Dna methylation of the glucocorticoid receptor gene predicts substance use in adolescence: longitudinal data from over 1000 young individuals
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Early life stress has been linked to increased methylation of the Nuclear Receptor Subfamily 3 Group C Member 1 (_NR3C1_) gene, which codes for the glucocorticoid receptor.
Moreover, early life stress has been associated with substance use initiation at a younger age, a risk factor for developing substance use disorders. However, no studies to date have
investigated whether _NR3C1_ methylation can predict substance use in young individuals. This study included adolescents 13–14 years of age that reported no history of substance use at
baseline, (_N_ = 1041; males = 46%). Participants contributed saliva DNA samples and were followed in middle adolescence as part of KUPOL, a prospective cohort study of 7th-grade students in
Sweden. Outcome variables were self-reports of (i) recent use, (ii) lifetime use, and (iii) use duration of (a) alcohol, (b) tobacco products, (c) cannabis, or (d) any substance. Outcomes
were measured annually for three consecutive years. The predictor variable was DNA methylation at the exon 1 F locus of _NR3C1_. Risk and rate ratios were calculated as measures of
association, with or without adjustment for internalizing symptoms and parental psychiatric disorders. For a subset of individuals (_N_ = 320), there were also morning and afternoon salivary
cortisol measurements available that were analyzed in relation to _NR3C1_ methylation levels. Baseline _NR3C1_ hypermethylation associated with future self-reports of recent use and use
duration of any substance, before and after adjustment for potential confounders. The overall estimates were attenuated when considering lifetime use. Sex-stratified analyses revealed the
strongest association for cigarette use in males. Cortisol analyses revealed associations between _NR3C1_ methylation and morning cortisol levels. Findings from this study suggest that
saliva _NR3C1_ hypermethylation can predict substance use in middle adolescence. Additional longitudinal studies are warranted to confirm these findings. SIMILAR CONTENT BEING VIEWED BY
OTHERS ALCOHOL CONSUMPTION, DEPRESSION, OVERWEIGHT AND CORTISOL LEVELS AS DETERMINING FACTORS FOR _NR3C1_ GENE METHYLATION Article Open access 24 March 2021 DNA METHYLATION AND GENERAL
PSYCHOPATHOLOGY IN CHILDHOOD: AN EPIGENOME-WIDE META-ANALYSIS FROM THE PACE CONSORTIUM Article 16 November 2022 METHYLATION AND EXPRESSION OF GLUCOCORTICOID RECEPTOR EXON-1 VARIANTS AND
FKBP5 IN TEENAGE SUICIDE-COMPLETERS Article Open access 13 February 2023 INTRODUCTION Adolescence represents a critical period for brain maturation and development that, if disrupted by
substance use, can enhance the risk for developing substance use disorders later in life [1]. Individuals who initiate substance use before the age of 14 have an estimated 34% prevalence
rate of lifetime abuse and, as individuals continue to mature until the age of 21, the risk drops 4–5% for each year that substance use initiation is delayed [2, 3]. Exposure to stressful
life events in childhood is a strong risk factor for a younger age of drug use onset and the emergence of problematic substance use already in adolescence [4,5,6,7,8]. However, the molecular
mechanisms that underlie the association between early life stress and adolescent substance use remain largely unknown. Stress activates the hypothalamic–pituitary–adrenal (HPA) axis and
leads to the production of glucocorticoids, e.g., cortisol in humans [9]. Glucocorticoids serve numerous functions essential to survival, including metabolic and inflammatory processes, and
they mediate the behavioral responses to stress [9]. Glucocorticoids also act in a negative feedback loop to suppress HPA activation and promote homeostasis when the stressor has subsided
[9]. On the molecular level, this negative feedback is achieved by binding of glucocorticoids to glucocorticoid receptors, transcription factors encoded by the Nuclear Receptor Subfamily 3
Group C Member 1 (_NR3C1_) gene [9]. Preclinical rodent models of early life stress, e.g., based on maternal separation, have linked prolonged activations of the HPA axis with the
development of anxiogenic and fearful behaviors [10]. The negative effects of maternal separation were found to be mediated by epigenetic changes in the rodent _Nr3c1_ gene. Specifically, a
relationship was found between maternal separation and DNA hypermethylation of the neuron-specific _Nr3c1_ promoter, at the exon 17 locus, which interferes with transcription factor binding
of the nerve growth factor-induced protein A (NGFI-A) and leads to decreased expression of _Nr3c1_ [11]. A number of human studies have also found that _NR3C1_ hypermethylation at the
equivalent human locus (i.e., exon 1 F) is associated with exposure to early life stress, including childhood maltreatment, parental loss, or parental disease, and being the victim of
bullying [12,13,14,15,16,17,18,19]. Moreover, several human studies have linked _NR3C1_ methylation levels with changes in HPA functioning, e.g., as reflected by aberrant cortisol stress
responses [15, 17, 20,21,22]. There is also increasing evidence demonstrating that an altered HPA axis, e.g., due to early life adversity, can contribute to key aspects of substance use
disorders [23, 24]. Specifically, a dysregulated HPA axis can enhance a drug’s positive reinforcing effects, thus promoting continued use that is necessary for the development of dependence
[25]. A dysregulated HPA axis can also strengthen the negative effects associated with abstinence and withdrawal, thus contributing to relapse and the maintenance of substance use through
negative reinforcement [26, 27]. Glucocorticoids, in particular, interact with various neurotransmitter and neuropeptide systems implicated in substance use disorders and are thought to play
a critical role in addiction, partly by modulating the formation of drug-related memories [28, 29]. A specific and causal role of NR3C1 in addictive behaviors has been demonstrated in
preclinical studies, where selective inactivation of _Nr3c1_ in mouse dopaminoceptive neurons was found to reduce the motivation of mice to self-administer cocaine [30]. Similarly,
pharmacological compounds targeting NR3C1, including NR3C1 antagonists and modulators, have been found to possess therapeutic properties in intervention studies for alcohol dependence [31,
32]. However, no large-scale human studies to date have employed a longitudinal approach to examine the association between _NR3C1_ methylation at the exon 1 F locus and later substance use
risk among adolescent individuals. The presented data, which derive from over 1000 adolescents with no reported history of substance use at baseline (i.e., at 13–14 years of age), suggest
that saliva _NR3C1_ hypermethylation can predict future substance use in middle adolescence. METHODS STUDY PARTICIPANTS The study participants were part of the KUPOL study, a prospective
cohort study that included _N_ = 3959 adolescents attending the 7th grade (13–14 years of age) of compulsory school in both urban and rural areas of eight regions in southern and central
Sweden. Detailed information on the KUPOL study has been published elsewhere [33]. In brief, participant recruitment took place during school years 2013–2014 and 2014–2015. Data were
collected through self-administered questionnaires that were completed annually by adolescents and their parents during the following three years, i.e., until 16–17 years of age. National
registries were also used to retrieve medical and socio-economic information on students and their parents. A sample of KUPOL students also contributed saliva specimens (_N_ = 1315). In the
present study, we analyzed participants who were naïve to any substance use at baseline and had successful measurements of _NR3C1_ methylation (_N_ = 1041). The KUPOL study was approved by
the Stockholm Ethics Review Board (reference numbers: 2012/1904-31/1 and 2016/1280-32). All students agreed to participate in the study and all legal guardians gave written informed consent.
SUBSTANCE USE DATA Self-reported substance use data were available for cigarettes, snus (a smokeless tobacco product popular in Scandinavia), alcohol, and cannabis. In the analyses, we also
included the compound substance measures of “tobacco use” and “any substance use”. “Tobacco use” was defined as cigarette smoking and/or snus use, and “any substance use” was defined as any
report of using tobacco, alcohol, or cannabis. For each substance, we analyzed three main outcomes, i.e., (i) recent use, (ii) lifetime use, and (iii) use duration, according to the
following criteria: (i) Recent tobacco or cannabis use was defined as a report of first-time use in the past 30 days [yes/no]. Recent alcohol use was defined as a first-time report of having
consumed alcohol at least once a month during the past year. (ii) Lifetime use was defined as the first-time report of having used tobacco or cannabis at least once in a lifetime (i.e.,
also capturing use prior to the past 30 days) or having consumed alcohol at least once in the past year (i.e., also capturing the use of less than once a month in the past year) [yes/no].
Since none of the individuals had used any substances at baseline, lifetime use referred to the entire lifetime of the individuals up to the examined point. (iii) Use duration of each
substance was calculated as the number of years reporting recent use in the 3-year follow-up (i.e., range between 0 and 3). DNA METHYLATION ANALYSIS Detailed procedures of DNA sample
preparation and DNA methylation analyses have been described previously [34]. In brief, saliva samples were collected from participants using whole-saliva collection kits (Oragene•DNA; DNA
Genotek Inc., Ottawa, Canada) and, following DNA extraction, bisulfite conversion of the DNA was performed using the EZ-96 DNA Methylation-Gold™ MagPrep kit (Zymo Research Corporation;
Irvine; USA). DNA methylation levels at the _NR3C1_ exon 1-F locus were quantified using pyrosequencing reagents on a Pyromark Q96 device (Qiagen, Hilden, Germany). Specifically, a 162-base
pair fragment was amplified using the PyroMark PCR Kit (Qiagen, Hilden, Germany) and the following primers: forward 5′- AGTTTTAGAGTGGGTTTGGAG-3′, reverse biotin-5′-CCCCCAACTCCCCAAAAA-3′.
Polymerase chain reaction conditions were optimized to yield distinct single bands and were as follows: (i) 94 °C for 15 min, (ii) 45 cycles of 94 °C for 30 s, 60 °C for 60 s and 72 °C for
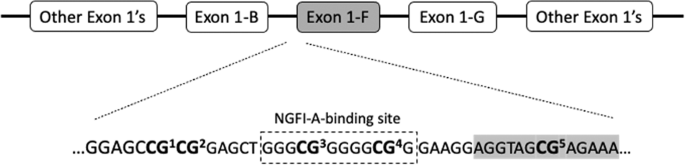
30 s, and (iii) final extension at 72 °C for 10 min. The sequencing primer, 5′-GAGTGGGTTTGGAGT-3′, was used for pyrosequencing, and data were collected on five CpG sites, denoted CpGs 1–5
(Fig. 1). As shown in Fig. 1, CpGs 3 and 4 belong to a binding site of NGFI-A, as reported by McGowan et al. [13]. Our CpG numbering follows the one reported by Oberlander et al. [17], where
CpG3 was also found to associate with infant stress cortisol reactivity. Of note, CpG3 constitutes a site that is not captured by the HumanMethylation450 and the EPIC BeadChips (Illumina;
Illumina Inc., San Diego, CA, USA). For reference, we also provide the 450 K/EPIC array probe IDs that correspond to our remaining CpGs of interest: CpG1 (cg15645634), CpG2 (not captured),
CpG3 (not captured), CpG4 (cg15910486), CpG5 (cg04111177). To validate the efficiency of our primers, amplification of unmethylated and methylated control DNA samples was performed using
human HCT116 DKO Non-Methylated and Methylated DNA (Zymo Research Corporation). The percentage methylation values obtained for the unmethylated control was in the 0–4% range, and for the
fully methylated control in the 86–95% range, for all five CpGs. Water samples gave no signal. The average between-plate coefficient of variation for CpGs 1–5 was 10.3% (range: 4.5–16.2%),
calculated from control samples containing 0, 6, 9, and 12% methylated DNA, analyzed in duplicate in each plate. Most of the analyzed samples (98%) yielded methylation values within a 0–12%
range at any given CpG site, which is within the commonly reported range (0–20%) described in previous pyrosequencing studies of the same NR3C1 region using blood or saliva samples from
infants, children, and adults [15, 17, 22, 35]. In our study, correlation analyses among the five CpG sites also showed that CpG3 was the site whose DNA methylation levels correlated the
least with the remaining four sites (Fig. S1). We also provide the overall distribution of methylation levels across the five CpG sites (Fig. S2), as well as the distributions of individual
CpG methylation levels in relation (i) to sex, internalizing symptoms, and parental history of psychiatric disorders (Fig. S3) and (ii) to substance use types (Fig. S4). All our analyses
were performed blinded to the phenotypes and, for analytical purposes, methylation levels for each CpG were categorized into three groups as previously described [34], i.e.,: (i)
unmethylated: participants with no detectable methylation levels (0%); (ii) low methylation: methylation below the median of detectable methylation levels; and (iii) high methylation:
methylation above the median of detectable methylation levels. SALIVARY CORTISOL ANALYSES Salivary cortisol data were available from 320 participants at baseline. The specimen collection and
analytic procedures for salivary cortisol measurements have been described previously [36]. In brief, specimens were collected in Salivette tubes (Sarstedt, Leicester, UK) twice during the
same day; in the morning (approximately 2 h after awakening) and in the afternoon (approximately 8 h after awakening). Samples were stored at −20 °C until subsequent cortisol measurements
using an enzyme-linked immune sorbent assay (Salivary Cortisol ELISA Kit; Salimetrics, UK). Each ELISA sample was run in duplicate, with morning and afternoon samples from the same
participant run on the same 96-well plate, and cortisol concentrations were expressed in micrograms per deciliter (μg/dL). Each plate also contained a cortisol concentration standard curve,
as well as interplate controls with known cortisol concentrations, run in duplicates. The correlation coefficients of the standard curves were >0.997 with a median of 0.999 for each
plate. The control samples revealed a between-plate coefficient of variation of 7.0% and the median within-plate coefficient of variation for all participant samples was 8.1%. COVARIATES We
used information on parents’ education, birthplace, and smoking habits to examine the social characteristics of the sample. Previous literature has found that methylation of _NR3C1_ at the
exon 1-F locus is associated with internalizing, but not externalizing, behavior problems [32, 34]. Moreover, parental depression has been associated with _NR3C1_ methylation in the
offspring [17]. Thus, adolescent internalizing symptoms and parental psychiatric disorders were considered potential confounders. However, it is also important to consider another possible
causal pathway. Specifically, since internalizing symptoms can stem from a dysregulated HPA axis, internalizing symptoms may be a mediator of the association between HPA-axis dysregulation
and substance use. Thus, considering internalizing symptoms as a covariate in the models may result in over adjustment. We included self-reported internalizing symptoms (as a continuous
variable) in the 7th grade, measured according to the Center for Epidemiologic Studies Depression Scale for Children (CES-DC) [37, 38]. The CES-DC is a 20-item, internationally validated
scale used in epidemiological studies of children and adolescents (6–17 years of age), with a total score ranging from zero to 60. This score was dichotomized using a cut-off score of ≥30,
which has been found to capture internalizing problems in Swedish adolescents [38, 39]. Parental history of mental, behavioral, and neurodevelopmental disorders was retrieved from the
National Patient Register according to the International Classification of Disease (ICD) 10 codes (F01-F99) [40] and categorized as a binary variable. STATISTICAL ANALYSES We used
descriptive statistics to summarize the main characteristics of the study sample. The associations of _NR3C1_ methylation with recent substance use, lifetime substance use, and substance use
duration were assessed on a univariate level for each CpG site separately and for all sites grouped together. Poisson regression models \(\left( {\log \left( {E\left( {Y{{{\mathrm{|}}}}X}
\right)} \right) = \alpha + \beta _i \ast X_i} \right)\), with or without sex stratification, were used to derive the rate ratios (RRs) and corresponding 95% confidence intervals (CIs) for
recent use and lifetime use, and the risk ratios (RRs) and 95% CIs for substance use duration and increasing methylation during the 3-year follow-up. In all analyses, the unmethylated group
constituted the reference. Models with and without adjusting for internalizing symptoms and parental history of mental, behavioral, and neurodevelopment disorders were compared. A
sensitivity analysis was performed to analyze the potential selection bias introduced by attrition in the third follow-up (non-compulsory education, 18.6%), limiting the analyses to the
first two years of follow-up (end of the Swedish compulsory education). In addition, multilevel models were used to take into account the clustering of students within schools. As multilevel
models did not reveal heterogeneity among schools, the results were reported according to the standard regression models. Bonferroni-corrected confidence intervals were included in
sensitivity analyses to adjust for multiple testing. Confidence intervals were set at 99.2% (1-alpha/_m_, where alpha = 0.05 [type I error] and _m_ = 6 [number of tests, 5 separate CpG sites
+ CpG sites grouped together]). The goodness of fit test and graphical checks of residuals based on residual deviance did not show a major departure from Poisson model assumptions. To
quantify the effect of unmeasured potential confounding factors, we also report the _E_-values for associations between CpG 3 site/CpG 1–5 sites and substance use as outcomes. The _E_-value
represents the minimal strength of association on the RR scale that an unmeasured confounder would need to have, with both the predictor and the outcome, to fully explain the association
between the two [41]. The association between the methylation levels across the five CpG sites and cortisol levels were examined using linear regression models. Due to the small sample size
(_n_ = 320), methylation levels were considered as a binary variable (methylated vs. unmethylated group). To account for potential selection because of the sampling approach, we performed
weighted linear regression models applying an inverse probability weight method. Sex, internalizing problems, parental history of mental, behavioral, and neurodevelopmental disorders, and
school was considered in weight calculation. As the results from weighted models were not different from those obtained with standard linear regression models, they are reported according to
this latter method. The code of the statistical analysis is available from the corresponding author on request. Considering a prevalence of methylation of 50% for CpG 3 site, a sample of
1040 students is sufficient to detect a 5% increase in smoking behaviors in those methylated compared to those non-methylated with a power of 80%. The Stata software package (StataCorp. LLC;
TX, USA) was used for all statistical analyses. RESULTS PARTICIPANT CHARACTERISTICS The analytical sample consisted of _N_ = 1041 students, 13–14 years of age, without a history of
substance use at baseline. Of these students, 73.5% had at least one parent with a university education, less than 20% had an immigration background with similar distribution between sexes
(Table 1). Also, 6.2% of students presented with internalizing symptoms according to CES-DC scoring, with a higher prevalence in females than in males (9.3% vs. 2.3%), and 11.5% of students
had parents with a history of psychiatric disorders. The prevalence of methylation varied across the five analyzed CpG sites of _NR3C1_ and CpG3, which belongs to the binding site of NGFI-A
in the promoter region of _NR3C1_’s exon 1-F (Fig. 1), was the site most prone to methylation (Table 1). The distribution of methylation levels was similar according to sex and internalizing
symptoms distribution, while the parental history of psychiatric disorders was associated with higher levels of methylation at CpG sites 1–3 (Fig. S3). During the 3-year follow-up, 166
students (15.9%) reported recent use of at least one substance. Specifically, 96 students (9.2%) reported recent smoking, 56 (5.4%) recent use of snus, 103 (9.8%) recent use of alcohol, and
14 (1.3%) recent use of cannabis. Totally, 76 (7.3%) students reported recent polysubstance use (Table S1). We observed slightly higher recent use rates among females than males (18.0%
versus 13.5% for at least one substance) particularly for cigarette smoking (11.3% versus 6.7%) and alcohol consumption (12.2% versus 7.1%). With regard to lifetime use, 193 students (18.5%)
reported ever having smoked, 118 (11.3%) reported lifetime use of snus, 486 (46.7%) reported lifetime use of alcohol, and 35 (3.4%) reported lifetime use of cannabis (Table S1). With regard
to substance use duration, about 10% of students used at least one substance for 1 year, 5% for 2 years, and 1% for 3 years (Table S1). Table S2 reports descriptive statistics on the
association between DNA methylation levels and current substance use. The groups with high methylation levels for CpGs 2 and 3, and CpGs1–5, show higher current substance use. The graphical
visualization of the distribution of methylation levels confirmed this pattern, mainly for CpG site 3 (Fig. S4). NR3C1 METHYLATION PREDICTS SUBSTANCE USE IN ADOLESCENCE The results from the
Poisson regression models that compared rates of recent substance use, in terms of baseline _NR3C1_ methylation levels, are presented in Fig. 2 and Table S3. Students with high baseline DNA
methylation levels at CpG site 3 had 1.7–2.3-fold higher rates of reporting recent use of cigarettes, snus, and alcohol. The estimates across the other CpG sites (2, 4, and grouped) were
lower, although the same patterns emerged, especially for the recent use of cigarettes. Thus, high baseline methylation in the combined CpG1–5 group is associated with an increased risk for
recent cigarette use. The sensitivity analysis showed that the corresponding _E_-value for methylation at CpG site 3 (RR = 2.30) and any CpG site (RR = 1.62) as exposure, and recent
cigarette use as an outcome, were 4.03 (lower confidence limit 2.26) and 2.62 (1.21), respectively (Table S4). The multivariable-adjusted analysis for internalizing symptoms and parental
history of psychiatric disorders, along with the Bonferroni-corrected estimates, yielded similar results but with less precise estimates (Tables S5 and S6). The only exception was for
cannabis use where the confidence intervals were too wide to draw any conclusion on the direction of the association. Site- and substance-specific associations were slightly different
between sexes (Tables S7 and S8). Risk estimates were stronger for CpG site 3 in males, and CpG site 2 in females. Moreover, the associations with cigarette and alcohol use were more
pronounced in males. The overall estimates were attenuated when considering lifetime substance use as the outcome (Fig. 3 and Table S9) also considering models adjusted for parental history
of psychiatric disorders and with Bonferroni corrected estimates (Tables S10 and S11). Nonetheless, the associations remained significant between CpG site 3 and cigarette smoking or cannabis
use. Moreover, sex-stratified analyses revealed associations for lifetime cigarette use in males (CpG site 3) and lifetime cannabis use in females (CpG sites 1 and 5) (Tables S12 and S13).
NR3C1 METHYLATION PREDICTS SUBSTANCE USE DURATION IN ADOLESCENCE Baseline _NR3C1_ methylation levels were also associated with higher risk for prolonged substance use duration (i.e., number
of years reporting recent use), with the highest estimates in those with high methylation levels at CpG site 3 in both unadjusted and adjusted models (Fig. 4, Tables S14–S16). Site- and
substance-specific associations were also confirmed for the duration outcome when stratifying by sex (Tables S17 and S18). Restricting analyses to the second follow-up resulted in estimates
that were in the same direction but with wider confidence intervals (data not shown). ASSOCIATION BETWEEN NR3C1 METHYLATION AND SALIVARY CORTISOL LEVELS Finally, we examined the association
of _NR3C1_ methylation at the five CpG sites under investigation with morning or afternoon salivary cortisol levels, which were available from a subset of participants (_N_ = 320). We found
that DNA methylation levels at CpG site 3 were associated with higher morning cortisol levels. The average increase of morning cortisol level was 0.027 μg/dL [95% CI: 0.003–0.050] in the
methylation group compared to the unmethylated group. No significant associations were found for the remaining CpG sites or with afternoon cortisol levels. DISCUSSION In this prospective
cohort study of over 1000 adolescent individuals, we found that the presence of hypermethylation at the exon 1-F promoter of _NR3C1_ in early adolescence, i.e., 13–14 years of age, was
associated with an increased risk of future self-reports of recent substance use and substance use duration during the 3-year follow-up. When considering lifetime substance use as an
outcome, the overall estimates were attenuated although some significances remained. Specifically, our findings were particularly consistent for hypermethylation of CpG site 3, which belongs
to a binding site of NGFI-A and has been associated with decreased _NR3C1_ expression as part of the exon 1-F promoter region [13]. In our study, hypermethylation of CpG site 3 predicted
future self-reports of (i) recent use of any substance (i.e., tobacco products, alcohol, or cannabis), (ii) lifetime use of tobacco products (cigarettes or snus), as well as (iii) substance
use duration of any substance. Sex-stratified analyses revealed a pronounced association in males between CpG3 hypermethylation and recent use of cigarettes (RR = 5.3). The equivalent
analyses in females revealed an association between hypermethylation of CpG2 and recent tobacco use (RR = 2.31). However, hypermethylation of CpG3 was associated again with a longer duration
of tobacco use in both males and females. Adjusting for potential confounders, such as internalizing problems and parental history of mental, behavioral, and neurodevelopmental disorders,
yielded results in the same direction. Lifetime substance use can be viewed as a proxy for risk-taking behavior often observed in adolescence, and with predisposing factors that include
temperament, neurobehavioral disinhibition, and peer behaviors [42]. As such, “lifetime use” can be considered a more benign outcome since it also captures individuals who are occasional
users or have experimented with a substance on single occasions. By contrast, the transition from occasional use to a substance use disorder constitutes a maladaptive process that requires
both heavy/repeated use and genetic predispositions [43]. Thus, the frequency of recent substance use reports over the years (reflected in our study using the outcome of substance use
duration), may be a better indicator of chronic problematic substance use, which increases the risk of substance use disorders [42, 44]. Given (i) the overall attenuated estimates for
lifetime use found in our study, and given (ii) that methylation quantitative trait loci (mQTLs) may underlie our observed changes in _NR3C1_ methylation (as discussed further below), this
may indicate that _NR3C1_ is involved more in those maladaptive processes that are related to substance abuse, and less in those processes that underlie experimental or occasional use.
However, further longitudinal studies, with more detailed information on substance use behaviors, are warranted to support this hypothesis. The first evidence that _NR3C1_ may be able to
modulate the initiation of substance use, was provided by genetic studies showing a significant association between polymorphisms in the _NR3C1_ gene and alcohol use initiation in
14‐year‐old adolescents [45]. However, our study is the first to our knowledge to employ a large-scale (_N_ > 1000) longitudinal approach to examine whether _NR3C1_ methylation at the
exon 1-F locus can predict three different substance use outcomes in middle adolescence. Importantly, the use of DNA samples from individuals with no lifetime history of substance use, makes
our findings unlikely to be explained by reverse causality [46, 47]. Moreover, since previous studies had found associations between _NR3C1_ methylation and cortisol stress responses [15,
17, 20,21,22], we also examined the correlations between the five CpGs under investigation and salivary cortisol levels available from a subset of individuals. We found that DNA methylation
at CpG 3 was associated with higher morning cortisol levels. Interestingly, a previous study using cord blood samples from infants found that increased methylation at CpG3 was the only CpG
site under investigation that was associated with infant stress cortisol reactivity [17]. The same study found that exposure to increased third-trimester depressed maternal mood was also
associated with increased neonatal methylation of CpG3 [17]. Similarly, an independent study using preschoolers found that hypermethylation at the same CpG site is associated with
internalizing behavior problems [35], providing further support for the biological relevance of this CpG site and its association to stress sensitivity, HPA functioning, and risk for
psychopathology. Overall, our data suggest that saliva _NR3C1_ hypermethylation can predict substance use outcomes in middle adolescence, and are consistent with our previous findings from
the KUPOL study, which demonstrated an association between morning cortisol levels and tobacco use initiation [36]. Moreover, our findings provide support for an epigenetic predisposition
that could drive vulnerability to addiction [48]. Although no prior substance-use studies employed a longitudinal approach to investigate _NR3C1_ methylation levels in adolescents, a number
of studies used cross-sectional approaches in adult cohorts, which provide support for our findings. Specifically, two studies investigating alcohol use found hypermethylation and lower
expression of _NR3C1_ in post-mortem brain tissue of adult individuals with a history of alcohol use disorder [49, 50]. Another study investigating cocaine use found lower peripheral levels
of _NR3C1_ gene expression in adult chronic cocaine users [51]. A study using healthy adults found that a history of a _past_ substance use disorder was associated with lower peripheral
levels of _NR3C1_ methylation [52]. However, a recent study that examined adults with cannabinoid use disorders found no associations with _NR3C1_ methylation levels [53]. The specific
contributors to _NR3C1_ hypermethylation in our study participants remain unknown. Although previous studies have provided support for the hypothesis that _NR3C1_ hypermethylation in
children can result from environmental adversities, such as early life stress [54], we were not able to address this possibility since our utilized cohort did not include information on
early life adversities. Moreover, we cannot exclude the possibility that genetic variations can also influence _NR3C1_ methylation levels, similar to findings from other stress-responsive
genes such as the FK506 binding protein 5 (_FKBP5_) gene [55, 56]. Moreover, it is unlikely that methylation of _NR3C1_ is the only epigenetic predisposing factor influencing substance use
risk in adolescence. This assumption is supported by the only additional prospective study of DNA methylation and substance use risk in adolescence, to our knowledge, which included 244
individuals with methylation data from cord blood at birth and whole blood at age 7 [57]. This study by Cecil and colleagues found no link with _NR3C1_ but reported instead of an association
between substance use during adolescence and cord blood methylation of 65 loci involved in neurodevelopmental processes, including _PACSIN1_, _NEUROD4_, and _NTRK_ [57]. Nonetheless, it
should be noted that the latter study was conducted using the Illumina HumanMethylation450 BeadChip, which does not capture our main CpG site of interest, i.e., CpG3 (see also Methods).
Moreover, although Illumina and pyrosequencing data have been found to be congruent to a large extent, deviations from this congruency may still occur and caution needs to be taken for
individual loci when translating beta-values directly into percent methylation levels [58]. A number of additional limitations should be kept in mind when interpreting the results from our
study. First, the level of DNA methylation was evaluated at a single time point, making the timing of the epigenetic process unclear. Second, DNA methylation levels detected in saliva do not
necessarily correspond to those in the brain since DNA methylation signatures are often tissue-specific. Nonetheless, previous studies examining the concordance of DNA methylation across
commonly used peripheral tissues (i.e., blood, saliva, and buccal tissue) with DNA methylation in the brain, found the highest correlations to the brain with saliva, at least when the
average global correlations were used [59]. Thus, it is also important to consider the nature of the cell types present in saliva samples, which may vary based on each study’s saliva
collection kit. For instance, the epithelial cell count in saliva samples collected with the Oragene·DNA kit, used in our study, can range from 20 to 70%, with the remaining cells being
mostly leukocytes and with the age of subject being one potential determining factor (personal correspondence with DNA Genotek). Furthermore, although it is customary to assess leukocyte
cell-type distributions in blood-based DNA methylation studies, such cell-type assessments are not made possible in saliva samples already collected with the Oragene·DNA kit that utilizes
lytic chemistry, meaning it will lyse most cells in the sample and degrade/denature proteins. To address this limitation, future studies can consider collecting a small amount of saliva into
a separate tube for cell sorting, since raw saliva samples can be used to perform cell-sorting assays and can be smeared on a slide to enumerate cells by microscopy. Nonetheless, it is
reassuring that the majority of our analyzed samples yielded methylation values within the commonly reported range described in previous pyrosequencing studies of _NR3C1_ using blood or
saliva samples [15, 17, 22, 35]. Third, the number of participants with drug use was relatively small, which could affect the precision of the results. In addition, self-reported substance
use tends to underestimate actual use [60, 61]. Fourth, randomness in methylation can affect measurement precision, while systematic measurement errors may bias estimates towards or away
from the null. Nonetheless, the large sample size contributed to reducing the role of random errors on final estimates. However, the small number of cases for cannabis use did not always
allow reaching conclusions on estimated directions. Moreover, possible measurement errors of methylation are unlikely to have occurred differentially according to substance use. Hence,
nondifferential misclassification would likely result in an underestimation of the associations under examination. Along the same lines, misclassification of substance use is unlikely to
have differed according to methylation levels. Nonetheless, caution needs to be taken when interpreting results from substance use duration, since a value of 1 at the first follow-up may
differ in meaning from a value of 1 at the third follow-up. Specifically, the former may indicate experimental use if it is not followed by substance use in the second follow-up, while the
latter may indicate either experimental use or the beginning of chronic use depending on substance intake during the subsequent (unstudied) years. Finally, unmeasured, unknown, or residual
confounding could not be excluded, for instance, due to parenting style and genetic liability to substance dependence. Consequently, we performed a sensitivity analysis to estimate the
magnitude of confounding (Table S4). For example, the observed RR of 1.62 (between grouped CpG sites and recent cigarette use) could be explained by an unmeasured confounder that was
associated both with the exposure and the outcome by a RR of 2.62 each (_E_-value), above and beyond the measured confounders. The confidence interval could be moved to include the null by
an unmeasured confounder that was associated both with the exposure and with the outcome by a RR of 1.21 each, above and beyond the measured confounders. Overall, _E_-values support the
robustness of the present findings. Indeed, genetic factors in the form of single nucleotide polymorphisms (SNPs) represent the main possible confounders in the pathway between HPA axis
dysregulation and substance use. However, it is unlikely that the magnitude of the association between a single SNP and both exposure/outcome will be higher than 2.62, given that psychiatric
traits are most polygenic, involving a continuum of small effects, and SNP heritability accounts for modest percentages in substance use behaviors [43, 62, 63]. Despite these limitations,
our study also had notable strengths. It was based on a large longitudinal cohort with low attrition at first and second follow-ups (8.9% and 12.7%, respectively), thus minimizing the risk
of reverse causality due to DNA methylation changes caused by substance use [64]. DNA methylation was assessed in early adolescence, a period when the use of substances is usually not yet
established, and the few subjects that reported substance use at baseline were excluded. Moreover, outcome information was assessed during three consecutive years. The adjustment was also
made for internalizing symptoms and parental history of mental, behavioral, and neurodevelopmental disorders at baseline, which were treated as potential confounders. However, it should also
be noted that controlling for internalizing symptoms may result in over-adjustment due to a possible causal pathway from _NR3C1_ methylation to internalizing symptoms to substance use.
Conclusively, our findings indicate that _NR3C1_ methylation levels can predict substance use outcomes in adolescence. However, additional longitudinal studies are warranted to confirm these
findings and to investigate the role of genes versus environment in affecting _NR3C1_ methylation. CODE AVAILABILITY The code of the statistical analysis is available under request to the
corresponding author (http://[email protected]). REFERENCES * Crews F, He J, Hodge C. Adolescent cortical development: a critical period of vulnerability for addiction. Pharmacol Biochem
Behav. 2007;86:189–99. Article CAS PubMed Google Scholar * Jordan CJ, Andersen SL. Sensitive periods of substance abuse: early risk for the transition to dependence. Dev Cognit Neurosci.
2017;25:29–44. Article Google Scholar * Grant BF. Age at smoking onset and its association with alcohol consumption and DSM-IV alcohol abuse and dependence: results from the National
Longitudinal Alcohol Epidemiologic Survey. J Subst Abus. 1998;10:59–73. Article CAS Google Scholar * Clark DB, Lesnick L, Hegedus AM. Traumas and other adverse life events in adolescents
with alcohol abuse and dependence. J Am Acad Child Adolesc Psychiatry. 1997;36:1744–51. Article CAS PubMed Google Scholar * Dube SR, Felitti VJ, Dong M, Chapman DP, Giles WH, Anda RF.
Childhood abuse, neglect, and household dysfunction and the risk of illicit drug use: the adverse childhood experiences study. Pediatrics 2003;111:564–72. Article PubMed Google Scholar *
Bensley LS, Spieker SJ, Van Eenwyk J, Schoder J. Self-reported abuse history and adolescent problem behaviors. II. Alcohol and drug use. J Adolesc Health. 1999;24:173–80. Article CAS
PubMed Google Scholar * Alves RL, Oliveira P, Lopes IM, Portugal CC, Alves CJ, Barbosa F, et al. Early-life stress affects drug abuse susceptibility in adolescent rat model independently
of depression vulnerability. Sci Rep. 2020;10:13326. Article CAS PubMed PubMed Central Google Scholar * Andersen SL. Stress, sensitive periods, and substance abuse. Neurobiol Stress.
2019;10:100140. Article PubMed Google Scholar * Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and
preparative actions. Endocr Rev. 2000;21:55–89. CAS PubMed Google Scholar * Wang D, Levine JLS, Avila-Quintero V, Bloch M, Kaffman A. Systematic review and meta-analysis: effects of
maternal separation on anxiety-like behavior in rodents. Transl Psychiatry. 2020;10:174. Article PubMed PubMed Central Google Scholar * Weaver IC, Cervoni N, Champagne FA, D’Alessio AC,
Sharma S, Seckl JR, et al. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–54. Article CAS PubMed Google Scholar * Cicchetti D, Handley ED. Methylation of the
glucocorticoid receptor gene, nuclear receptor subfamily 3, group C, member 1 (NR3C1), in maltreated and nonmaltreated children: associations with behavioral undercontrol, emotional
lability/negativity, and externalizing and internalizing symptoms. Dev Psychopathol. 2017;29:1795–806. Article PubMed PubMed Central Google Scholar * McGowan PO, Sasaki A, D’Alessio AC,
Dymov S, Labonté B, Szyf M, et al. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat Neurosci. 2009;12:342–48. Article CAS PubMed
PubMed Central Google Scholar * Perroud N, Paoloni-Giacobino A, Prada P, Olié E, Salzmann A, Nicastro R, et al. Increased methylation of glucocorticoid receptor gene (NR3C1) in adults with
a history of childhood maltreatment: a link with the severity and type of trauma. Transl Psychiatry. 2011;1:e59. Article CAS PubMed PubMed Central Google Scholar * Tyrka AR, Price LH,
Marsit C, Walters OC, Carpenter LL. Childhood adversity and epigenetic modulation of the leukocyte glucocorticoid receptor: preliminary findings in healthy adults. PLoS ONE.
2012;7:e30148–e48. Article CAS PubMed PubMed Central Google Scholar * Melas PA, Wei Y, Wong CC, Sjöholm LK, Åberg E, Mill J, et al. Genetic and epigenetic associations of MAOA and NR3C1
with depression and childhood adversities. Int J Neuropsychopharmacol. 2013;16:1513–28. Article CAS PubMed Google Scholar * Oberlander TF, Weinberg J, Papsdorf M, Grunau R, Misri S,
Devlin AM. Prenatal exposure to maternal depression, neonatal methylation of human glucocorticoid receptor gene (NR3C1) and infant cortisol stress responses. Epigenetics 2008;3:97–106.
Article PubMed Google Scholar * van der Knaap LJ, Riese H, Hudziak JJ, Verbiest MM, Verhulst FC, Oldehinkel AJ, et al. Glucocorticoid receptor gene (NR3C1) methylation following stressful
events between birth and adolescence. The TRAILS study. Transl Psychiatry. 2014;4:e381. Article PubMed PubMed Central Google Scholar * Farrell C, Doolin K, O’ Leary N, Jairaj C, Roddy
D, Tozzi L, et al. DNA methylation differences at the glucocorticoid receptor gene in depression are related to functional alterations in hypothalamic-pituitary-adrenal axis activity and to
early life emotional abuse. Psychiatry Res. 2018;265:341–48. Article CAS PubMed Google Scholar * Lewis CR, Breitenstein RS, Henderson A, Sowards HA, Piras IS, Huentelman MJ, et al. Harsh
parenting predicts novel HPA receptor gene methylation and NR3C1 methylation predicts cortisol daily slope in middle childhood. Cell Mol Neurobiol. 2021;41:783–93. Article CAS PubMed
Google Scholar * Conradt E, Fei M, LaGasse L, Tronick E, Guerin D, Gorman D, et al. Prenatal predictors of infant self-regulation: the contributions of placental DNA methylation of NR3C1
and neuroendocrine activity. Front Behav Neurosci. 2015;9:130. Article PubMed PubMed Central Google Scholar * Alexander N, Kirschbaum C, Wankerl M, Stauch BJ, Stalder T,
Steudte-Schmiedgen S, et al. Glucocorticoid receptor gene methylation moderates the association of childhood trauma and cortisol stress reactivity. Psychoneuroendocrinology 2018;90:68–75.
Article CAS PubMed Google Scholar * al’Absi M, Lemieux A, Westra R, Allen S. Early life adversity influences stress response association with smoking relapse. Psychopharmacology
2017;234:3375–84. Article PubMed PubMed Central Google Scholar * Lovallo WR. The hypothalamic-pituitary-adrenocortical axis in addiction. Int J Psychophysiol. 2006;59:193–4. Article
PubMed PubMed Central Google Scholar * Sinha R. Chronic stress, drug use, and vulnerability to addiction. Ann N Y Acad Sci. 2008;1141:105–30. Article CAS PubMed PubMed Central Google
Scholar * Buchmann AF, Laucht M, Schmid B, Wiedemann K, Mann K, Zimmermann US. Cigarette craving increases after a psychosocial stress test and is related to cortisol stress response but
not to dependence scores in daily smokers. J Psychopharmacol (Oxf, Engl). 2010;24:247–55. Article CAS Google Scholar * Sinha R. The role of stress in addiction relapse. Curr Psychiatry
Rep. 2007;9:388–95. Article PubMed Google Scholar * Srinivasan S, Shariff M, Bartlett SE. The role of the glucocorticoids in developing resilience to stress and addiction. Front
Psychiatry. 2013;4:68. Article PubMed PubMed Central Google Scholar * Goldfarb EV, Sinha R. Drug-induced glucocorticoids and memory for substance use. Trends Neurosci. 2018;41:853–68.
Article CAS PubMed PubMed Central Google Scholar * Ambroggi F, Turiault M, Milet A, Deroche-Gamonet V, Parnaudeau S, Balado E, et al. Stress and addiction: glucocorticoid receptor in
dopaminoceptive neurons facilitates cocaine seeking. Nat Neurosci. 2009;12:247–9. Article CAS PubMed Google Scholar * McGinn MA, Tunstall BJ, Schlosburg JE, Gregory-Flores A, George O,
de Guglielmo G, et al. Glucocorticoid receptor modulators decrease alcohol self-administration in male rats. Neuropharmacology 2021;188:108510. Article CAS PubMed Google Scholar *
Vendruscolo LF, Estey D, Goodell V, Macshane LG, Logrip ML, Schlosburg JE, et al. Glucocorticoid receptor antagonism decreases alcohol seeking in alcohol-dependent individuals. J Clin
Investig. 2015;125:3193–7. Article PubMed PubMed Central Google Scholar * Galanti MR, Hultin H, Dalman C, Engström K, Ferrer-Wreder L, Forsell Y, et al. School environment and mental
health in early adolescence—a longitudinal study in Sweden (KUPOL). BMC Psychiatry. 2016;16:243. Article PubMed PubMed Central Google Scholar * Efstathopoulos P, Andersson F, Melas PA,
Yang LL, Villaescusa JC, Rȕegg J, et al. NR3C1 hypermethylation in depressed and bullied adolescents. Transl Psychiatry. 2018;8:121. Article PubMed PubMed Central Google Scholar * Parade
SH, Ridout KK, Seifer R, Armstrong DA, Marsit CJ, McWilliams MA, et al. Methylation of the glucocorticoid receptor gene promoter in preschoolers: links with internalizing behavior problems.
Child Dev. 2016;87:86–97. Article PubMed PubMed Central Google Scholar * Raffetti E, Landgren AJ, Andersson F, Donato F, Lavebratt C, Forsell Y, et al. Cortisol concentration as
predictor of tobacco initiation in adolescents: results from a population-based Swedish cohort. J Adolesc Health. 2021;68:758–64. Article PubMed Google Scholar * Olsson G, von Knorring
AL. Depression among Swedish adolescents measured by the self-rating scale Center for Epidemiology Studies-Depression Child (CES-DC). Eur Child Adolesc Psychiatry. 1997;6:81–7. CAS PubMed
Google Scholar * Fendrich M, Weissman MM, Warner V. Screening for depressive disorder in children and adolescents: validating the Center for Epidemiologic Studies Depression Scale for
Children. Am J Epidemiol. 1990;131:538–51. Article CAS PubMed Google Scholar * Olsson G, von Knotting AL. Depression among Swedish adolescents measured by the self rating scale Center
for Epidemiology Studies - Depression Child (CES-DC). Eur Child Adolesc Psychiatry. 1997;6:81–7. CAS PubMed Google Scholar * Giordano G, Colaneri M, Di Filippo A, Blanchini F, Bolzern P,
De Nicolao G, et al. Modeling vaccination rollouts, SARS-CoV-2 variants and the requirement for non-pharmaceutical interventions in Italy. Nat Med. 2021;27:993–98. Article CAS PubMed
PubMed Central Google Scholar * Vanderweele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med. 2017;167:268–74. Article PubMed Google
Scholar * Hanson KL, Medina KL, Padula CB, Tapert SF, Brown SA. Impact of adolescent alcohol and drug use on neuropsychological functioning in young adulthood: 10-year outcomes. J Child
Adolesc Subst Abus. 2011;20:135–54. Article Google Scholar * Kranzler HR, Zhou H, Kember RL, Smith RV, Justice AC, Damrauer S, et al. Author correction: genome-wide association study of
alcohol consumption and use disorder in 274,424 individuals from multiple populations. Nat Commun. 2019;10:4050. Article PubMed PubMed Central Google Scholar * Lewinsohn PM, Rohde P,
Brown RA. Level of current and past adolescent cigarette smoking as predictors of future substance use disorders in young adulthood. Addiction. 1999;94:913–21. Article CAS PubMed Google
Scholar * Desrivières S, Lourdusamy A, Müller C, Ducci F, Wong CP, Kaakinen M, et al. Glucocorticoid receptor (NR3C1) gene polymorphisms and onset of alcohol abuse in adolescents. Addiction
Biol. 2011;16:510–3. Article Google Scholar * Philibert RA, Beach SR, Brody GH. The DNA methylation signature of smoking: an archetype for the identification of biomarkers for behavioral
illness. Neb Symp Motiv Neb Symp Motiv. 2014;61:109–27. Google Scholar * Zakhari S. Alcohol metabolism and epigenetics changes. Alcohol Res. 2013;35:6–16. PubMed PubMed Central Google
Scholar * Nielsen DA, Utrankar A, Reyes JA, Simons DD, Kosten TR. Epigenetics of drug abuse: predisposition or response. Pharmacogenomics. 2012;13:1149–60. Article CAS PubMed Google
Scholar * McClintick JN, Xuei X, Tischfield JA, Goate A, Foroud T, Wetherill L, et al. Stress-response pathways are altered in the hippocampus of chronic alcoholics. Alcohol.
2013;47:505–15. Article CAS PubMed Google Scholar * Gatta E, Grayson DR, Auta J, Saudagar V, Dong E, Chen Y, et al. Genome-wide methylation in alcohol use disorder subjects: implications
for an epigenetic regulation of the cortico-limbic glucocorticoid receptors (NR3C1). Mol Psychiatry. 2021;26:1029–41. Article CAS PubMed Google Scholar * Schote AB, Jäger K, Kroll SL,
Vonmoos M, Hulka LM, Preller KH, et al. Glucocorticoid receptor gene variants and lower expression of NR3C1 are associated with cocaine use. Addiction Biol. 2019;24:730–42. Article CAS
Google Scholar * Tyrka AR, Parade SH, Welch ES, Ridout KK, Price LH, Marsit C, et al. Methylation of the leukocyte glucocorticoid receptor gene promoter in adults: associations with early
adversity and depressive, anxiety and substance-use disorders. Transl Psychiatry. 2016;6:e848. Article CAS PubMed PubMed Central Google Scholar * Pehlivan S, Aytac HM, Cetinay Aydin P,
Nursal AF, Pehlivan M. Global and glucocorticoid receptor gene-specific (NR3C1) DNA methylation analysis in patients with cannabinoid or synthetic cannabinoid use disorder. Psychiatry Res.
2021;298:113774. Article CAS PubMed Google Scholar * Parade SH, Huffhines L, Daniels TE, Stroud LR, Nugent NR, Tyrka AR. A systematic review of childhood maltreatment and DNA
methylation: candidate gene and epigenome-wide approaches. Transl Psychiatry. 2021;11:134. Article PubMed PubMed Central Google Scholar * Klengel T, Mehta D, Anacker C, Rex-Haffner M,
Pruessner JC, Pariante CM, et al. Allele-specific FKBP5 DNA demethylation mediates gene-childhood trauma interactions. Nat Neurosci. 2013;16:33–41. Article CAS PubMed Google Scholar *
Klinger-König J, Hertel J, Van der Auwera S, Frenzel S, Pfeiffer L, Waldenberger M, et al. Methylation of the FKBP5 gene in association with FKBP5 genotypes, childhood maltreatment and
depression. Neuropsychopharmacology. 2019;44:930–8. Article PubMed PubMed Central Google Scholar * Cecil CA, Walton E, Smith RG, Viding E, McCrory EJ, Relton CL, et al. DNA methylation
and substance-use risk: a prospective, genome-wide study spanning gestation to adolescence. Transl Psychiatry. 2016;6:e976. Article CAS PubMed PubMed Central Google Scholar * Roessler
J, Ammerpohl O, Gutwein J, Hasemeier B, Anwar SL, Kreipe H, et al. Quantitative cross-validation and content analysis of the 450k DNA methylation array from Illumina, Inc. BMC Res Notes.
2012;5:210. Article CAS PubMed PubMed Central Google Scholar * Braun PR, Han S, Hing B, Nagahama Y, Gaul LN, Heinzman JT, et al. Genome-wide DNA methylation comparison between live
human brain and peripheral tissues within individuals. Transl Psychiatry. 2019;9:47. Article PubMed PubMed Central Google Scholar * Connor Gorber S, Schofield-Hurwitz S, Hardt J,
Levasseur G, Tremblay M. The accuracy of self-reported smoking: a systematic review of the relationship between self-reported and cotinine-assessed smoking status. Nicotine Tob Res.
2009;11:12–24. Article PubMed Google Scholar * Jain R, Jhanjee S, Jain V, Gupta T, Mittal S, Chauhan P, et al. Biochemical validation of self-reported smokeless tobacco abstinence among
smokeless tobacco users: results from a clinical trial of varenicline in India. J Psychoact Drugs. 2015;47:331–5. Article Google Scholar * Zhang Y, Qi G, Park JH, Chatterjee N. Estimation
of complex effect-size distributions using summary-level statistics from genome-wide association studies across 32 complex traits. Nat Genet. 2018;50:1318–26. Article CAS PubMed Google
Scholar * Liu M, Jiang Y, Wedow R, Li Y, Brazel DM, Chen F, et al. Association studies of up to 1.2 million individuals yield new insights into the genetic etiology of tobacco and alcohol
use. Nat Genet. 2019;51:237–44. Article CAS PubMed PubMed Central Google Scholar * Sugden K, Hannon EJ, Arseneault L, Belsky DW, Broadbent JM, Corcoran DL, et al. Establishing a
generalized polyepigenetic biomarker for tobacco smoking. Transl Psychiatry. 2019;9:92. Article PubMed PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS The authors
would like to thank the participating adolescents, families, and schools, and the executive team of the KUPOL study who make our research possible. The KUPOL study is funded by a grant (No.
259-2012-48) containing funds from the Swedish Research Council Formas, the Swedish Research Council for Health, Working Life and Welfare, and the Swedish Research Council-Vetenskapsrådet
awarded to M.R.G. The funders had no role in study design, data collection, and analysis, decision to publish, or preparation of the article. FUNDING Open access funding provided by
Karolinska Institute. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Global Public Health, Karolinska Institutet, Stockholm, Sweden Elena Raffetti, Filip Andersson, Yvonne
Forsell & Maria Rosaria Galanti * Center for Psychiatry Research, Department of Clinical Neuroscience, Karolinska Institutet & Stockholm Health Care Services, Stockholm, Sweden
Philippe Anastasios Melas * Center for Molecular Medicine, Karolinska University Hospital, Stockholm, Sweden Philippe Anastasios Melas & Catharina Lavebratt * Region Västra Götaland,
Research and Development Primary Health Care, Gothenburg, Sweden Anton Jonatan Landgren * Department of Rheumatology and Inflammation Research, Institute of Medicine, Sahlgrenska Academy,
University of Gothenburg, Gothenburg, Sweden Anton Jonatan Landgren * Centre for Epidemiology and Community Medicine, Stockholm Health Care District, Stockholm Region, Stockholm, Sweden
Filip Andersson, Yvonne Forsell & Maria Rosaria Galanti * Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden Catharina Lavebratt Authors * Elena
Raffetti View author publications You can also search for this author inPubMed Google Scholar * Philippe Anastasios Melas View author publications You can also search for this author
inPubMed Google Scholar * Anton Jonatan Landgren View author publications You can also search for this author inPubMed Google Scholar * Filip Andersson View author publications You can also
search for this author inPubMed Google Scholar * Yvonne Forsell View author publications You can also search for this author inPubMed Google Scholar * Catharina Lavebratt View author
publications You can also search for this author inPubMed Google Scholar * Maria Rosaria Galanti View author publications You can also search for this author inPubMed Google Scholar
CONTRIBUTIONS E.R., C.L., Y.F., and M.R.G. conceived the study, E.R., P.A.M., A.J.L., and M.R.G. designed the analysis. C.L. analyzed the methylation data, E.R. and F.A. performed the
statistical analysis. P.A.M. and E.R. prepared the figure. E.R. wrote the first draft of the paper, to which all authors contributed. All authors revised the final paper. CORRESPONDING
AUTHOR Correspondence to Elena Raffetti. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains
neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTAL TABLES AND FIGURES RIGHTS AND PERMISSIONS OPEN ACCESS
This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as
long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third
party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the
article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright
holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Raffetti, E., Melas, P.A., Landgren,
A.J. _et al._ DNA methylation of the glucocorticoid receptor gene predicts substance use in adolescence: longitudinal data from over 1000 young individuals. _Transl Psychiatry_ 11, 477
(2021). https://doi.org/10.1038/s41398-021-01601-6 Download citation * Received: 14 August 2021 * Revised: 19 August 2021 * Accepted: 01 September 2021 * Published: 15 September 2021 * DOI:
https://doi.org/10.1038/s41398-021-01601-6 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative