Cell deformation by single-beam acoustic trapping: a promising tool for measurements of cell mechanics
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT We demonstrate a noncontact single-beam acoustic trapping method for the quantification of the mechanical properties of a single suspended cell with label-free. Experimentally
results show that the single-beam acoustic trapping force results in morphological deformation of a trapped cell. While a cancer cell was trapped in an acoustic beam focus, the morphological
changes of the immobilized cell were monitored using bright-field imaging. The cell deformability was then compared with that of a trapped polystyrene microbead as a function of the applied
acoustic pressure for a better understanding of the relationship between the pressure and degree of cell deformation. Cell deformation was found to become more pronounced as higher pressure
levels were applied. Furthermore, to determine if this acoustic trapping method can be exploited in quantifying the cell mechanics in a suspension and in a non-contact manner, the
deformability levels of breast cancer cells with different degrees of invasiveness due to acoustic trapping were compared. It was found that highly-invasive breast cancer cells exhibited
greater deformability than weakly-invasive breast cancer cells. These results clearly demonstrate that the single-beam acoustic trapping technique is a promising tool for non-contact
quantitative assessments of the mechanical properties of single cells in suspensions with label-free. SIMILAR CONTENT BEING VIEWED BY OTHERS ACOUSTIC TWEEZERS FOR HIGH-THROUGHPUT SINGLE-CELL
ANALYSIS Article 19 July 2023 SPATIALLY SELECTIVE MANIPULATION OF CELLS WITH SINGLE-BEAM ACOUSTICAL TWEEZERS Article Open access 25 August 2020 VISCOELASTICITY OF DIVERSE BIOLOGICAL SAMPLES
QUANTIFIED BY ACOUSTIC FORCE MICRORHEOLOGY (AFMR) Article Open access 04 June 2024 INTRODUCTION The mechanical properties of cells play a key role in various cellular functions, such as
proliferation, migration, and gene expression1,2,3. Also, they can be altered by diseases or by the external environment4. For instance, a red blood cell infected by malaria activates the
erythrocytic stages of its life cycle, resulting in the cell’s progressive stiffening. Therefore, the stiffness of a red blood cell can be used for the determination of malaria infection5.
Also, the mechanics of cancer cells have been measured to determine cancer cell invasiveness, as highly invasive cancer cells are typically softer than weakly invasive cancer cells, allowing
them to migrate more easily6. As a result, the mechanical properties of a cell can serve as useful biomarkers for the detection of various diseases and in identifications of cell
phenotypes, necessitating the development of biophysical tools to quantify cell mechanics. Many tools capable of probing cell mechanics, including atomic force microscopy (AFM)7,8, optical
tweezers9,10, and magnetic tweezers11,12, have been developed. AFM utilizes a nano-sized probe to measure the local stiffness of cells13, but it is limited to the measurements of the
mechanics of cells with a Young’s modulus greater than 50 Pa. One of its shortcomings is that it requires the probe to be in contact with a cell; moreover, isolation from surrounding
vibrations is required to achieve reliable outcomes7,8. On the other hand, optical tweezers enable one to trap a single cell in a tightly focused laser beam. They have been successfully used
to measure the mechanical properties of red blood cells by pulling microspheres attached to these cells14. However, they can result in cell damage due to the temperature rise induced by the
applied laser14. In addition, the trapping force generated by optical tweezers is limited to the pico-Newton range, thus allowing only the trapping of tiny biological samples. Magnetic
tweezers have been also shown to be promising for the probing of the mechanical properties of individual molecules, inter-molecular bonds, and whole cells. With this technique, the complex
modulus of elasticity of a cell can be quantified and the local viscoelasticity of a cell can be measured15. A major drawback of this approach is that spherical magnetic beads of varying
diameters must be loaded into the cytoplasm of a cell16. In addition to the tools described above, several ultrasonic techniques have been developed to measure cell mechanics. A
high-frequency acoustic-radiation force-impulse microscopic method which works via the photoacoustic detection (PA-ARFI) of a functionalized carbon nanotube attached to the cell membrane was
developed to measure cell mechanics17. With the PA-ARFI technique, the mechanics of breast cancer cells of different phenotypes can be successfully quantified. A single-beam acoustic
trapping technique with a 193 MHz press-focused lithium niobate (LiNbO3) transducer was also utilized to study the mechanical properties of a breast cancer cell. In that study, a 5 μm
fibronectin-coated polystyrene microbead acoustically trapped was attached to a target cell and was then pulled with acoustic tweezers in order to measure the elastic properties of the
cell18. Compared to optical tweezers, the single-beam acoustic trapping technique offers several advantages, such as the generation of stronger force of a few nano-Newtons, causing less cell
damage, and the use of relatively simple setups, thus demonstrating that it is a promising alternative19,20. Although these ultrasonic techniques have the potential to measure cell
mechanics, they have a few limitations. First, they need either microbeads or carbon nanotubes to be attached to a target cell, necessitating mastery of the reproduction approach with regard
to bead attachment and the uniform distribution and attachment of carbon nanotubes onto the cell membrane. Second, the use of these techniques is limited to the measurement of the
mechanical properties of cultured cells. Therefore, they may not be suitable for the measurement of a suspended cell. Note that the suspended state of a cell is more relevant to practical
applications of cellular biophysics to medicine21. Hence, it would be desirable to develop an ultrasonic technique capable of measuring the mechanical properties of a suspended cell without
any materials attached to the cell. In this paper, we therefore demonstrate a non-contact single-beam acoustic trapping method for the quantification of the mechanical properties of a
suspended cell without any materials attached to the cell. The isolation of a suspended target cell from other adjacent cells is achieved by the acoustic trapping of the target cell using a
30 MHz lithium niobate (LiNbO3) highly focused ultrasound transducer. In addition, we examine whether a trapped cell is deformed due to the acoustic pressure generated during acoustic
trapping and whether the cell deformability due to the acoustic trapping is dependent on its mechanical properties. Furthermore, the deformability levels of breast cancer cells with
different degrees of invasiveness were quantitatively compared upon changes in the acoustic trapping force. The results clearly demonstrate the feasibility of the single-beam acoustic
trapping technique for quantifying cell mechanics in a non-contact manner without the need for labeling. RESULTS CELL DEFORMATION BY ACOUSTIC TRAPPING In order to investigate whether a cell
could be deformed due to acoustic trapping, a breast cancer cell was trapped and changes in the cell size in a transverse direction were then monitored using the system described in the
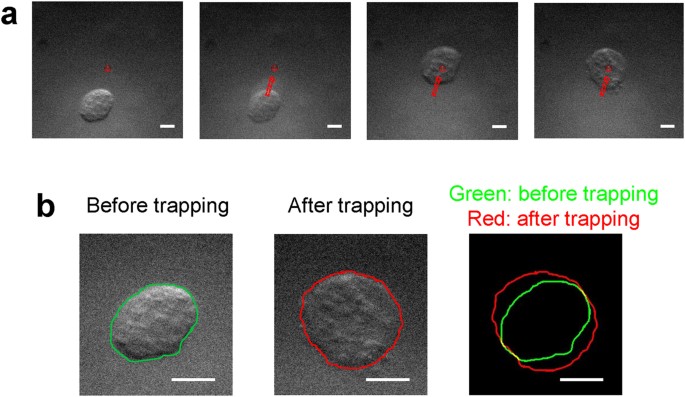
method section. Figure 1(a,b) illustrates how the breast cancer cell (BT-474) is moved to the focus and then held by the beam when the trap is switched on [Fig. 1(a) and Supplementary Video
1]. It was found here that the transverse size of a trapped cell becomes larger than that of the cell prior to acoustic trapping. For a detailed examination of the change in the cell size
before and after acoustic trapping, the boundaries of the cell before and after acoustic trapping were extracted with ImageJ. As shown in Fig. 1b (right), the cell boundary after acoustic
trapping (the red solid line) is larger than that before acoustic trapping (the red solid line). These results clearly demonstrate that the single cell acoustic trapping method results in
morphological deformation of the trapped cell under the given condition (1.64 MPa). DEFORMATION OF A TRAPPED CELL WITH DIFFERENT MECHANICAL PROPERTIES DUE TO ACOUSTIC TRAPPING. In addition,
the diameters of acoustically trapped breast cancer cells, including BT-474 (weakly- invasive), MCF-7 (weakly- invasive), and MDA-MB-231 (highly-invasive) cells, which typically exhibit
different mechanical properties, were measured and compared as a function of the acoustic trapping force. Figure 2 illustrates the morphological changes of acoustically trapped cells at the
indicated acoustic pressures. The diameters of cells were found to increase as the acoustic pressure was increased (Fig. 2a). The red dotted line indicates the original diameter of the cell
before acoustic trapping, whereas the blue dotted line indicates the increased diameter of the cell after acoustic trapping at the given acoustic pressures. The gap between the red and blue
dotted line represents the increased length of the diameter of a trapped cell. At 0.71 MPa, the diameters of the trapped cells were observed to be clearly larger than those of the cells
before acoustic trapping (0 MPa). As the acoustic pressure was increased further to 1.22 MPa, the diameters of the trapped cells became longer, whereas that of the polystyrene microbead did
not change. The normalized diameters of the BT-474 cells were measured and found to be approximately 1.07, 1.08, and 1.09 at 0.71, 0.97, and 1.22 MPa, respectively. The normalized diameters
of the MCF-7 cells were measured to be approximately 1.08, 1.11, and 1.12 at 0.71, 0.97, and 1.22 MPa, respectively. In contrast, the corresponding normalized diameters of the MDA-MB-231
cells were approximately 1.09, 1.14, and 1.16 at those acoustic pressures, (Fig. 2b). The normalized diameter of the MDA-MB-231 cell was observed to be larger than that of the MCF-7 and
BT-474 cell at 1.22 MPa. These results show that the changes in the diameter of the trapped cells due to acoustic trapping depend on the applied acoustic pressure and on the mechanical
properties of an acoustically trapped sample. DEFORMABILITY OF HIGHLY AND WEAKLY INVASIVE BREAST CANCER CELLS DUE TO ACOUSTIC TRAPPING A quantitative analysis of the deformability due to
acoustic trapping of trapped highly (MDA-MB-231) and weakly invasive (MCF-7 and BT-474) breast cancer cells was carried out in order to investigate whether the single-beam acoustic trapping
technique allowed discrimination between mechanical properties of breast cancer cells of different phenotypes. Note that MDA-MB-231 cells are known to be softer than MCF-7 and BT-474
cells17. Figure 3 illustrates the change in the deformability of the trapped cells due to acoustic trapping. The MDA-MB-231 cells exhibit greater deformability than the MCF-7 and BT-474
cells and the polystyrene microbeads (control) upon acoustic trapping at 1.22 MPa [p-value = 0.006 (MCF-7) and 0.003 (BT-474) < 0.01]. In addition, the areas of both the highly and weakly
invasive breast cancer cells (sample number: 9) increased as the acoustic pressure increased, whereas that of the trapped polystyrene microbeads did not increase. The mean deformability
values of MDA-MB-231 cells here were found to be 0.064, 0.114, and 0.149. In contrast, the mean deformability values for the MCF-7 cells were ~0.046, ~0.050, and ~0.068 at acoustic pressure
levels of 0.71, 0.97, and 1.22 MPa and the mean deformability values for the BT-474 cells were ~0.022 ~0.047, and ~0.041 at acoustic pressure levels of 0.71, 0.97, and 1.22 MPa,
respectively. The changes in the area of the polystyrene microbeads due to acoustic trapping were negligible. These results thus demonstrate that the single-beam acoustic trapping technique
may be a promising tool for quantifying the mechanical properties of a suspended cell, as it allows discrimination between highly and weakly invasive breast cancer cells by quantifying the
deformability of these cells due to acoustic trapping. CHANGES IN CELL VIABILITY DUE TO ACOUSTIC TRAPPING Finally, the viability of breast cancer cells was compared before and after acoustic
trapping at 1.22 MPa. Figure 4 illustrates the normalized mean viability of each type of acoustically trapped breast cancer cell before and 30 minutes after acoustic trapping. It was found
that the viability levels of MDA-MB-231, MCF-7, and BT-474 cells 30 minutes after acoustic trapping were slightly lower than those of the cells before acoustic trapping, but the difference
was not significant (p-values > 0.01). The normalized mean viability levels for the MDA-MB-231, MCF-7, and BT-474 cells were measured to be 0.943 (standard deviation: ±0.1400), 0.991
(standard deviation: ±0.057), and 0.908 (standard deviation: ±0.1406), respectively. Therefore, these results indicate that the cell functions were not significantly compromised by the force
exerted during acoustic trapping at the indicated acoustic pressure [p-value = 0.23 (MDA-MB-231), 0.64 (MCF-7), and 0.07 (BT-474) > 0.01]. DISCUSSION This paper reports that single-beam
acoustic trapping within a certain range of acoustic pressure results in the deformation of an acoustically trapped cell. We clearly observed that a breast cancer cell was deformed due to
acoustic trapping at the given acoustic pressures, whereas this was not the case for a polystyrene microbead (Supplementary Video 2). Note that the typical Young’s modulus of a polystyrene
microbead is 2.28–3.28 GPa22, much greater than that of a breast cancer cell, which has a known Young’s modulus of a few hundreds of Pa to a few kPa23. Therefore, these results clearly
indicate that the proposed trapping technique under the given condition leads to morphological deformation in a trapped cell but not in a trapped polystyrene bead, further suggesting that
deformation in trapped samples may be dependent on their mechanical characteristics. We also quantitatively compared the deformability of breast cancer cells of different phenotypes due to
acoustic trapping at different acoustic pressures. In this experiment, the applied acoustic pressures were limited to much less than ~1.64 MPa for a quantitative analysis of the
deformability of trapped cells, as we observed cell blebbing, which may have been caused mainly by decoupling of the cytoskeleton from the plasma membrane, in several acoustically trapped
cells when the cells were trapped in acoustic beams at levels exceeding ~1.64 MPa [Supplementary Video 1]. Hence, we examined the deformability of breast cancer cells due to acoustic
trapping at less than 1.22 MPa. We here observed that the area of the trapped cells increased as the applied acoustic pressure was increased to 1.22 MPa. In contrast, the polystyrene
microbead with a higher stiffness level did not exhibit any significant changes in its area as the acoustic pressure was increased. These results therefore demonstrate that the deformation
during the acoustic trapping was likely dependent on the applied acoustic pressure and on the stiffness of the trapped sample. Moreover, we could discriminate between the mechanical
properties of highly (MDA-MB-231) and weakly invasive (MCF-7 and BT-474) breast cancer cells in a suspension by comparing their deformability levels due to acoustic trapping at the given
axial acoustic pressure (Fig. 3). Previous reports showed that the Young’s modulus of the MDA-MB-231 cells was found to be 500–750 Pa while Young’s modulus of MCF-7 cells was found to be
800–1300 Pa23. In addition, MDA-MB-231 cells exhibited a larger membrane displacement than MCF-7 cells upon ARFI application17,24, indicating that MDA-MB-231 cells were softer than MCF-7
cells. In this study, the MDA-MB-231 cells exhibited greater deformability than the MCF-7 and BT-474 cells, thus indicating that MDA-MB-231 cells are softer than MCF-7 and BT-474 cells.
These results are in good agreement with the outcomes obtained by other ultrasonic17,24 and atomic microscopic techniques23,24. Note that the single-beam acoustic trapping technique assessed
here does not require additional agents such as microbeads or carbon nanotubes to quantify the mechanical properties of cells compared to earlier ultrasonic techniques. Therefore, the
present results demonstrate that the single-beam acoustic trapping technique would be very well suited for contact-free, marker-free measurements of the mechanics of a cell in a suspension.
Among other cell previously developed manipulation tools with similar functions, an optical stretcher has become well established as a contact- and marker-free tool for the measurement of
cell mechanics. This method utilizes two dual-opposed, slightly divergent, identical laser beams to trap an object and then stretch it. However, in the optical stretcher, micro-fluidic
channels are needed for a high-throughput cell analysis, and relatively high laser powers of ~800 mW for each beam are typically required to realize surface forces up to hundreds of
pico-Newtons25. In contrast, acoustic trapping with a single highly focused acoustic beam results in cell deformation, which may be caused by the squeezing of the cell due to the applied
axial acoustic pressure, unlike the stretching of a cell on an optical stretcher. Moreover, the single-beam acoustic tweezers are capable of generating stronger trapping forces up to several
nano-Newtons as compared to an optical stretcher, with a relatively simple and inexpensive setup19, suggesting that the acoustic trapping technique may be suitable for manipulation of
relatively large and stiff biological samples and for quantifications of the mechanics of such samples. In our previous study related to cancer cells18, the cell function was observed to be
intact even 24 hours after acoustic trapping force of several hundred megahertz was exerted onto breast cancer cells. Akin to the results shown in our previous studies, the acoustically
trapped cells here were still viable after acoustic trapping for five minutes at 1.22 MPa. In this study, we applied tightly focused ultrasound microbeams to the cells for five minutes in
the examination of deformability of highly and weakly invasive breast cancer cells due to acoustic trapping at different acoustic pressures. Therefore, we performed the cell viability test
after five-minute ultrasound microbeam application to the cells. Note that ultrasound microbeam application for a few seconds in the acoustic trapping of a cell at the acoustic pressure used
was sufficient to deform the cell [Supplementary Video 1]. Thus, the acoustic trapping duration required for the measurement of the cell mechanics could be reduced to much less than five
minutes. These results indicate that the mechanical properties of a cell can be measured in a considerably healthy condition with the single-beam acoustic trapping technique at the given
acoustic pressure level. CONCLUSIONS In this paper, we demonstrate that the acoustic trapping technique has the potential to become a valuable tool with which to quantify the mechanical
properties of a cell in a suspension without the need for contact or labeling. It was found that acoustic trapping results in the deformation of a trapped cell, which is likely to be caused
by the squeezing of the cell. In addition, the deformation of a trapped cell was found to depend on the applied acoustic pressure and on its mechanical properties. Measurement of the
mechanical properties of a single cell has been of increasing interest as part of the effort to gain a better understanding of various cell functions, such as motility, division, and
adhesion26. In particular, the mechanical characteristics of cancer cells have been shown to be highly correlated with their metastatic potentials in various cases6. In previous studies, it
was reported that a highly invasive cancer cell exhibited softer mechanical characteristics than a weakly invasive cancer cell23. Therefore, this unique acoustic trapping technique may be
useful for the determination of the invasion potential of cancer cells given its ability to measure the cancer cell mechanics. However, to measure absolute mechanical properties of a cell by
using the technique, it would be needed to calibrate the deformability of a cell due to acoustic trapping with cell-mimic phantoms with different mechanical properties, including Young’s
Modulus, Poisson ratio, and etc. The cell mimicking phantoms with different mechanical properties are not available at this time and future efforts will be dedicated to obtain absolute
mechanical properties of a cell by the single-beam acoustic trapping technique. METHODS AND MATERIALS CELL PREPARATION AND MATERIALS MDA-MB-231, MCF-7, and BT-474 human breast cancer cell
lines were obtained from ATCC (Manassas, VA) and were maintained in Dulbecco’s modified eagle medium (DMEM) containing 10% fetal bovine serum. Hank’s balanced salt solution (HBSS) was
purchased from Invitrogen (Grand Island, NY) to maintain the cells during the cell experiment. A trypsin-EDTA solution (Invitrogen, Grand Island, NY) in which to suspend the cells was
prepared. ACOUSTIC TWEEZER SYSTEM A press-focused LiNbO3 transducer at a center frequency of 30 MHz was fabricated with the conventional procedure described previously (Fig. 5a)27. For
fabrication of the transducer, a 36° rotated Y-cut LiNbO3 plate (LiNbO3; Boston Piezo-Optics, MA, USA) with a thickness of ~76 μm was first coated with chrome (Cr)/gold (Au) electrodes
(Cr/Au; \Nano-Master, TX, USA). The first matching layer was made from a mixture of Insulcast 501 epoxy (Insulcast 501; American Safety Technologies, PA, USA) and 2–3 μm silver particles
(Silver; Aldrich Chemical Co., MO, USA) and then lapped to a thickness of 12 μm, followed by the attachment of the layer on one side of the LiNbO3 plate. Conductive silver epoxy (E-Solder
3022; Von Roll Isola Inc., USA) was cast on the other side of the LiNbO3 for construction of a backing. The LiNbO3-matching-backing stack was concentrically placed in the brass housing. The
stack and the housing were electrically insulated by filling an epoxy (Epo-Tek 301; Epoxy Technologies, MA, USA) between them. It was press-focused and sputtered with Cr/Au electrodes. A
parylene film (14 μm) was then coated over the aperture. The aperture diameter and focal length of the transducer were 4 mm and 3 mm, respectively (_f_-number = 0.75). The lateral and axial
beam widths were measured correspondingly to be 30 μm and 200 μm (−3 dB) with a needle hydrophone (Precision Acoustics, UK), [Fig. 5(b,c)]28. In addition, two-dimensional lateral and axial
spatial peak temporal average intensity (ISPTA) levels were examined [Frequency: 30 MHz, number of cycles: 10, the pulse repetition frequency (PRF): 500 Hz] [Fig. 5(d,e)]. The acoustic beam
intensity was shown to be nearly symmetric at the focal point in the transverse direction [Fig. 5d, inset], whereas the axial beam intensity was found to be slightly non-symmetric [Fig. 5e,
inset]. The transducer was mounted on a three-axis motorized stage (LMG26 T50MM; OptoSigma, Santa Ana, CA, USA) equipped with an inverted fluorescence microscope (IX71, Olympus, Center
Valley, PA, USA) to align the focus precisely with a target suspended cell by using a pulser-receiver (5910PR; Olympus, Center Valley, PA, USA). In the adjustment of the focus with a target
suspended cell, the transducer was focused onto a Mylar film where a target cell was located and then adjusted the tilt of the transducer by examination of echo signal amplitudes from the
Mylar film. Note that the echo signal amplitude from the Mylar film became maximal when the transducer was perpendicularly focused on the Mylar film. In order to acoustically trap a target
suspended cell, 30 MHz sinusoidal bursts from a function generator (SG382; Stanford Research Systems, CA, USA) were amplified in a 50-dB power amplifier (525LA; E&I ltd., Rochester,
USA). The amplified sinusoidal bursts were then input to the transducer. The resultant peak-to-peak (V_pp_) voltages of the bursts were adjusted to 0, 4.7, 9.5, and 14.2 Vpp. The duty cycles
were tuned to 500, and PRF was 500 Hz. During the acoustic trapping of the cell, the cell deformation in the lateral direction was monitored and then recorded by a CCD camera (ORCA-Flash
2.8; Hamamatsu Photonics, Hamamatsu, Japan) attached to the microscope (Fig. 6). QUANTIFICATION OF THE MECHANICAL PROPERTIES OF A TRAPPED CELL Breast cancer cells were plated onto 35mm petri
dishes and incubated in the complete medium at 37˚C for 36 hours prior to the cell experiment. The cells were then suspended by the addition of 2ml of the trypsin-EDTA solution into the
petri dish after aspiration of the complete medium. This step was followed by the thorough washing of the cells with HBSS. The cells suspended in 2ml of HBSS were added to a 35mm petri dish
of which the bottom plate was replaced with Mylar film. A target suspended cell was then isolated from other adjacent cells by acoustic trapping of the cell. Bright-field imaging was
conducted to monitor the cell deformation due to acoustic trapping, followed by measurement of the size of the cell in the transverse direction. The size of the cell was also calculated at
different acoustic pressures [30 MHz, duty cycles: 500, PRF: 500 Hz, input voltages to the transducer: 0, 4.7, 9.5, and 14.2 Vpp (corresponding acoustic pressures: 0, 0.71, 0.97, and 1.22
MPa)]. CALCULATION OF THE DEFORMABILITY OF A TRAPPED CELL The deformability (D) was calculated according to the following equation, where Atrap and Abefore_trap represent the measured area
of the cell after and before the acoustic trapping, respectively. For a detailed examination of the changes in the deformability of the cells before and after acoustic trapping, the area of
the cells was precisely quantified using the ImageJ software package (NIH, Bethesda, Maryland, USA). CELL VIABILITY TEST Cell viability tests before and after the acoustic trapping of a
target cell were carried out in order to examine whether the applied acoustic trapping force compromised the trapped cell. For each cell viability test, a cell-permeant dye, Calcein AM
(Invitrogen, Carlsbad, CA, USA) was loaded into the cell as described previously18. After the loading of the dye into the cells, acoustic trapping of the target cell was then carried out for
five minutes. For an examination of changes in the cell viability after acoustic trapping, fluorescence imaging of the cell (Ex: 480 nm, Em: 530 nm) was performed before and 30 minutes
after the acoustic trapping of the cell. For a statistical analysis, the mean and standard deviation of the fluorescence level at each time interval were obtained with a sample size of n =
10. A two-tailed paired t-test was carried out with the level of significance set as follows: _p-value_ < 0.01. ADDITIONAL INFORMATION HOW TO CITE THIS ARTICLE: Hwang, J. Y. _et al._ Cell
Deformation by Single-beam Acoustic Trapping: A Promising Tool for Measurements of Cell Mechanics. _Sci. Rep._ 6, 27238; doi: 10.1038/srep27238 (2016). REFERENCES * Deguchi, S. & Sato,
M. _Cellular mechanotransduction: diverse perspectives from molecules to tissues_. 1st edn, (eds Mofrad, M. R. K. et al.) 220–233 (Cambridge University Press, 2010). * Engler, A. J. et al.
_Cell Mechanics_. VOL. 83 (eds Wang, Y. L. et al.) 522–544 (Academic Press, 2007). Google Scholar * Titushkin, I. & Cho, M. Regulation of cell cytoskeleton and membrane mechanics by
electric field: role of linker proteins. _Biophysical journal_ 96, 717–728, doi: 10.1016/j.bpj.2008.09.035 (2009). Article CAS ADS PubMed PubMed Central Google Scholar * Lee, G. Y.
& Lim, C. T. Biomechanics approaches to studying human diseases. _Trends in biotechnology_ 25, 111–118, doi: 10.1016/j.tibtech.2007.01.005 (2007). Article CAS PubMed Google Scholar *
Hou, H. W. et al. Deformability based cell margination–a simple microfluidic design for malaria-infected erythrocyte separation. _Lab Chip_ 10, 2605–2613, doi: 10.1039/c003873c (2010).
Article CAS PubMed Google Scholar * Xu, W. et al. Cell stiffness is a biomarker of the metastatic potential of ovarian cancer cells. _PLoS One_ 7, e46609, doi:
10.1371/journal.pone.0046609 (2012). Article CAS ADS PubMed PubMed Central Google Scholar * Rico, F. et al. Probing mechanical properties of living cells by atomic force microscopy
with blunted pyramidal cantilever tips. _Phys Rev E Stat Nonlin Soft Matter Phys_ 72, 021914, doi: http://dx.doi.org/10.1103/PhysRevE.72.021914 (2005). Article Google Scholar * Titushkin,
I. & Cho, M. Modulation of cellular mechanics during osteogenic differentiation of human mesenchymal stem cells. _Biophys J_ 93, 3693–3702, doi: 10.1529/biophysj.107.107797 (2007).
Article CAS PubMed PubMed Central Google Scholar * Titushkin, I. & Cho, M. Distinct membrane mechanical properties of human mesenchymal stem cells determined using laser optical
tweezers. _Biophys J_ 90, 2582–2591, doi: 10.1529/biophysj.105.073775 (2006). Article CAS PubMed PubMed Central Google Scholar * Zhang, H. & Liu, K. K. Optical tweezers for single
cells. _J R Soc Interface_ 5, 671–690, doi: 10.1098/rsif.2008.0052 (2008). Article CAS PubMed PubMed Central Google Scholar * Kasza, K. E., Vader, D., Köster, S., Wang, N. & Weitz,
D. A. Magnetic twisting cytometry. _Cold Spring Harb Protoc_ 2011, 421–422, doi: 10.1101/pdb.prot5599 (2011). Article Google Scholar * Fabry, B., Maksym, G. N., Hubmayr, R. D., Butler, J.
P. & Fredberg, J. J. Implications of heterogeneous bead behavior on cell mechanical properties measured with magnetic twisting cytometry. _J Magn Magn Mater_ 194, 120–125, doi:
10.1016/S0304-8853(98)00564-2 (1999). Article CAS ADS Google Scholar * Raman, A. et al. Mapping nanomechanical properties of live cells using multi-harmonic atomic force microscopy.
_Nature nanotechnology_ 6, 809–814, doi: 10.1038/nnano.2011.186 (2011). Article CAS ADS PubMed Google Scholar * Musielak, M. Red blood cell-deformability measurement: review of
techniques. _Clinical hemorheology and microcirculation_ 42, 47–64, doi: 10.3233/CH-2009-1187 (2009). Article CAS PubMed Google Scholar * Kilinc, D. & Lee, G. U. Advances in magnetic
tweezers for single molecule and cell biophysics. _Integrative biology: quantitative biosciences from nano to macro_ 6, 27–34, doi: 10.1039/c3ib40185e (2014). Article CAS Google Scholar
* Bausch, A. R., Moller, W. & Sackmann, E. Measurement of local viscoelasticity and forces in living cells by magnetic tweezers. _Biophysical journal_ 76, 573–579, doi:
10.1016/S0006-3495(99)77225-5 (1999). Article CAS ADS PubMed PubMed Central Google Scholar * Hwang, J. Y. et al. Non-contact acoustic radiation force impulse microscopy via
photoacoustic detection for probing breast cancer cell mechanics. _Biomedical optics express_ 6, 11–22, doi: 10.1364/BOE.6.000011 (2015). Article CAS PubMed Google Scholar * Hwang, J. Y.
et al. Cell membrane deformation induced by a fibronectin-coated polystyrene microbead in a 200-MHz acoustic trap. _IEEE Trans Ultrason Ferroelectr Freq Control_ 61, 399–406, doi:
10.1109/TUFFC.2014.2925 (2014). Article PubMed PubMed Central Google Scholar * Lee, J. et al. Single beam acoustic trapping. _Applied physics letters_ 95, 73701, doi: 10.1063/1.3206910
(2009). Article CAS PubMed Google Scholar * Lee, J., Lee, C. & Shung, K. K. Calibration of sound forces in acoustic traps. _IEEE transactions on ultrasonics, ferroelectrics, and
frequency control_ 57, 2305–2310, doi: 10.1109/TUFFC.2010.1691 (2010). Article PubMed PubMed Central Google Scholar * Maloney, J. M., Lehnhardt, E., Long, A. F. & Van Vliet, K. J.
Mechanical fluidity of fully suspended biological cells. _Biophysical journal_ 105, 1767–1777, doi: 10.1016/j.bpj.2013.08.040 (2013). Article CAS ADS PubMed PubMed Central Google
Scholar * Callister, W. D. & Rethwisch, D. G. _Materials Science and Engineering: An Introduction_. 9th edn, (eds Callister, W. D. et al.) Ch. 15, 580–587 (Wiley, 2013). * Swaminathan,
V. et al. Mechanical stiffness grades metastatic potential in patient tumor cells and in cancer cell lines. _Cancer research_ 71, 5075–5080, doi: 10.1158/0008-5472.CAN-11-0247 (2011).
Article CAS PubMed PubMed Central Google Scholar * Kang, B. J., Yoon, C., Park, J. M., Hwang, J. Y. & Shung, K. K. Jitter reduction technique for acoustic radiation force impulse
microscopy via photoacoustic detection. _Optics express_ 23, 19166–19175, doi: 10.1364/OE.23.019166 (2015). Article CAS ADS PubMed PubMed Central Google Scholar * Guck, J. et al. The
optical stretcher: a novel laser tool to micromanipulate cells. _Biophysical journal_ 81, 767–784, doi: 10.1016/S0006-3495(01)75740-2 (2001). Article CAS ADS PubMed PubMed Central
Google Scholar * Kirmizis, D. & Logothetidis, S. Atomic force microscopy probing in the measurement of cell mechanics. _International journal of nanomedicine_ 5, 137–145, doi:
http://dx.doi.org/10.2147/IJN.S5787 (2010). Article Google Scholar * Cannata, J. M., Ritter, T. A., Chen, W. H., Silverman, R. H. & Shung, K. K. Design of efficient, broadband
single-element (20-80 MHz) ultrasonic transducers for medical imaging applications. _IEEE transactions on ultrasonics, ferroelectrics, and frequency control_ 50, 1548–1557, doi:
10.1109/TUFFC.2003.1251138 (2003). Article PubMed Google Scholar * Huang, B. & Shung, K. K. Characterization of high-frequency, single-element focused transducers with wire target and
hydrophone. _IEEE transactions on ultrasonics, ferroelectrics, and frequency control_ 52, 1608–1612, doi: 10.1109/TUFFC.2005.1516034 (2005). Article PubMed PubMed Central Google Scholar
Download references ACKNOWLEDGEMENTS This work has been supported by DGIST and National Research Foundation of Korea (NRF) (15-01-HRLA-01, NRF-2014R1A1A2054934 and NRF-2014M3A9D7070668)
for J.Y. Hwang at DGIST, NRF (NRF-2012R1A1A1015778 and -2015R1D1A1A01060074) and the Research Grant of Kwangwoon University in 2015 for J.W. Lee at KWU and by a NIH grant (P41-EB002182) for
K.K. Shung at USC. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Information and Communication Engineering, Daegu Gyeongbuk Institute of Science & Technology, Daegu,
Republic of Korea Jae Youn Hwang, Jihun Kim & Jin Man Park * Department of Biomedical Engineering, NIH Resource Center for Medical Ultrasonic Transducer Technology, University of
Southern California, Los Angeles, CA, USA Changyang Lee, Hayong Jung & K. Kirk Shung * Department of Electronic Engineering, Kwangwoon University, Seoul, Republic of Korea Jungwoo Lee
Authors * Jae Youn Hwang View author publications You can also search for this author inPubMed Google Scholar * Jihun Kim View author publications You can also search for this author
inPubMed Google Scholar * Jin Man Park View author publications You can also search for this author inPubMed Google Scholar * Changyang Lee View author publications You can also search for
this author inPubMed Google Scholar * Hayong Jung View author publications You can also search for this author inPubMed Google Scholar * Jungwoo Lee View author publications You can also
search for this author inPubMed Google Scholar * K. Kirk Shung View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS J.Y.H developed initial
concept; J.Y.H., J.L. and K.K.S. designed research; J.Y.H., J.K., C.L., J.M.P. and H.J. performed research; J.Y.H., J.L. and K.K.S. analyzed data; J.Y.H., J.L. and K.K.S. wrote the paper.
CORRESPONDING AUTHORS Correspondence to Jae Youn Hwang or Jungwoo Lee. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing financial interests. SUPPLEMENTARY INFORMATION
SUPPLEMENTARY INFORMATION (PDF 84 KB) SUPPLEMENTARY VIDEO 1 (AVI 30939 KB) SUPPLEMENTARY VIDEO 2 (AVI 1884 KB) RIGHTS AND PERMISSIONS This work is licensed under a Creative Commons
Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the
credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of
this license, visit http://creativecommons.org/licenses/by/4.0/ Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Hwang, J., Kim, J., Park, J. _et al._ Cell Deformation by
Single-beam Acoustic Trapping: A Promising Tool for Measurements of Cell Mechanics. _Sci Rep_ 6, 27238 (2016). https://doi.org/10.1038/srep27238 Download citation * Received: 22 January 2016
* Accepted: 13 May 2016 * Published: 08 June 2016 * DOI: https://doi.org/10.1038/srep27238 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get
shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative