Short day length-induced decrease of cesium uptake without altering potassium uptake manner in poplar
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Short day length-induced alteration of potassium (K) localization in perennial trees is believed to be a mechanism for surviving and adapting to severe winters. To investigate the
relationship between cesium (Cs) and K localizations, a model tree poplar, hybrid aspen T89, was employed. Under short day length conditions, the amount of 137Cs absorbed through the root
and translocated to the root was drastically reduced, but 42K was not. Potassium uptake from the rhizosphere is mediated mainly by KUP/HAK/KT and CNGC transporters. In poplar, however, these
genes were constantly expressed under short-day conditions except for a slight increase in the expression a _KUP/HAK/KT_ gene six weeks after the onset of the short-day treatment. These
results indicated that the suppression of 137Cs uptake was triggered by short day length but not regulated by competitive Cs+ and K+ transport. We hypothesize that there are separately
regulated Cs+ and K+ transport systems in poplar. SIMILAR CONTENT BEING VIEWED BY OTHERS INTERACTIVE EFFECT OF POTASSIUM AND CADMIUM ON GROWTH, ROOT MORPHOLOGY AND CHLOROPHYLL _A_
FLUORESCENCE IN TOMATO PLANT Article Open access 08 March 2021 REGULATING ROLE OF ABSCISIC ACID ON CADMIUM ENRICHMENT IN RAMIE (_BOEHMERIA NIVEA_ L.) Article Open access 11 November 2021
LEAF NUTRIENT RESORPTION IN LUCERNE DECREASES WITH RELIEF OF RELATIVE SOIL NUTRIENT LIMITATION UNDER PHOSPHORUS AND POTASSIUM FERTILIZATION WITH IRRIGATION Article Open access 29 June 2020
INTRODUCTION In 2011, the Fukushima Daiichi Nuclear Power Plant accident released a large amount of radionuclides into the environment. Due to its long half-life, radioactive cesium (137Cs)
was considered the main contaminant. To estimate the transfer process of 137Cs in the terrestrial environment, we focused on its behavior in woody plants because the transfer process within
the forest ecosystem is much slower than it is in other areas1. After the forest contamination by 137Cs, depositions to tree canopies, leaf- and/or bark uptake, acropetal branch
translocation, etc. were energetically investigated2,3,4. Little is known about the physiology of Cs transfer and distribution within trees. Cesium chemically resembles potassium (K) but it
is not an essential nutrient for plant growth. Avery reported that Cs+ inhibits the inward-K+ channels in the plasma membrane and is therefore considered toxic to plants5. In general,
rhizosphere Cs+ consists mostly of stable 133Cs and its concentration is <200 μM, which is not toxic to plants6. Cesium uptake and translocation within the plant body are thought to be
mediated by K+ transport systems6. _Arabidopsis_ HAK5 (AtHAK5) is a K+ uptake transporter in roots and is up-regulated under K+ deficiency7,8. The _AtHAK5_ T-DNA insertion mutant, _athak5_,
showed a significantly decreased K content and a tolerance to 300 μM Cs+ treatment under low K conditions9,10. AtCNGC2 demonstrated cation transport activity using transgenic transfected
human embryonic kidney cells. _AtCNGC1_ is the candidate gene for Cs+ uptake and was identified by quantitative trait locus analysis in _Arabidopsis_11,12. In this study, we investigated
137Cs and 42K localizations using a model tree, poplar, under both long- and short photoperiods. To estimate the 137Cs retention time within the forest ecosystem, the Cs content of each tree
organ must be determined under seasonal conditions since Cs is circulated via root uptake, translocation, and leaf abscission. Poplar is a perennial deciduous tree with a characteristic
seasonal cycle of growth and dormancy. The phase shift from growth to dormancy is a winter adaptation13. The transition of meristems into dormant buds is crucial to protect them against
hazardous frosts. Woody plants shift their growth stage when they perceive changes in photoperiod and temperature14. The initiation of cold acclimation under short day length increases
endogenous abscisic acid levels and reduces endogenous gibberellic acid levels15,16,17. In beech tree (_Fagus sylvatica_ L.) leaf senescence, leaf K content decreases before shedding and the
recovered K is deposited in the stem cortex and pith18. Japanese native poplar (_Populus maximowiczii_) also showed a decrease in leaf K concentration following dormant bud formation19. An
increase in K+ concentration in xylem sap was observed during the winter season in field-grown _Populus nigra_20. These behaviors imply the existence of re-translocation mechanisms for K,
and it is assumed that the potassium is transported to the organs that require it once it is resorbed. Potassium is transported through various systems within the plant body21. Epstein _et
al_. showed that K+ absorption in barley roots is mediated by both a high-affinity- and a low-affinity biphasic transport process22. The high-affinity transport system (HATS) is up-regulated
by a decrease in external K+ concentration. The low-affinity transport system (LATS), however, operates even when there is sufficient external K+ 23. Potassium ion uptake by the root
symplast via HATS is mediated by the KUP/HAK/KT transporter family. There are thirteen such transporters in _Arabidopsis_24,25 and twenty-seven in rice26. Non-selective cation transport
mechanisms such as voltage-independent cation channels (VICC) are categorized as LATS. In _Arabidopsis, AtCNGC_ encodes VICC type channels. Twenty types of AtCNGC are present in the
_Arabidopsis_ genome. Based on the above, it is assumed that the expression and function of K+ permeable transporters also regulate Cs+ translocation in various plant species and situations.
Therefore, we investigated the relationship between the change in K localization induced by short day length and the behavior of Cs absorbed from the rhizosphere. To this end, 137Cs and 42K
accumulations and gene expression patterns of major K+ transporters were analyzed using a model tree poplar, hybrid aspen T89. RESULTS AMOUNT OF 137CS IN SHOOTS WAS DOWN-REGULATED UNDER
SHORT-DAY CONDITIONS Under a controlled growth cycle system in _Populus alba_ L., the shift from long-day (LD) to short-day (SD) conditions decreased phosphate in the lower leaves27. This
change suggests the existence of mechanisms for the re-translocation of phosphate from older- to younger (upper) leaves in response to with seasonal changes. Furukawa _et al_. indicated Ca2+
transport from root to shoot in _Populus maximowiczii_ is also regulated by the shift from LD to SD19. Based on these facts, the uptake of Cs+ within the root and its behavior within the
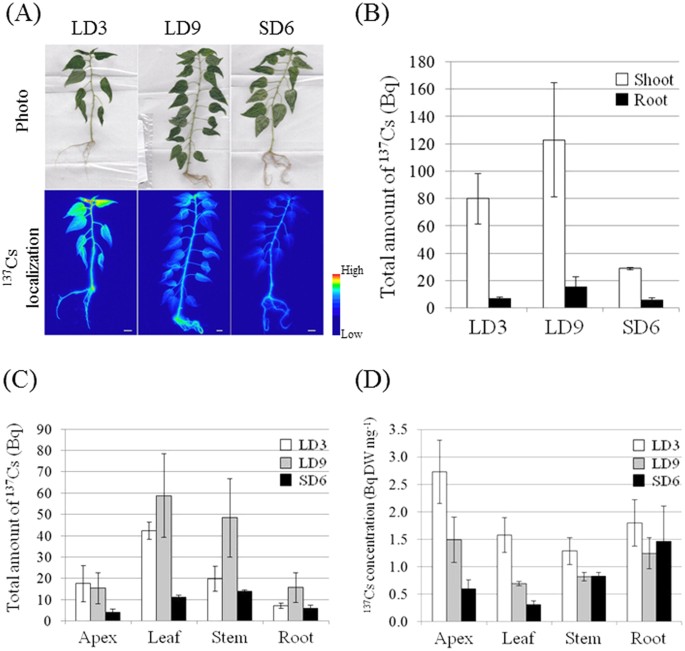
plant body in LD and SD conditions were compared. To measure seasonal variations in Cs+ uptake, a 137Cs+ solution was added to the growth media under LD3, LD9, and SD6 conditions (see
Methods). Figure 1A shows the localization of 137Cs by root absorption under LD3, LD9, and SD6 conditions. In the LD3 plants, 137Cs was localized entirely and the radiation intensity around
the apex was highest there. The LD9 plants were the same age as the SD6 plants and showed the same 137Cs behavior as the LD3 plants. In the SD6 plants, 137Cs was localized mainly in the stem
and root and the total 137Cs was lower than that for the other plants. In LD3, LD9, and especially SD6 plants, all the nodes showed high amounts of 137Cs. The quantity of 137Cs in the
shoots of SD6 plants was about 36.3% and 23.6% lower than that in the LD3 and LD9 shoots, respectively (Fig. 1B). On the other hand, the amount of 137Cs in the roots was similar for all
three conditions. Cesium-137 accumulated mainly (48.8%) in the leaves under LD3 conditions (Fig. 1C). In LD9 conditions, 137Cs also accumulated to a large extent in the leaves (42.5%).
Nevertheless, under SD6 conditions, the leaf 137Cs content was 32.1%, and organs containing the most 137Cs were the stems (39.7%). For the shoot apices, 137Cs levels were lower in SD6 plants
than they were in LD3 and LD9, but the difference was not significant. The concentrations of 137Cs were highest in the apices of the LD3 and LD9 plants (Fig. 1D). Nevertheless, the
decreases in the 137Cs concentrations in the apices and the leaves under SD6 conditions were significant, and the transition to SD suppressed Cs transport into the apices and the leaves.
POTASSIUM-42 UPTAKE WAS CONSTANT UNDER LD AND SD CONDITIONS Based on the 137Cs uptake activity assays, it was expected that the amount of 42K absorbed through the root would also be
down-regulated by the transition to SD. Poplar roots were treated with exogenous 42K and the amounts of 42K in the shoots and roots under LD3, SD2, SD4, and SD6 conditions were measured
after 24 h incubation (Fig. 2). No difference was found in the amount of 42K in the roots among four conditions. The amount of 42K in the shoots at the early stage of SD was almost
equivalent to that in the shoots at LD3. In contrast, the amount of 42K in the SD6 plant was slightly higher than it was in the other conditions, but the difference was not significant.
These data suggest that the demand for K in the rhizosphere neither increased nor decreased by the transition to SD in the poplar for up to six weeks. A comparison of Figs 1B and 2 indicates
that 137Cs accumulation significantly decreased under SD6 condition, but 42K accumulation remained almost constant through the SD transition. This fact indicates that the decrease in Cs
levels in the shoot is not explained by a simple reduction in transpiration rate under SD conditions and that important K+ uptake systems in poplar might be independently regulating Cs
accumulations in it. It is also implied that the transporter responsible for Cs+ uptake in poplar might have only limited involvement in K+ uptake since no decrease in K accumulation was
observed when Cs accumulation was low. _PTTHAK-LIKE1_ WAS UP-REGULATED BY TRANSITION TO SHORT-DAY We investigated under SD conditions the expression patterns of some candidate genes related
to K+ transport. We focused on the KUP/HAK/KT family K+ transporters and a cyclic-nucleotide-gated channel (CNGC) type K+ channels. As for KUP/HAK/KT family genes, we concentrated on three
genes in poplar. One of these was _Populus tremula_ K+ _uptake transporter 1_ or _PtKUP1_ (Accession number, AJ299422; POPTR_0003s13370) which was identified in hybrid aspen28. _PtKUP1_ was
used in a complementation test with a K+ -uptake-deficient _E. coli_ mutant. The addition of Cs+ to the culture media strongly inhibited the growth of _E. coli_ expressing PtKUP128. We also
looked at two KUP/HAK/KT family transporters resembling AtHAK5. _Populus trichocarpa_, whose genome was elucidated in 200629, has nine _AtHAK5_ homolog genes in its genome, including
_PtKUP1_. To evaluate the similarity between these putative K+ transporters, we constructed a phylogenetic tree of these genes and AtHAK5 homologs reported to be involved in K+ and Cs+
transport. We used their amino acid sequences (Fig. S1A). An _AtHAK5_ homolog in barley, _HvHAK1_, also up-regulated its expression under K deficiency, and transgenic yeast expressing the
_HvHAK1_ gene showed an enhanced growth rate30. Like _HvHAK1_, rice _OsHAK5_ also exhibits a high homology to _AtHAK5_31. The poplar gene POPTR_0010s10450 had the highest amino acid sequence
homology with AtHAK5. POPTR_0001s00580 had the second highest. We identified POPTR_0010s10450 and POPTR_0001s00580 orthologues in the hybrid aspen T89 and named them _PttHAK-like1_ and
_PttHAK-like2_, respectively. CNGC (cyclic-nucleotide-gated channel) may be a non-selective K+ channel which mediates K+ uptake by the root symplast21. In _Arabidopsis,_ the CNGC channel
AtCNGC2 shows K+ permeability32. A quantitative trait locus analysis indicated that _AtCNGC1_ is associated with shoot K and Cs concentrations in _Arabidopsis_12,33. In _P. trichocarpa_,
nine genes were selected as _AtCNGC1_ homologs based on their amino acid sequences (see Supplementary Fig. S1B). Proteome BLAST analysis showed that POPTR_0012s01690 and POPTR_0015s02090
scored significantly higher than did others and the orthologues in hybrid aspen T89 were named as _PttCNGC1-like1_ and _PttCNGC1-like2_, respectively. To determine which K+ uptake related
gene is the most abundantly expressed among these five transporters, we evaluated the expression level of each gene under LD3 conditions. In poplar roots, no obvious differences were found
in the expression levels of the genes selected (Fig. 3A). There may be redundancy in the expression of these K+ influx transporters under LD3 conditions. During the transition to the SD
conditions, the expression of _PtKUP1_ did not significantly change (Fig. 3B). _PttHAK-like1_ showed steady expression until the transition to SD4 conditions and was up-regulated by about
1.5-fold under SD6 conditions (Fig. 3C). _PttHAK-like2_ expression tended to decrease in SD2 and SD4 plants but maintained statistically steady-state transcription levels through SD
transition (Fig. 3D). The expressions of _PttCNGC1-like1, PttCNGC1-like2_ were also relatively constant under SD conditions (Fig. 3E,F). There was a small but statistically significant
increase in the expression level of _PttHAK-like1_ under SD6 conditions but the amount of K absorbed through the root did not change with the transition to SD (Figs 2 and 3C). This
inconsistency may be accounted for by the low elevation level of _PttHAK-like1_ expression and the significant differences in the amino acid sequence of the poplar genes. To confirm, we
sequenced the entire _PttHAK-like1_ gene from the hybrid aspen T89. AtHAK5 and POPTR_0010s10450 in _P. trichocarpa_ and PttHAK-like1 in hybrid aspen T89 and their alignment are shown in
Supplementary Fig. S2. The AtHAK5 amino acid sequence showed 44.5% homology to POPTR_0010s10450 and 44.2% to PttHAK-like1. For the poplar, PttHAK-like1 and POPTR_0010s10450 shared 97.8%
homology. The GEGGTFALY domain (AtHAK5-type transporters) is important for K+ and Cs+ selectivity34. Of these three genes, the GEGGTFALY domain was completely conserved and, consequently,
there is no obvious explanation for the functional divergence. Despite the steady 42K uptake manner through seasonal transitions, Cs accumulation activity was down-regulated under SD6
conditions. Therefore, the Cs+ and K+ transport systems are probably separately regulated in poplar. DISCUSSION Potassium is one of the most abundant essential plant nutrients. It is
required for metabolism, photosynthesis, the tricarboxylic acid (TCA) cycle, glycolysis, and amino acid biosynthesis35. Maintaining enough K within the plant body is therefore quite
important. For example, in K-deficient sunflowers, the carbon flux into the TCA cycle decreased due to changes in carbon distribution36. Potassium deficiency also inhibited sugar
translocation in several plants37. Thus, the amount of K is closely tied to processes that maintain homeostasis in plants such as charge balance, pH regulation, and osmotic potential35.
Potassium is the dominant solute in the xylem- and phloem saps of _Ricinus communis_ and the circulation of K is required for plant growth and development38. In this study, the relative
amounts of 42K accumulated were compared over seasonal transitions. It was found that 42K accumulation remained constant until SD6. Dormant buds formed up to four weeks after the onset of
the short-day treatment (data not shown); therefore, K re-translocation should have already started at SD6. Nevertheless, the results showed that 42K accumulation from root uptake and the
expression of genes related to the root K+ uptake were almost constant (Figs 2 and 3B–F). It has been reported that the induction of AtHAK5 was enhanced by K+ deficiency7,8 or by Cs+
applications when there was sufficient K+ 39. Therefore, the slight increase in _PttHAK-like1_ expression under SD6 might be a response to K+ starvation during the long growth period.
Despite the constant K accumulation pattern under SD conditions, Cs accumulation drastically decreased in SD6 plants (Fig. 1A,B). Cesium ion uptake and translocation are considered to be
regulated by the plant K+ transport system but no down-regulation in the genes related to K+ uptake was identified during SD transition (Fig. 3B–F). It is known that plant mineral uptake
mechanisms are regulated by protein activity level as well as gene expression6. Further nutrient transport activity analysis is necessary but these results suggest the possible existence of
a novel uptake transporter which carries Cs+ much more efficiently than it does K+. Since a decrease in Cs was observed only in the shoot (Fig. 1B), attention should be given to the
transporters involved in Cs+ transfer between the root cells adjacent to the xylem and the xylem vessels themselves. Potassium-42 translocation from the root to the shoot was not affected by
the transition to SD (Fig. 2). Therefore, K+ xylem loading might not be down-regulated, and there could be a Cs+ re-uptake pathway from the xylem sap to the root cells. This type of
regulation was hypothesized for the Zn2+ transport system adjacent to root xylem vessels, and it may serve to keep shoot Zn2+ concentrations below toxic levels40. This mechanism would only
be plausible if Cs+ specific transporters exist near the root xylem vessels—and these have not yet been found. We did not find the K+ uptake transporter which was obviously up- or down
regulated in the transition to SD. We used _Arabidopsis_ eFP Browser (http://bar.utoronto.ca/efp/cgi-bin/efpWeb.cgi), which provides detailed information about the _Arabidopsis_ gene
expression site and various gene induction factors such as organic- and inorganic stressors. Expressions of the homologous _Arabidopsis_ genes _AtKUP1, AtHAK5,_ and _AtCNGC1_ are not changed
by short-day treatment or by exogenous abscisic acid. These expression patterns are consistent with our results and imply that short day length-induced regulation of K+ uptake is also
unnecessary in poplar. Unlike K accumulation, Cs accumulation did not remain constant, but drastically changed by day length transition. Therefore, the Cs+ uptake and translocation
mechanisms differ from those for K+ in poplar. It was not determined why Cs accumulation was down-regulated but K accumulation was constant under the transition to SD conditions. It is
clear, however, that Cs accumulation was affected by photoperiod. METHODS PLANT MATERIAL AND GROWTH CONDITIONS Hybrid aspen T89 (_Populus tremula_ x _tremuloides_) (kindly provided by Prof,
B. Sundberg, Swedish University of Agricultural Sciences, Sweden) were cultured in sterile pots in half-strength Murashige & Skoog (MS) medium under light- and temperature-controlled
conditions (light 16 h, darkness 8 h, 23 °C; light intensity 37.5 μmol m−1 s−1). Each month, all plants were cut about five centimeters below the shoot apex and replanted in fresh MS medium.
MEASUREMENT OF 137CS AND 42K DISTRIBUTIONS IN POPLAR Poplars were grown under long-day (LD) conditions for three- and nine weeks in light- and temperature-controlled conditions (LD3 and
LD9). Long-day conditions were as follows: light-period 16 h (light intensity 37.5 μmol m−1 s−1), dark-period 8 h, temperature 23 °C. To investigate the effects of seasonal transitions, the
culture conditions were shifted to short-day (SD) for an additional two, four, and six weeks (SD2, SD4, and SD6) after the end of LD3 cultivation. Short-day conditions were as follows:
light-period 8 h (light intensity 37.5 μmol m−1 s−1), dark-period 16 h, temperature 23 °C. 137CsCl (25 kBq, with 0.1 μM 133CsCl) or 42K (8 kBq, with 0.1 μM 39KCl) solutions were then added
to the growth media to trace root absorption. The 42KCl solution was prepared using an 42Ar+-42K+ generator41,42. The purity of the 42K+ was verified from the gamma-ray spectra emitted by
the test solutions using a germanium detector (GEM-type, ORTEC, USA). The decay of the 42K+ spectral peak (1525 keV) was monitored for 7 d as described in Kobayashi _et al_.43. The
half-lives of the test solutions were measured with a liquid scintillation counter (LSC-6100, Hitachi Aloka Medical, Japan) and were theoretically identical to the actual half-life of 42K.
Plants grown under LD and SD conditions were incubated with radioisotopes under the same photoperiods. Incubation times were 48 h and 24 h for the 137Cs and 42K experiments, respectively.
Shoots and roots were separated and dried for 3 d at 50 °C. In the 137Cs assay using SD6 plants, plants were cut into four parts: apex (shoot apex and top three leaves), leaf (remaining
leaves and petioles), stem, and root. To measure 137Cs and 42K radioactivity, the gamma counters AccuFLEX γ7001 (Hitachi Aloka Medical, Japan) and ARC-300 (Hitachi Aloka Medical, Japan) were
used, respectively. The details of handling and measuring 137Cs and 42K were described in Kobayashi _et al_.43. Cesium-137 distribution was also investigated autoradiographically with a
laser imaging scanner (FLA-9500, GE Healthcare, UK) in LD3, LD9, and SD6 plants. Significant differences between 42K and 137Cs quantities for each organ type and under each photoperiod were
evaluated using one-way ANOVA. ACQUISITION OF K INFLUX TRANSPORTER HOMOLOGOUS GENE NUCLEOTIDE SEQUENCES Full-length _AtHAK5_ (At4G13420) and _AtCNGC1_ (At5G53130) coding sequences were
obtained from the _Arabidopsis_ sequence database (TAIR; https://www.arabidopsis.org/). Eight homologous _HAK5_ genes and nine homologous _CNGC1_ genes were identified in poplar from the
plant genomic resource (Phytozome; https://phytozom.jgi.doe.gov/pz/portal.html). _OsHAK1_ (Os04g0401700) and _OsHAK5_ (Os01g0930400) coding sequences were identified from RAP-DB,
(http://rapdb.dna.affrc.go.jp/). The _HvHAK1_ (Accession number: AF025292) coding sequence was searched using nucleotide BLAST in NCBI (http://www.ncbi.nlm.nih.gov/). CONSTRUCTING THE
PHYLOGENETIC TREE The full-length coding sequences for the _Populus HAK5_ and _CNGC1_ homologs were converted to amino acid sequences, and then phylogenetic trees were created using the
Maximum Likelihood method in MEGA application (Molecular Evolutionary Genetics Analysis, _ver._ 5.05). GENE EXPRESSION ANALYSIS Plant roots were flash-frozen in liquid nitrogen then
pulverized using a mixer mill (QIAGEN, Germany). Total RNA was extracted using RNeasy Plant Mini Kit (QIAGEN). Quantitative real-time reverse-transcription PCR (qRT-PCR) was performed using
One Step SYBR PrimeScript RT-PCR Kit ІІ (Takara, Japan) and 7300 Real Time PCR System (Applied Biosystems, USA). Three biological replicates were run for each photoperiod. An ubiquitin gene
(Accession number: AF240445) was used as a reference gene in hybrid aspen T89. The _Ubiqutin_ primers for qRT-PCR were the following: UBIQUTIN-F (5′-TGAACCAAATGATACCATTGATAG-3′) and
UBIQUTIN-R (5′-GTAGTCGCGAGCTGTCTTG-3′). The gene expression analysis primers for _PtKUP1, PttHAK-like1, PttHAK-like2, PttCNGC1-like1,_ and _PttCNGC1-like2_ are listed in Table 1. Significant
differences between each gene expression level were confirmed by one-way ANOVA. CLONING OF THE _PTTHAK-LIKE1_ CODING SEQUENCE The full-length coding sequence of _PttHAK-like1_ was cloned.
The whole root of Hybrid aspen T89 grown until SD6 was harvested and stored at −80 °C. Total RNA was extracted using RNeasy Plant Mini Kit, and cDNA was synthesized from the extracted RNA
with a ReverTra Ace (TOYOBO, Japan). The primers used for cloning _PttHAK-like1_ were the following: _PttHAK-like1_: (5′-ATGGAAGGAGATGATGATCG-3′) and (5′-TTAGACCATGTATGTCATCCC-3′). The
full-length coding sequence was amplified by PrimeSTAR GXL DNA Polymerase (Takara, Japan) and purified by Wizard SV Gel and PCR Clean-Up System (Promega, USA). _PttHAK-like1_ was inserted
into a pGEM T-Easy cloning vector by TA cloning (Promega, USA). The nucleotide sequences of _PttHAK-like1_ were determined using a DNA sequencer (3130 Genetic Analyzer; Applied Biosystems,
USA) and a Big-Dye terminator v3.1 sequencing standard Kit (Applied Biosystems, USA). The sequence was analyzed with Finch TV and BioEdit. ADDITIONAL INFORMATION HOW TO CITE THIS ARTICLE:
Noda, Y. _et al_. Short day length-induced decrease of cesium uptake without altering potassium uptake manner in poplar. _Sci. Rep._ 6, 38360; doi: 10.1038/srep38360 (2016). PUBLISHER'S
NOTE: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. REFERENCES * Shaw, G. Radionuclides in forest ecosystems.
Radioactivity in the Environment. 10, 127–155 (2007). Article CAS Google Scholar * Kato, H., Onda, Y. & Gomi, T. Interception of the Fukushima reactor accident-derived 137Cs, 134Cs,
and 131I by coniferous forest canopies. Geophys. Res. Lett. 39, doi: 10.1029/2012GL052928 (2012). * Takata, D. Distribution of radiocesium from the radioactive fallout in fruit trees. In:
Nakanishi, T. M., Tanoi, K. (ed) Agricultural implications of the Fukushima nuclear accident (Springer, New York 143–162 2013). * Kanasashi, T. et al. Radiocesium distribution in sugi
(_Cryptomeria japonica_) in eastern Japan: translocation from needles to pollen. J. Environ. Radioact. 139, 398–406 (2015). Article CAS PubMed Google Scholar * Avery, S. V. Cesium
accumulation by microorganisms: uptake mechanisms, cation competition, compartmentalization and toxicity. J. Ind. Microbiol. 14, 76–84 (1995). CAS PubMed Google Scholar * White, P. J.
& Broadley, M. R. Mechanisms of cesium uptake by plants. New Phytol. 147, 241–256 (2000). Article CAS Google Scholar * Ahn, S. J., Shin, R. & Schachtman, D. P. Expression of
_KT/KUP_ genes in Arabidopsis and the role of root hairs in K+ uptake. Plant Physiol. 134, 1135–1145 (2004). Article CAS PubMed PubMed Central Google Scholar * Jung, J. Y., Shin, R.
& Schachtman, D. P. Ethylene mediates response and tolerance to potassium deprivation in _Arabidopsis_. Plant Cell 21, 607–621 (2009). Article CAS PubMed PubMed Central Google
Scholar * Gierth, M., Mäser, P. & Schroeder, J. I. The potassium transporter _AtHAK5_ functions in K+ deprivation-induced high-affinity K+ uptake and AKT1 K+ channel contribution to K+
uptake kinetics in Arabidopsis roots. Plant Physiol. 137, 1105–14 (2005). Article CAS PubMed PubMed Central Google Scholar * Qi, Z. et al. The high affinity K+ transporter AtHAK5 plays
a physiological role in planta at very low K+ concentrations and provides a cesium uptake pathway in _Arabidopsis_. J. Exp. Bot. 59, 595–607 (2008). Article CAS PubMed Google Scholar *
Leng, Q., Mercier, R. W., Yao, W. & Berkowitz, G. A. Cloning and first functional characterization of a plant cyclic nucleotide-gated cation channel. Plant Physiol. 121, 753–761 (1999).
Article CAS PubMed PubMed Central Google Scholar * Kanter, U. et al. Cesium and strontium accumulation in shoots of _Arabidopsis thaliana_: Genetic and physiological aspects. J. Exp.
Bot. 61, 3995–4009 (2010). Article ADS CAS PubMed PubMed Central Google Scholar * Jansson, S., Bhalerao, R. P. & Groover, A. T. Genetics and genomics of populus. (Springer, New
York 2010). * Welling, A., Moritz, T., Palva, E. T. & Junttila, O. Independent activation of cold acclimation by low temperature and short photoperiod in hybrid aspen. Plant Physiol.
129, 1633–1641 (2002). Article CAS PubMed PubMed Central Google Scholar * Olsen, J. E. et al. Ectopic expression of oat phytochrome A in hybrid aspen changes critical day length for
growth and prevents cold acclimatization. Plant J. 12, 1339–1350 (1997). Article CAS Google Scholar * Welling, A., Kaikuranta, P. & Rinne, P. Photoperiodic induction of dormancy and
freezing tolerance in _Betula pubescens._ Involvement of ABA and dehydrins. Physiol. Plant. 100, 119–125 (1997). Article CAS Google Scholar * Mølmann, J. A. et al. Low night temperature
and inhibition of gibberellin biosynthesis override phytochrome action and induce bud set and cold acclimation, but not dormancy, in _PHYA_ overexpressors and wild-type of hybrid aspen.
Plant Cell Environ. 28, 1579–1588 (2005). Article Google Scholar * Eschrich, W., Fromm, J. & Essiamah, S. Mineral partitioning in the phloem during autumn senescence of beech leaves.
Trees 2, 73–83 (1988). Article CAS Google Scholar * Furukawa, J., Kanazawa, M. & Satoh, S. Dormancy-induced temporal up-regulation of root activity in calcium translocation to shoot
in _Populus maximowiczii_. Plant Root 6, 10–18 (2012). Article CAS Google Scholar * Furukawa, J. et al. Seasonal fluctuation of organic and inorganic components in xylem sap of _Populus
nigra_. Plant Root 5, 56–62 (2011). Article CAS Google Scholar * Ahmad, I. & Maathuis, F. J. M. Cellular and tissue distribution of potassium: Physiological relevance, mechanisms and
regulation. J. Plant Physiol. 171, 708–714 (2014). Article CAS PubMed Google Scholar * Epstein, E., Rains, D. W. & Elzam, O. E. Resolution of dual mechanisms of potassium absorption
by barley roots. Proc. N. A. S. 49, 684–692 (1963). Article ADS CAS Google Scholar * Maathuis, F. J. M. & Sanders, D. Regulation of K+ absorption in plant root cells by external K+:
interplay of different plasma membrane K+ transporters. J. Exp. Bot. 48, 451–458 (1997). Article CAS PubMed Google Scholar * Rubio, F., Santa-Maria, G. E. & Rodriguez-Navarro, A.
Cloning of _Arabidopsis_ and barley cDNAs encoding HAK potassium transporters in root and shoot cells. Physiol. Plant. 109, 34–43 (2000). Article CAS Google Scholar * Mäser, P. et al.
Phylogenetic relationships within cation transporter families of Arabidopsis. Plant Physiol. 126, 1646–1667 (2001). Article PubMed PubMed Central Google Scholar * Gupta, M. et al.
KT/HAK/KUP potassium transporters gene family and their whole life cycle expression profile in rice (_Oryza sativa_). Mol. Genet. Genomics 280, 437–452 (2008). Article CAS PubMed Google
Scholar * Kurita, Y. et al. Establishment of a shortened annual cycle system; a tool for the analysis of annual re-translocation of phosphorus in the deciduous woody plant (_Populus alba_
L.). J. Plant Res. 127, 545–551 (2014). Article CAS PubMed Google Scholar * Langer, K. et al. Poplar potassium transporters capable of controlling K+ homeostasis and K+ -dependent
xylogenesis. Plant J. 32, 997–1009 (2002). Article CAS PubMed Google Scholar * Tuskan, G. A. et al. The genome of black cottonwood, _Populus trichocarpa_ (Torr. & Gray). Science 313,
1596–1604 (2006). Article ADS CAS PubMed Google Scholar * Santa-María, G. E., Rubio, F., Dubcovsky, J. & Rodríguez-Navarro, A. The _HAK1_ gene of barley is a member of a large gene
family and encodes a high-affinity potassium transporter. Plant Cell 9, 2281–2289 (1997). PubMed PubMed Central Google Scholar * Yang, T. et al. The role of a potassium transporter
OsHAK5 in potassium acquisition and transport from roots to shoots in rice at low potassium supply levels. Plant Physiol. 166, 945–959 (2014). Article PubMed PubMed Central Google Scholar
* Hua, B. G., Mercier, R. W., Leng, Q. & Berkowitz, G. A. Plants do it differently. A new basis for potassium/sodium selectivity in the pore of an ion channel. Plant Physiol. 132,
1353–1361 (2003). Article CAS PubMed PubMed Central Google Scholar * Harada, H. & Leigh, R. A. Genetic mapping of natural variation in potassium concentrations in shoots of
_Arabidopsis thaliana_. J. Exp. Bot. 57, 953–960 (2006). Article CAS PubMed Google Scholar * Alemán, F. et al. The F130S point mutation in the Arabidopsis high-affinity K+ transporter
AtHAK5 increases K+ over Na+ and Cs+ selectivity and confers Na+ and Cs+ tolerance to yeast under heterologous expression. Front. Plant Sci. 5, 430, doi: 10.3389/fpls.2014.00430.eCollection
(2014). Article PubMed PubMed Central Google Scholar * Amtmann, A. & Rubio, F. Potassium in plants. eLS, doi: 10.1002/9780470015902.a0023737 (2012). * Yamada, S. et al. Effect of
potassium nutrition on current photosynthesized carbon distribution to carbon and nitrogen compounds among rice, soybean and sunflower. J. Plant Nutr. 25, 1957–1973 (2002). Article CAS
Google Scholar * Amtmann, A., Troufflard, S. & Armengaud, P. The effect of potassium nutrition on pest and disease resistance in plants. Physiol. Plant. 133, 682–691 (2008). Article
CAS PubMed Google Scholar * Marschner, H., Kirkby, E. A. & Engels, C. Importance of cycling and recycling of mineral nutrients within plants for growth and development. Botanica.
Acta. 110, 265–273 (1997). Article CAS Google Scholar * Adams, E., Abdollahi, P. & Shin, R. Cesium inhibits plant growth through jasmonate signaling in _Arabidopsis thaliana_. Int. J.
Mol. Sci. 14, 4545–4559 (2013). Article CAS PubMed PubMed Central Google Scholar * Burleigh, S. H., Kristensen, B. K. & Bechmann, I. E. A plasma membrane zinc transporter from
_Medicago truncatula_ is up-regulated in roots by Zn fertilization, yet down-regulated by arbuscular mycorrhizal colonization. Plant Mol. Biol. 52, 1077–1088 (2003). Article CAS PubMed
Google Scholar * Homareda, H. & Matsui H. Biochemical utilization of 42Ar-42K Generator. Radioisotopes 35, 543–546 (1986). Article CAS PubMed Google Scholar * Aramaki, T. et al.
Application of 42K to Arabidopsis Tissues Using Real-Time Radioisotope Imaging System (RRIS). Radioisotopes 64, 169–176 (2015). Article CAS Google Scholar * Kobayashi, N. I. et al. Tracer
experiment using 42K+ and 137Cs+ revealed the different transport rates of potassium and caesium within rice roots. Functional Plant Biology 43, 151–160 (2015). Article Google Scholar
Download references ACKNOWLEDGEMENTS This work was financially supported in part by KAKENHI Grant Number 24110007 awarded to J.F. We would like to thank Editage (www.editage.jp) for English
language editing. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Graduate school of Life and Environmental Sciences, University of Tsukuba, Tsukuba, 305-8572, Japan Yusaku Noda * Faculty of
Life and Environmental Sciences, University of Tsukuba, Tsukuba, 305-8572, Japan Jun Furukawa, Tsutomu Aohara & Shinobu Satoh * Center for Research in Isotopes and Environmental
Dynamics, University of Tsukuba, Tsukuba, 305-8577, Japan Jun Furukawa * Graduate school of Agricultural and Life Sciences, The University of Tokyo, Tokyo, 113-8657, Japan Naoto Nihei,
Atsushi Hirose, Keitaro Tanoi & Tomoko M. Nakanishi * PRESTO, Japan Science and Technology Agency (JST), Kawaguchi, 332-0012, Japan Keitaro Tanoi Authors * Yusaku Noda View author
publications You can also search for this author inPubMed Google Scholar * Jun Furukawa View author publications You can also search for this author inPubMed Google Scholar * Tsutomu Aohara
View author publications You can also search for this author inPubMed Google Scholar * Naoto Nihei View author publications You can also search for this author inPubMed Google Scholar *
Atsushi Hirose View author publications You can also search for this author inPubMed Google Scholar * Keitaro Tanoi View author publications You can also search for this author inPubMed
Google Scholar * Tomoko M. Nakanishi View author publications You can also search for this author inPubMed Google Scholar * Shinobu Satoh View author publications You can also search for
this author inPubMed Google Scholar CONTRIBUTIONS Y.N. and J.F. contributed equally to this work. Y.N. and J.F. proposed and organized the project. Y.N., J.F., T.A., N.N., K.T., and S.S.
discussed and designed the experiment. Y.N., J.F., T.A., N.N., A.H., and K.T. carried out the experiments. Y.N., J.F., T.A., N.N., A.H., K.T. T.M.N., and S.S. analyzed and interpreted the
data. Y.N. and J.F. wrote the main manuscript text. All the authors revised the manuscript and participated in discussions of the research. ETHICS DECLARATIONS COMPETING INTERESTS The
authors declare no competing financial interests. ELECTRONIC SUPPLEMENTARY MATERIAL SUPPLEMENTARY FIGURES RIGHTS AND PERMISSIONS This work is licensed under a Creative Commons Attribution
4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if
the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit
http://creativecommons.org/licenses/by/4.0/ Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Noda, Y., Furukawa, J., Aohara, T. _et al._ Short day length-induced decrease of
cesium uptake without altering potassium uptake manner in poplar. _Sci Rep_ 6, 38360 (2016). https://doi.org/10.1038/srep38360 Download citation * Received: 05 May 2016 * Accepted: 09
November 2016 * Published: 07 December 2016 * DOI: https://doi.org/10.1038/srep38360 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get
shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative