination therapy for cancer treatment.
_Curr. Drug Metab._ 17, 37–51 (2015). Article CAS PubMed Google Scholar * Lafont, E. et al. Ordering of ceramide formation and caspase-9 activation in CD95L-induced Jurkat leukemia T
cell apoptosis. _Biochim. Biophys. Acta_ 1821, 684–693 (2012). Article CAS PubMed Google Scholar * Abdel Shakor, A. B., Atia, M., Alshehri, A. S., Sobota, A. & Kwiatkowska, K.
Ceramide generation during curcumin-induced apoptosis is controlled by crosstalk among Bcl-2, Bcl-xL, caspases and glutathione. _Cell Signal._ 27, 2220–2230 (2015). Article CAS PubMed
Google Scholar * Dauner, K., Eid, W., Raghupathy, R., Presley, J. F. & Zha, X. mTOR complex 1 activity is required to maintain the canonical endocytic recycling pathway against
lysosomal delivery. _J. Biol. Chem._ 292, 5737–5747 (2017). Article CAS PubMed PubMed Central Google Scholar * Teres, S. et al. 2-Hydroxyoleate, a nontoxic membrane binding anticancer
drug, induces glioma cell differentiation and autophagy. _Proc. Natl Acad. Sci. USA_ 109, 8489–8494 (2012). Article CAS PubMed PubMed Central Google Scholar * Tafesse, F. G. et al. Both
sphingomyelin synthases SMS1 and SMS2 are required for sphingomyelin homeostasis and growth in human HeLa cells. _J. Biol. Chem._ 282, 17537–17547 (2007). Article CAS PubMed Google
Scholar * Itoh, M. et al. Possible role of ceramide as an indicator of chemoresistance: decrease of the ceramide content via activation of glucosylceramide synthase and sphingomyelin
synthase in chemoresistant leukemia. _Clin. Cancer Res._ 9, 415–423 (2003). CAS PubMed Google Scholar * Lafont, E., Kitatani, K., Okazaki, T. & Segui, B. Regulation of death and
growth signals at the plasma membrane by sphingomyelin synthesis: implications for hematological malignancies. _Recent. Pat. Anticancer Drug Discov._ 6, 324–333 (2011). Article CAS PubMed
Google Scholar * Miro-Obradors, M. J., Osada, J., Aylagas, H., Sanchez-Vegazo, I. & Palacios-Alaiz, E. Microsomal sphingomyelin accumulation in thioacetamide-injured regenerating rat
liver: involvement of sphingomyelin synthase activity. _Carcinogenesis_ 14, 941–946 (1993). Article CAS PubMed Google Scholar * Riboni, L., Viani, P., Bassi, R., Giussani, P. &
Tettamanti, G. Basic fibroblast growth factor-induced proliferation of primary astrocytes. evidence for the involvement of sphingomyelin biosynthesis. _J. Biol. Chem._ 276, 12797–12804
(2001). Article CAS PubMed Google Scholar * Luberto, C. & Hannun, Y. A. Sphingomyelin synthase, a potential regulator of intracellular levels of ceramide and diacylglycerol during
SV40 transformation. Does sphingomyelin synthase account for the putative phosphatidylcholine-specific phospholipase C? _J. Biol. Chem._ 273, 14550–14559 (1998). Article CAS PubMed Google
Scholar * Trapp E. K. et al. LKB1 pro-oncogenic activity triggers cell survival in circulating tumor cells. _Mol. Oncol._ 11, 1508–1526 (2017). * Hopkins A., Coatham M. L., Berry F. B.
FOXC1 Regulates FGFR1 isoform switching to promote invasion following TGFbeta-induced EMT. _Mol. Cancer Res._ 15, 1341–1353 (2017). * Taherian, A., Li, X., Liu, Y. & Haas, T. A.
Differences in integrin expression and signaling within human breast cancer cells. _BMC Cancer_ 11, 293 (2011). Article CAS PubMed PubMed Central Google Scholar * Kalluri, R. &
Weinberg, R. A. The basics of epithelial–mesenchymal transition. _J. Clin. Invest._ 119, 1420–1428 (2009). Article CAS PubMed PubMed Central Google Scholar * Gupta, P. & Srivastava,
S. K. HER2 mediated de novo production of TGFbeta leads to SNAIL driven epithelial-to-mesenchymal transition and metastasis of breast cancer. _Mol. Oncol._ 8, 1532–1547 (2014). Article CAS
PubMed PubMed Central Google Scholar * Katsuno, Y., Lamouille, S. & Derynck, R. TGF-beta signaling and epithelial–mesenchymal transition in cancer progression. _Curr. Opin. Oncol._
25, 76–84 (2013). Article CAS PubMed Google Scholar * Wu, T. et al. Let7a suppresses cell proliferation via the TGFbeta/SMAD signaling pathway in cervical cancer. _Oncol. Rep._ 36,
3275–3282 (2016). Article CAS PubMed Google Scholar * Yao, W. et al. Tetrahydroxystilbene glucoside improves TNF-alpha-induced endothelial dysfunction: involvement of TGFbeta/Smad
pathway and inhibition of vimentin expression. _Am. J. Chin. Med.._ 43, 183–198 (2015). Article CAS PubMed Google Scholar * Deng, Y., Rivera-Molina, F. E., Toomre, D. K. & Burd, C.
G. Sphingomyelin is sorted at the trans Golgi network into a distinct class of secretory vesicle. _Proc. Natl Acad. Sci. USA_ 113, 6677–6682 (2016). Article CAS PubMed PubMed Central
Google Scholar * Subathra, M., Qureshi, A. & Luberto, C. Sphingomyelin synthases regulate protein trafficking and secretion. _PLoS One_ 6, e23644 (2011). Article CAS PubMed PubMed
Central Google Scholar * Qureshi, A. et al. Role of sphingomyelin synthase in controlling the antimicrobial activity of neutrophils against _Cryptococcus neoformans_. _PLoS One_ 5, e15587
(2010). Article CAS PubMed PubMed Central Google Scholar * Soreghan, B., Thomas, S. N. & Yang, A. J. Aberrant sphingomyelin/ceramide metabolic-induced neuronal endosomal/lysosomal
dysfunction: potential pathological consequences in age-related neurodegeneration. _Adv. Drug Deliv. Rev._ 55, 1515–1524 (2003). Article CAS PubMed Google Scholar * Tafesse, F. G. et al.
Intact sphingomyelin biosynthetic pathway is essential for intracellular transport of influenza virus glycoproteins. _Proc. Natl Acad. Sci. USA_ 110, 6406–6411 (2013). Article CAS PubMed
PubMed Central Google Scholar * Ermini, L. et al. A single sphingomyelin species promotes exosomal release of endoglin into the maternal circulation in preeclampsia. _Sci. Rep._ 7, 12172
(2017). Article PubMed PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS This work was supported by Natural Science Foundation of Guangdong Province, China
(2014A030313334). AUTHORS’ CONTRIBUTIONS K.Z. contributed to data acquisition, analysis and interpretation of data, drafting of the manuscript, and statistical analysis; Z.C. and H.F.
contributed to data acquisition, analysis and interpretation and statistical analysis; Y.C. and C.Z. contributed to acquisition of data and materials; J.Y. contributed to data analysis and
statistical analysis; Y.L., L.Z. and X.J. contributed to improvement of the study concept and study supervision, also L.Z. and X.J. contributed to the revision of the manuscript; F.S.
contributed to the study concept and design and study supervision, obtained funding and critically revised the manuscript for important intellectual content. All authors read and approved
the final manuscript. AUTHOR INFORMATION Author notes * These authors contributed equally: Kehong Zheng, Zetao Chen, Haizhan Feng AUTHORS AND AFFILIATIONS * Department of General Surgery,
Zhujiang Hospital, Southern Medical University, Guangzhou, China Kehong Zheng, Haizhan Feng, Ying Chen, Cheng Zhang, Jinlong Yu, Yunfeng Luo & Fujun Shi * Division of Laboratory
Medicine, Zhujiang Hospital, Southern Medical University, Guangzhou, China Kehong Zheng * Department of Pathology, Nanfang Hospital, Southern Medical University, Guangzhou, China Zetao Chen
& Liang Zhao * Department of Pathology, School of Basic Medical Sciences, Southern Medical University, Guangzhou, China Zetao Chen & Liang Zhao * Department of Cell biology,
Downstate Medical Centre, State University of New York, New York, NY, USA Xiancheng Jiang Authors * Kehong Zheng View author publications You can also search for this author inPubMed Google
Scholar * Zetao Chen View author publications You can also search for this author inPubMed Google Scholar * Haizhan Feng View author publications You can also search for this author inPubMed
Google Scholar * Ying Chen View author publications You can also search for this author inPubMed Google Scholar * Cheng Zhang View author publications You can also search for this author
inPubMed Google Scholar * Jinlong Yu View author publications You can also search for this author inPubMed Google Scholar * Yunfeng Luo View author publications You can also search for this
author inPubMed Google Scholar * Liang Zhao View author publications You can also search for this author inPubMed Google Scholar * Xiancheng Jiang View author publications You can also
search for this author inPubMed Google Scholar * Fujun Shi View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Fujun
Shi. ETHICS DECLARATIONS ETHICS APPROVAL Fresh primary breast cancer specimens and paired noncancerous breast tissue specimens were provided by the Department of General Surgery, Zhujiang
Hospital, Southern Medical University in Guangdong, China. The study was approved by the Ethics Committee of Southern Medical University, and all aspects of the study comply with the
criteria of the Declaration of Helsinki. The Committee approved the collection of tissue without requiring informed consent, given that the data would be analysed anonymously. CONSENT FOR
PUBLICATION Yes. CONFLICT OF INTEREST The authors declare that they have no conflict of interest. ADDITIONAL INFORMATION PUBLISHER’S NOTE: Springer Nature remains neutral with regard to
jurisdictional claims in published maps and institutional affiliations. Edited by G.M. Fimia SUPPLEMENTARY INFORMATION SUPPLEMENTARY RESULTS SUPPLEMENTARY MATERIALS AND METHOD SUPPLEMENTARY
TABLE RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and
reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes
were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If
material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain
permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS
ARTICLE Zheng, K., Chen, Z., Feng, H. _et al._ Sphingomyelin synthase 2 promotes an aggressive breast cancer phenotype by disrupting the homoeostasis of ceramide and sphingomyelin. _Cell
Death Dis_ 10, 157 (2019). https://doi.org/10.1038/s41419-019-1303-0 Download citation * Received: 09 May 2018 * Revised: 14 December 2018 * Accepted: 02 January 2019 * Published: 15
February 2019 * DOI: https://doi.org/10.1038/s41419-019-1303-0 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a
shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative