Simple surface engineering of polydimethylsiloxane with polydopamine for stabilized mesenchymal stem cell adhesion and multipotency
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Polydimethylsiloxane (PDMS) has been extensively exploited to study stem cell physiology in the field of mechanobiology and microfluidic chips due to their transparency, low cost
and ease of fabrication. However, its intrinsic high hydrophobicity renders a surface incompatible for prolonged cell adhesion and proliferation. Plasma-treated or protein-coated PDMS shows
some improvement but these strategies are often short-lived with either cell aggregates formation or cell sheet dissociation. Recently, chemical functionalization of PDMS surfaces has proved
to be able to stabilize long-term culture but the chemicals and procedures involved are not user- and eco-friendly. Herein, we aim to tailor greener and biocompatible PDMS surfaces by
developing a one-step bio-inspired polydopamine coating strategy to stabilize long-term bone marrow stromal cell culture on PDMS substrates. Characterization of the polydopamine-coated PDMS
surfaces has revealed changes in surface wettability and presence of hydroxyl and secondary amines as compared to uncoated surfaces. These changes in PDMS surface profile contribute to the
stability in BMSCs adhesion, proliferation and multipotency. This simple methodology can significantly enhance the biocompatibility of PDMS-based microfluidic devices for long-term cell
analysis or mechanobiological studies. SIMILAR CONTENT BEING VIEWED BY OTHERS SELECTIVE BIOFUNCTIONALIZATION OF 3D CELL-IMPRINTED PDMS WITH COLLAGEN IMMOBILIZATION FOR TARGETED CELL
ATTACHMENT Article Open access 27 July 2022 NANO-LITER PERFUSION MICROFLUIDIC DEVICE MADE ENTIRELY BY TWO-PHOTON POLYMERIZATION FOR DYNAMIC CELL CULTURE WITH EASY CELL RECOVERY Article Open
access 11 January 2023 CELL-CONTROLLED DYNAMIC SURFACES FOR SKELETAL STEM CELL GROWTH AND DIFFERENTIATION Article Open access 17 May 2022 INTRODUCTION Polydimethylsiloxane (PDMS) silicone
elastomer has been receiving much attention as a popular material for developing substrate platforms in mechanobiological1,2,3,4 and microfluidic applications5,6,7, owing to its numerous
advantages over other fabrication materials. The salient characteristics of PDMS giving rise to wide applications include its tunable elastomeric properties, low cost, gas permeability,
optical transparency, low auto fluorescence, nano-scale precision and easy moldability8,9,10. However, the use of PDMS for cell culture often poses several challenges over long-term studies.
The intrinsic high surface hydrophobicity of PDMS has been identified by many studies to be the primary factor that causes poor cell adhesion, creating and dissociating islands of cell
aggregates11,12,13. Therefore, it is highly demanded to improve the surface biocompatibility of PDMS to facilitate long-term cell studies. Earlier attempts to reduce surface hydrophobicity
by oxygen plasma treatment have shown to improve cell adhesion but the effect was often short-lived due to hydrophobic recovery14,15. While extracellular matrix protein coating could
potentially enhance cell adhesion and proliferation, cell sheet aggregation and detachment was usually observed after prolonged culture due to protein dissociation13,16. Recently, PDMS
surface functionalization with (3-aminopropyl)triethoxy silane (APTES) with glutaraldehyde as crosslinker and coupled with protein coating has shown to improve cell adhesion, proliferation16
and osteogenic differentiation over 3 weeks13. Although this surface treatment method is very effective, the process involves time-consuming intermediate steps and the human error
accumulated between each step may potentially lead to batch-to-batch inconsistencies. Furthermore, the use of toxic chemicals such as (3-Aminopropyl)triethoxysilane (APTES) and
glutaraldehyde poses potential health hazards and generate chemical wastes that are toxic to the environment. Therefore, a simple, environmentally safe and effective surface
functionalization strategy is crucial for rendering biocompatible PDMS surfaces for long-term cell investigation on PDMS-based lab-on-a-chip devices. In recent years, the bio-inspired
polydopamine (PD) has been widely utilized in the areas of energy, environmental and biomedical sciences17. Dopamine undergoes oxidative polymerization in alkaline conditions and has a
strong adsorption onto a wide variety of substrates through covalent bonding and strong intermolecular interactions18,19. Investigations have shown that PD coating reduces substrate surface
hydrophobicity19 and promotes _in-vitro_ tissue development20,21,22. More importantly, PD has been shown to reduce the _in-vivo_ toxicity of implanted biomaterials and thus recommended as a
surface coating reagent for cell studies23. Nevertheless, the feasibility of PDMS surface functionalization with PD for long-term stem cell culture remains unclear. Bone marrow stromal cells
(BMSCs) are unspecialized stem cells that originate from the mesoderm with self-renewal and multilineage differentiation ability. Furthermore, BMSCs pose minimal issues with regard to
ethical concerns or teratoma formation as compared to other types of stem cells, such as embryonic stem cells and induced pluripotent stem cells24. These intrinsic features of BMSCs have
made them a promising cell source for stem cell therapy and regenerative medicine, thus increasing the number of clinical trials involving BMSCs24. To date, many fundamental understandings
of BMSCs still remain vague and require extensive studies to resolve numerous scientific questions raised in the attempts of real applications. The regular studies on the differentiation of
BMSCs often span over a duration of at least 3 weeks. However, the native PDMS surface property as described previously has limited the experimental studies of BMSC differentiation in
microfluidic lab-on-a-chip devices due to its inability to support long-term cell culture. Inspired by the numerous advantages of polydopamine, it is of great interest to investigate the
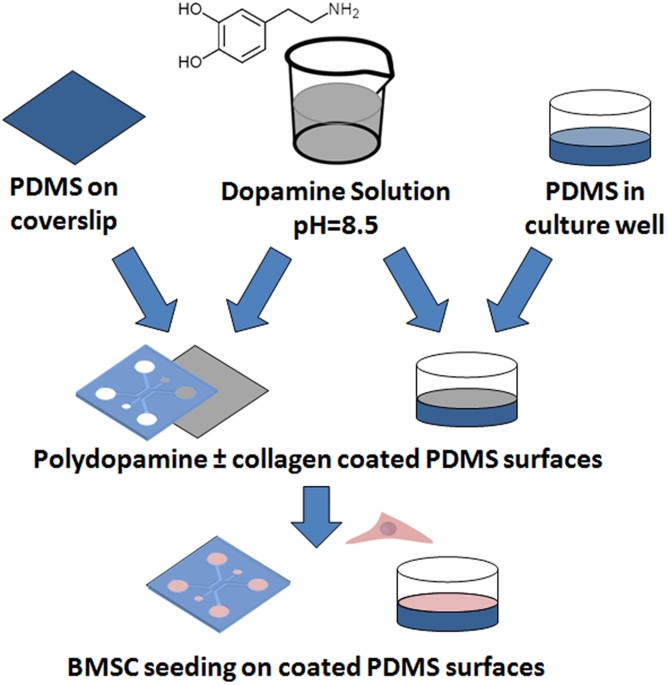
efficiency of PDMS substrate functionalization to stabilize long-term cell culture, especially in the field of BMSC-based cell studies. The PD concentration, coating time, the resulting
wettability and surface chemistry on the PD-coated PDMS are experimentally characterized to obtain fundamental insights into this surface treatment and its effect on the multipotency and
stability of BMSC culture over several weeks (Fig. 1). The PD-based surface modification can be utilized as a simple and efficient alternative to promote long-term BMSC culture while
maintaining their multipotency on the PDMS substrates. RESULTS AND DISCUSSION CONCENTRATION AND TIME OF PD COATING PD has been recently exploited to improve cell behaviour on various
substrates25,26,27,28. Nevertheless, the effect of PD concentration and coating time on the stability of BMSC growth remains poorly understood when functionalizing native PDMS substrates,
which is vital to develop a surface modification strategy for stable and long-term BMSC culture. To address this important issue, a series of PD concentrations increasing from 0 to 1.0%
(w/v) was coated on the native PDMS substrates for 24 h and assessed for the initial cell adhesion as well as the prolonged cell proliferation. When the native PDMS surface was coated with
PD at various concentrations, we observed at least a 40-fold increase in cell adhesion (p-value = 6 × 10−14, one way ANOVA) as compared to the uncoated PDMS surfaces (Fig. 2A and
Supplementary Fig. S1A), which was a strong evidence suggesting that the presence of PD facilitated initial cell adhesion. On the native uncoated PDMS surface, cell population did not
increase in 2 weeks (Fig. 2B) and islands of cell clumps were observed in the culture (Fig. 2C). Similarly, cell proliferation on PDMS coated with more than 0.1% (w/v) PD was not optimal
(Supplementary Fig. S1B). Although the cell proliferation was enhanced on PDMS surfaces coated with 0.025–0.05% (w/v) PD (p value = 1 × 10−4, one way ANOVA) for the first 10 days, this
promoting effect was however short-lived showing decrease in cell population from Day 11 onwards (Fig. 2B and Supplementary Fig S1B). Live-Dead Staining revealed minimal cell death on all
PD-coated PDMS (Supplementary Fig. S2), suggesting that the PD coating was not toxic to the BMSCs. This in turn implied that the reduction of cell proliferation after Day 10 might be related
to unstable cell adhesion. The decrease in cell population on PDMS coated with 0.025% and 0.050% (w/v) PD was further confirmed by microscopic imaging, which revealed sites of cell
aggregation and peeling-off upon confluence (Fig. 2C and Supplementary Fig. S3). On the other hand, the cell population on PDMS coated with more than 0.100% (w/v) PD did not reach confluence
(Fig. 2C and Supplementary Fig. S3) after 2 weeks of culture. These results hereby indicated that PD coating concentrations higher than 0.025% (w/v) were not able to maintain a stable
long-term BMSC culture on the PDMS surface. Amongst the various concentrations studied, only 0.01% (w/v) PD-coated PDMS surface exhibited continuous enhancement to the cell proliferation
(Fig. 2B, p-value < 1 × 10−4, one way ANOVA) with stabilized and confluent BMSC cell sheet for over 2 weeks (Fig. 2C). The coating time of 0.01% (w/v) PD varied from 0 to 24 h to
investigate its effect on both BMSC adhesion and proliferation. The results indicated at least 80-fold increase in initial cell adhesion as compared to native PDMS (p-value = 5.45 × 10−10,
one way ANOVA) if the coating time exceeded 1 h (Fig. 3A). Although the highest cell adhesion was observed on the PDMS with prolonged PD coating of 24 h, faster coating between 1 to 8 h did
not show significantly different effects. Interestingly, the long-term BMSC proliferation rate over 2 weeks was promoted on all PD-coated PDMS while not sensitive to the coating time (Fig.
3B, p-value = 1.35 × 10−9, one way ANOVA). PD COATING IN COMBINATION WITH COLLAGEN COATING The extracellular matrix plays many critical biological roles29,30,31 and has been shown to be
involved in cell adhesion32,33,34 and differentiation29,35. It is very common to coat the substrate with extracellular matrix proteins to provide amicable microenvironment for better cell
growth. Collagen is one of the most abundant proteins in the mammalian tissues and has been widely utilized as a coating material to improve the biocompatibility of various culture
substrates29,30,32,33,34,35 and the stem cell differentiation29,35. Nevertheless, conventional collagen coating by physical adsorption on PDMS surfaces was not optimistic in supporting
long-term cell culture due to formation of cell clusters and cell sheet dissociation after confluence13,16. This was further confirmed by our experimental observation (Fig. 4). As shown
above, PD coating for 1–24 h could improve cell adhesion and proliferation on PDMS (Fig. 3). It is thus essential to investigate if prior surface treatment with PD coating could also enhance
the performance of collagen coating on BMSC behaviour. Herein the effect of collagen coating combined with either 1 h or 24 h PD coating on BMSCs was further investigated. In a control
experiment, collagen coating on native PDMS had shown to enhance initial cell adhesion (Fig. 4A). However, the long-term cell growth with collagen coating only was not optimal (Fig. 4B) with
formation cell clusters that could cause cell dissociation easily (Fig. 4C). Subsequent collagen coating on PD-coated PDMS significantly improved cell adhesion (p-value = 0.001, Tukey HSD
test) and proliferation rate of BMSCs (p-value < 3.67 × 10−10, Tukey HSD test) as compared to both native PDMS and collagen-coated PDMS (Fig. 4), although the PD coating time did not show
notable different effect between 1 h and 24 h. These results suggested that PD coating was compatible and could be used in combination with popular surface functionalization with matrix
proteins. SURFACE CHARACTERIZATION The cell-surface interaction plays an important role in mediating BMSC adhesion, proliferation and differentiation36,37,38, which may explain the improved
MSC behaviour on the PD-coated PDMS observed above. Thus, it is imperative to characterize the surface properties to gain fundamental insights on the cell-surface interaction. There are
numerous studies highlighting the inherent hydrophobicity of PDMS as one of the major factors to cause poor maintenance of stable cell culture11,12,13. Modifying the hydrophobic surfaces to
hydrophilic can potentially promote cell adhesion and proliferation. Uncoated PDMS exhibited a water contact angle of 116.7 ± 2.21o (Fig. 5A). Increasing the PD concentration resulted in
decreased contact angle (40–70o, p value < 0.001, Tukey HSD test) on the coated PDMS surfaces (Fig. 5A). All these surfaces had shown to improve initial cell adhesion, while only 0.010%
(w/v) PD-coated PDMS surface was able to prolong the stability of BMSC proliferation over 2 weeks (Fig. 2B,C). The study using various coating time with 0.010% (w/v) PD from 1 to 24 h showed
that prolonged coating of 24 h significantly reduced the water contact angle (p-value = 0.001, Tukey HSD test) and changed the surfaces from hydrophobic to hydrophilic (Fig. 5B), which
could be the major reason that encouraged the initial cell adhesion (Fig. 3A). Interestingly, although 0.010% (w/v) PD coating between 1 to 8 h showed similar hydrophobicity profile as
compared to the uncoated PDMS surface (Fig. 5B), all these PD-coated hydrophobic surfaces promoted both BMSC adhesion and proliferation (Fig. 3). These findings suggested that surface
wettability might not be a critical factor that contributed to the improved behaviour of BMSCs in this study. The subsequent collagen coating after 24 h PD coating significantly increased
the PDMS surface hydrophobicity (Fig. 5C, p-value = 0.001, Tukey HSD test) but did not influence initial BMSC adhesion and proliferation notably (Fig. 4A,B). Surface chemistry is another
major factor that could influence cell behaviour37. Thus, we further evaluated the surface chemistry profile of the PD-coated PDMS with energy dispersive X-ray spectroscopy (EDS). The carbon
region scan of PD-coated PDMS obtained from EDS revealed higher relative loading of carbon (Fig. 5D), which implicated the successive coating due to the presence of organic PD molecules. As
native PDMS lacks the nitrogen atoms, the detection of nitrogen atoms on various PD-coated PDMS further confirmed the existence of PD (Fig. 5E) due to the presence of amine group. Prior
studies have suggested that the cell adhesion and growth can be possibly enhanced by the presence of free amine group39,40, which can be detected by Ninhydrin-based colorimetric assay41,42.
Herein we found a linear relation between the absorbance at 570 nm and the PD concentration (0−0.2%) using ninhydrin reagent for detecting free amine groups in solution (Supplementary Fig.
S4). Furthermore, the absorbance analysis on the PD-coated PDMS surfaces under various coating concentrations displayed a distinct peak at 0.100% (w/v) (Fig. 5F) and EDS also verified that
the highest amount of nitrogen appeared under this PD coating concentration (Fig. 5E). Particularly, the absorbance varied almost linearly to the PD coating concentration up to 0.1% (Fig.
5F). These results indicated that 0.1% (w/v) coating concentration could maximize the absorption of PD on native PDMS. Considering the previous results, it was implied that the presence of
amine groups could generally enhance initial cell adhesion as compared to the native PDMS (Fig. 2A), whereas the stability of BMSC culture was more favourable on surfaces with lower amine
contents (Fig. 2B). When the coating time of 0.01% PD was reduced to 1–8 h, the surface hydrophobicity did not change notably (Fig. 5B) while the coated surfaces exhibited similar promoting
effect on cell proliferation for long-term BMSC culture (Fig. 3B). These results suggested that the presence of amine groups had predominant effect over surface wettability for the enhanced
BMSC adhesion and proliferation. BMSC DIFFERENTIATION AND MULTIPOTENCY BMSC differentiation was widely investigated on various substrate platforms for more than 2 weeks43,44,45,46. It is
vital that the PD-coated PDMS surfaces can maintain the prolonged stability of cells adhesion and capability of BMSC multi-lineage differentiation in long-term induced differentiation
culture. The adhesion stability of cell population on the substrates mainly depends on the cell-substrate interaction through adhesins, such as integrins47,48. To understand the underlying
mechanism of the interaction between BMSCs and the PD-coated surfaces, we further analysed the expression of β1-integrin (CD29) and α5-integrin (CD49e) though real time gene expression. The
differentiated cells on unmodified surfaces (without collagen and PD coating) were not analysed due to the absence of stabilized cell population. β1-integrin and α5-integrin were observed to
be upregulated on most PD-coated PDMS as compared to PDMS simply coated with collagen in both osteogenic and adipogenic induced BMSCs (Supplementary Fig. S5), thus suggesting that the
presence of polydopamine could upregulate β1-integrin and α5-integrin to mediate the enhanced cell adhesion on PDMS substrate. Meanwhile, additional coating of collagen on PD-coated PDMS did
not show significant influence on the expression of β1-integrin but demonstrated significant upregulation of α5-integrin expression on 24 h PD-coated PDMS (p-value = 0.0395, Tukey HSD test,
Supplementary Fig. S5). To further verify the effect of cell adhesion stability on MSC differentiation, MSCs on different PDMS surfaces were subjected to either osteogenic or adipogenic
differentiation. Previous studies have demonstrated that increased β1-integrin and α5-integrin can enhance osteogenic and adipogenic differentiations of MSCs through the
phosphoinositide-3-kinase (PI3K) signalling pathway49,50,51,52. Particularly, the osteogenic capability of MSCs stimulated by PD film could be reduced in the presence of a potent PI3K
inhibitor (LY294002)51, which further implicated that PD-coated surfaces favoured MSC osteogenesis through the activation of both integrin and PI3K signalling pathways. In the present study,
the gene expression of phosphoinositide-3-kinase, catalytic, gamma polypeptide (PI3KCG) within MSCs cultured on PD-coated PDMS surfaces was found to be considerably higher (Supplementary
Fig. S5), which was consistent to the prior studies and verified the favoured MSC differentiations through promoted PI3K signalling pathway. Alizarin red staining was used to detect the
presence of brick-red nodules, which could indicate the bio-mineralization of BMSCs after 3 weeks of osteogenesis on different surfaces (Fig. 6A). On the other hand, Oil Red O staining was
used to identify the accumulation of triglyceride after 3 weeks of adipogenesis, which was presented as red oil droplets within the adipogenic cells (Fig. 6B). The native PDMS surface was
reported to discourage the stabilization of differentiated cell population13. This issue was also verified in this study for both osteogenic and adipogenic cell populations (C1− and Plain in
Fig. 6A,B). Coating the native PDMS substrates only with collagen type 1 (C1) exhibited minimal positive histological stain in both osteogenic and adipogenic lineages indicating attenuated
multipotency of the cell population (C1 + and Plain in Fig. 6A,B) due to their poor adhesion stability (Fig. 4C). In contrast, a layer of stabilized osteogenic or adipogenic cell population
was formed after 3 weeks of culture on the PDMS coated with 0.01% (w/v) PD (Fig. 6A,B). A previous study reported that surface amine groups can promote osteogenic lineage53, which was
consistent with our finding that PD-coated PDMS promoted BMSC osteogenesis considering the abundant amine groups contained in PD. With respect to the PD coating time, although prolonged
coating (24 h) enhanced BMSC adhesion and proliferation (Fig. 3) and reduced PDMS hydrophobicity significantly (Fig. 5B,C), the osteogenic lineage exhibited stronger stability (Alizarin Red
staining) on the surface under 1 h fast coating (Fig. 6A). These results were consistent to the gene expression by real-time PCR assays, which revealed that although Col1 expression was
upregulated on PDMS with either prolonged or fast PD coating (Supplementary Fig. S6), the other major osteogenic marker ALP expression was much higher under fast PD coating (Fig. 6C). It was
reported that the hydrophobicity of a biomaterial was able to encourage bone healing by promoting adherence of cells and adsorption of proteins54, which might explain our observation that
the hydrophobic PD-coated surface (with shorter coating time) facilitated better bio-mineralization. Real-time gene expression analysis further suggested that fast PD coating rendered a PDMS
surface that facilitated a higher stabilization of cell adhesion and osteogenic capability through the promotion of α-5 integrin expression and PI3K signalling pathway (p-value < 0.0384,
Tukey HSD test, Supplementary Fig. S5). Meanwhile, there was no much difference in Oil Red staining between 1 h and 24 h PD-coated PDMS (Fig. 6B) and the leptin expression on all PD ± C1
coated PDMS was upregulated as compared to C1-coated PDMS (Fig. 6C) in adipogenic lineage. This implied that both hydrophobic and hydrophilic PD coated PDMS could support adipogenic lineage
in long-term culture. We also investigated the effect of secondary coating with matrix proteins on the BMSC differentiation. It was found that the secondary coating with C1 on PD-coated
(0.010% w/v, 24h) PDMS improved the stabilization of osteogenic lineage (Fig. 6A) and increased alkaline phosphatase (ALP) gene expression (Fig. 6C), while having no such promoting effect on
the adipogenic lineage (Fig. 6B,C). The differentiated cells on unmodified surfaces were not analysed in the study due to the lack of stabilized cell population. The results suggested that
the surface chemistry and hydrophobicity had a combinatorial effect to stabilize and promote BMSC differentiation. Additionally, rapid PD coating of 1 h was sufficient to render a
biocompatible PDMS surface for prolonged study of BMSCs. It is noteworthy that PDMS could adsorb a variety of chemical components introduced by some common supplements (e.g., 10% FBS) to the
cell culture medium, such as amino acids, growth factors, vitamins and hormones55, which in turn could potentially alter the PDMS surface chemistry and interfere with the PD coating.
However, as indicated in this study, the promoting effect of PD coating to the stabilization of cell adhesion, proliferation and multipotency was not affected as compared to the non-coated
and collagen-coated PDMS in the presence of FBS-based culture medium. Therefore, these results suggested that the adsorption of a variety of chemical components contained in the culture
supplements onto the PDMS substrate did not interfere with the PD coating for stabilized cell culture. Moreover, previous studies have also shown that the use of supplemented culture medium
on modified PDMS substrates with (3-aminopropyl)triethoxysilane + glutaraldehyde + C1 did not deteriorate the MSC adhesion, proliferation and differentiation as compared to the commercial
tissue culture plates13,16. PD COATING FOR MICROFLUIDIC CHIPS There is an increasing demand for long-term stem cell studies using PDMS-based microfluidic systems56,57,58, which however has
been limited by the inherent properties of native PDMS. Previously, many microfluidic devices with multi-channel design were used to achieve separation and confinement of different cells
types in different compartments for on-chip studies of co-culture, cell migration and other cellular responses7,59,60. As shown above, PD-coated PDMS was able to support long-term BMSC
differentiation. Hereby we further investigated if the same surface modification could be applied to an enclosed 3-channel microfluidic system with minimal supply of culture medium and thus
allowing prolonged study of stem cell biology within a microfluidic chip. Similar to the open PDMS substrates, the native PDMS surface did not encourage the proliferation of BMSCs (Fig. 7A)
in the microfluidic system and thus was unfavourable for both osteogenic and adipogenic differentiations (Fig. 7B,C). While simple collagen coating improved the proliferation of BMSCs in the
microfluidic system, histological staining indicated minimal region on the substrate to stabilize osteogenic or adipogenic differentiation of BMSCs. 1 h PD coating further enhanced the BMSC
proliferation and notably improved the osteogenesis. Nevertheless, combining PD and collagen coatings rendered a microfluidic system with optimal BMSC proliferation, osteogenic or
adipogenic differentiation as compared to single coating with only one of the reagents. CONCLUSIONS Surface modification with PD and/or C1 was investigated in this study to improve the
biocompatibility of PDMS substrates for long-term BMSC culture. Although increasing the PD coating concentration and time could reduce the hydrophobicity of PDMS, a hydrophobic PDMS surface
coated with 0.01% PD for 1 h was shown to induce the optimal enhancement of BMSC adhesion, proliferation, stability and differentiations in long-term culture. These results suggested that
modification of PDMS surface chemistry by PD and matrix protein coatings could have predominant effects on the BMSC culture compared to the variation of hydrophobicity. This simple and rapid
surface treatment is applicable to open PDMS substrates as well as enclosed PDMS-based microfluidic systems, which can be widely applied in many _in-vitro_ studies of cellular physiology,
especially in the areas of regenerative medicine and mechanobiology. METHODS MATERIALS Laboratory chemicals were purchased from Sigma Aldrich (Singapore) and cell culture reagents were
purchased from Life Technologies (Singapore) unless stated otherwise. PDMS SUBSTRATE FABRICATION PDMS substrates were prepared by mixing ten parts of silicone elastomer base with one part of
curing agent (SYLGARD, Dow Corning, USA) and stirred. The mixture was then poured into well plates or culture dishes, degassed for 30 min in a vacuum oven to remove air bubbles, followed by
heat-curing at 70 °C for 100 min. Surface modification was performed by immersing the native PDMS surface in dopamine solution (Sigma Aldrich, Singapore) prepared in 10 mM Tris-HCl (pH
8.5). PD concentration (0.000%w/v–0.100%w/v) and duration of coating (0–24 h) were varied to investigate the effect of these parameters on the stability of BMSC adhesion and proliferation.
After PD coating, the surfaces were rinsed twice with 1X Phosphate Buffered Saline (PBS) to remove unattached PD molecules. Additional coating of 20 μg/ml collagen type 1 (Life Technologies,
Singapore) was performed to evaluate the combinatorial effect of PD and collagen on BMSC behaviour. Lastly, the uncoated and PD-coated PDMS substrates were UV-sterilized for 1 h prior to
cell culture. MESENCHYMAL STEM CELL CULTURE Human BMSCs were harvested under an IRB-approved protocol as described previously61. Primary BMSCs were allowed to grow till confluence in
expansion medium comprising low glucose Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% Fetal Bovine Serum (FBS), Penicillin (100 U/ml) and Streptomycin (100 μg/ml) mixture and
2 mM GlutamaxTM at 37 °C in humidified atmosphere with 5% CO2. BMSCs of passages 2−4 were used in the experiments for this study. CELL ADHESION ASSAY 3000 cells per cm2 was seeded and
maintained in expansion medium at 37 °C in humidified atmosphere with 5% CO2. For cell adhesion assay, samples were collected 1 h after cell seeding, washed twice with 1X PBS to remove
non-adherent cells. The adherent cells was frozen at −80 °C for 1 h, thawed and subsequently lysed with a cell lysis buffer comprising 1X CyQUANT GR Dye from the CyQUANT Cell Proliferation
Assay Kit (Life Technologies, Singapore) for 5 min. Fluorescence intensity of sample aliquots were measured with Infinite M200 series microplate reader (Tecan, Singapore) under excitation at
485 nm and emission at 535 nm, which reflected the relative amount of DNA in each sample. As the DNA content was constant within each cell, this measurement provided a simple and
deterministic means to correlate the total DNA content to the cell numbers and thus provided an accurate quantitation of the adhered cells on the surface. The reading was expressed as a fold
difference relative to the fluorescence intensity to the plain PDMS. CELL PROLIFERATION ASSAY AND FLUORESCENCE IMAGING The cell proliferation activity of BMSCs on uncoated and coated PDMS
surfaces were assessed with PrestoBlue cell viability reagent (Life Technologies, Singapore) according to the manufacturer’s protocol. Briefly, the expansion medium was removed and the cells
were washed twice with 1X PBS before incubating with expansion medium containing 10% PrestoBlue reagent for 1 h at 37 °C in humidified atmosphere with 5% CO2. Expansion medium containing
10% PrestoBlue reagent in the wells with no cells served as the blank control for this assay. The absorbance of the reduced PrestoBlue reagent for each sample aliquot was read with Infinite
M200 series microplate reader under excitation at 570 nm and emission at 600 nm. As the total viable cells correlated with the PrestoBlue dye reduction level, the percentage reduction of the
PrestoBlue reagent was calculated according to the manufacturer’s protocol. To visualize the BMSC populations on different PDMS surfaces, cell cultures after two weeks were fixed with 10%
formalin for 20 min, permeabilized with 0.1% Triton X-100 and stained with rhodamine phalloidin (for F-actin localization) and DAPI (for nucleus localization). To assess the viability of the
BMSCs on different PDMS surfaces, the unfixed cell population was directly stained with 20 ug/ml fluorescein diacetate (FDA) (Sigma Aldrich, Singapore) and 100 ug/ml propidium iodide (PI)
(Sigma, Singapore). Fluorescence images of the cells were captured with an inverted microscope (Olympus IX71, Singapore). SURFACE CHARACTERIZATION Uncoated and PD-coated PDMS surfaces were
assessed for wettability and surface chemical composition. All samples were assessed with the same sampling size (n = 4) to ensure the reproducibility of the production process. The water
contact angles were measured with a FTA 200 Contact Angle Analyzer (First Ten Angstroms, Virginia, US). The surface chemical composition was analysed using energy dispersive X-ray
spectroscopy (EDS). Briefly, PDMS samples were sputter-coated with a layer of platinum and analyzed with EDS (X-ManN, Oxford Instruments, Oxfordshire, UK) equipped with JSM-6701F Field
Emission Scanning Electron Microscope (JEOL, Singapore). The samples were measured at a magnification of 500X, with beam current and ion energy fixed at 11 μAh and 15 kV respectively. For
each sample surface, three different points were scanned and averaged to obtain the atomic composition of carbon, oxygen and nitrogen. Free amine groups were quantified by immersing the
uncoated and PD-coated PDMS with 2% Ninhydrin Reagent at 70 °C for 90 min, followed by measuring the absorbance at 570 nm. The correlation of free amine group within a concentration range of
polydopamine solution was also determined with ninhydrin assay. MICROFLUIDIC CHIP FABRICATION The photomask for chip fabrication was designed with AUTOCAD 2012 and printed on
high-resolution transparency films (CAD/Art Services Inc., Oregon, US) which was later used in contact photolithography to produce a master on a 4-inch silicon wafer. In photolithography,
SU8-2050 photoresist was spin-coated at 1500 rpm to create an evenly spread film, soft baked to evaporate the solvent and densified before being covered by the photomask and exposed to
highly collimated UV light. After UV exposure, the SU-8 was further subjected to post exposure baking to selectively cross-link the exposed portion of the photoresist. The non-exposed
portion of the film was etched away by immersing in a developer solution. PDMS microfluidic chip was fabricated by soft lithography whereby ten parts of silicone elastomer base was mixed to
one part of curing agents, poured over the master, degassed by vacuum and cured at 70 °C for 2 h before the solidified PDMS was peeled off from the master. The PDMS microfluidic chip was
plasma-treated prior to bonding with the uncoated and PD-coated PDMS. Bonded microfluidic chips were UV sterilized before BMSC seeding. OSTEOGENIC AND ADIPOGENIC DIFFERENTIATION 3000 cells
per cm2 were seeded and cultured for 1 day in expansion medium before being subjected to osteogenic or adipogenic differentiation. For osteogenic differentiation, BMSCs were cultured in
osteogenic medium comprising low glucose DMEM, 10% FBS, 1 mM sodium pyruvate, 50 μg ml-1 ascorbic acid, 1X glutamax, 100 U/100 μg penicillin/streptomycin, 10 mM β-glycerophosphate and 10−7 M
dexamethasone. For adipogenic differentiation, BMSCs were cultured in adipogenic medium comprising high glucose DMEM, 10% FBS, 0.01 mg ml−1 Insulin, 0.5 mM IBMX, 0.2 mM Indomethacin, 10−6
Dex, 1X glutamax and 100 U/100 μg penicillin/streptomycin. All BMSCs were cultured at 37 °C in humidified atmosphere with 5% CO2 with the differentiation medium changed every 2-3 days. GENE
EXPRESSION Differentiated BMSCs after two weeks of osteogenic or adipogenic differentiation were collected for specific differentiated lineage gene expression. The total RNA was harvested
with PureLink (R) RNA Mini Kit and its concentration was quantified with Nanodrop ND2000 (Thermo Scientific, Delaware, USA). 100 ng of total RNA was reverse transcripted with iScript™
Reverse Transcription Supermix kit (Biorad Laboratories, Singapore). mRNA expression level of α5-integrin, β1-integrin, PI3KCG, osteogenesis- and adipogenesis-associated gene markers were
quantified by real time polymerase chain reaction (PCR) assays with SYBR PCR Master Mix Kit (Life Technologies, Singapore) and StepOnePlus™ Real Time PCR Systems (Life Technologies,
Singapore). The primers used to quantify the specific gene expression were alkaline phosphatase (ALP) and collagen type 1 (Col1) for osteogenic differentiated cells and Leptin for adipogenic
differentiated cells (Table S1). The real-time PCR was initiated at 95 °C for 10 min, followed by 40-cycle amplification that included a denaturation step at 95 °C for 15 s and an extension
step at 60 °C for 1 min. All data were normalized to the GAPDH mRNA level and later expressed as the mRNA relative change with reference to the BMSCs prior to differentiation using the
Livak method. HISTOLOGICAL STAINING The differentiated BMSCs were fixed with 10% formalin overnight prior to histological staining. For the assessment of osteogenesis on different surfaces,
fixed BMSCs were immersed in Alizarin red solution for 5 min followed by gentle washing with distilled water until nonspecific staining was removed. For the assessment of adipogenesis on
different surfaces, fixed BMSCs were washed with 70% ethanol, stained with Oil Red O solution and counterstained with Haematoxylin. All microscopic images were captured with an Olympus IX71
inverted microscope (Olympus, Singapore). All macroscopic images were captured with a Canon 50D DSLR Camera (Canon, Singapore). STATISTICAL ANALYSIS Statistical analysis of all quantitative
data was performed by one way analysis of variance (ANOVA), while pairwise comparison of data was determined by Tukey’s HSD (Honestly Significant Difference) test. Statistical significance
was set at p-value < 0.05. ADDITIONAL INFORMATION HOW TO CITE THIS ARTICLE: Chuah, Y. J. _et al_. Simple surface engineering of polydimethylsiloxane with polydopamine for stabilized
mesenchymal stem cell adhesion and multipotency. _Sci. Rep_. 5, 18162; doi: 10.1038/srep18162 (2015). REFERENCES * Brown, X. Q., Ookawa, K. & Wong, J. Y. Evaluation of
polydimethylsiloxane scaffolds with physiologically-relevant elastic moduli: interplay of substrate mechanics and surface chemistry effects on vascular smooth muscle cell response.
Biomaterials 26, 3123–3129 (2005). Article CAS Google Scholar * Park, J. Y., Yoo, S. J., Lee, E. J., Lee, D. H. & Young, J. Increased poly(dimethylsiloxane) stiffness improves
viability and morphology of mouse fibroblast cells. BioChip J. 4, 230–236 (2010). Article CAS Google Scholar * Palchesko, R. N., Zhang, L., Sun, Y. & Fenberg, A. W. Development of
Polydimethylsiloxane Substrates with Tunable Elastic Modulus to Study Cell Mechanobiology in Muscle and Nerve. PLoS One 7, e51499-1–e51499-13 (2012). Article ADS Google Scholar * Chuah,
Y. J., Wu, Y., Yang, Z., Lee, E. H. & Kang, Y. Geometrical, Topographical and Mechanical Cues on Stem Cell Fate in a Micro-/Nano-environment. Encyclopedia of Microfluidics and
Nanofluidics, 1–8, doi: 10.1007/978-3-642-27758-0_1739-7 (2014). * Chung, S. et al. Cell migration into scaffolds under co-culture conditions in a microfluidic platform. Lab Chip 9, 269–275
(2009). Article CAS Google Scholar * Huh, D., Hamilton, G. A. & Ingber, D. E. From 3D cell culture to organs-on-chips. Trends Cell Biol. 21, 745–754 (2011). Article CAS Google
Scholar * Menon, N. V., Chuah, Y. J., Cao, B., Lim, M. & Kang, Y. A microfluidic co-culture system to monitor tumor-stromal interactions on a chip. Biomicrofluidics 8, 064118 (2014).
Article Google Scholar * Duffy, D. C., McDonald, J. C., Schueller, O. J. A. & Whitesides, G. M. Rapid prototyping of microfluidic systems in poly(dimethylsiloxane). Anal. Chem. 70,
4974–4984 (1998). Article CAS Google Scholar * McDonald, J. C. & Whitesides, G. M. Poly(dimethylsiloxane) as a material for fabricating microfluidic devices. Acc. Chem. Res. 35,
491–499 (2002). Article CAS Google Scholar * Mata, A., Fleischman, A. J. & Roy, S. Characterization of polydimethylsiloxane (PDMS) properties for biomedical micro/nanosystems. Biomed.
Microdevices 7, 281–293 (2005). Article CAS Google Scholar * Lee, J. N., Jiang, X., Ryan, D. & Whitesides, G. M. Compatibility of mammalian cells on surfaces of
poly(dimethylsiloxane). Langmuir 20, 11684–11691 (2004). Article CAS Google Scholar * Fuard, D., Chevolleau, T. T., Decossas, S., Tracqui, P. & Schiavone, P. Optimization of
poly-di-methyl-siloxane (PDMS) substrates for studying cellular adhesion and motility. Microelectron Eng. 85, 1289–1293 (2008). Article CAS Google Scholar * Chuah, Y. J., Kuddannaya, S.,
Lee, M. H. A., Zhang, Y. & Kang, Y., The Effects of Poly(dimethylsiloxane) Surface Silanization on Mesenchymal Stem Cell Fate. Biomater. Sci. 3, 383–390 (2015). Article CAS Google
Scholar * Hillborg, H. & Gedde, U. W. Hydrophobicity recovery of polydimethylsiloxane after exposure to corona discharges. Polymer 39, 1991–1998 (1998). Article CAS Google Scholar *
Tan, S. H., Nguyen, N. T., Chua, Y. C. & Kang, T. G. Oxygen plasma treatment for reducing hydrophobicity of a sealed polydimethylsiloxane microchannel. Biomicrofluidics 4, 32204 (2010).
Article Google Scholar * Kuddannaya, S. et al. Surface chemical modification of Poly(dimethylsiloxane) surface for enhanced adhesion and proliferation of Mesenchymal Stem Cells. ACS Appl.
Mater. Inter. 5, 9777–9784 (2013). Article CAS Google Scholar * Liu, Y., Ai, K. & Lu, L. Polydopamine and Its Derivative Materials: Synthesis and Promising Applications in Energy,
Environmental and Biomedical Fields. Chem. Rev. 114, 5057–5115 (2014). Article CAS Google Scholar * Lee, H., Dellatore, S. M., Miller, W. M. & Messersmith, P. B. Mussel-Inspired
Surface Chemistry for Multifunctional Coatings. Science 318, 426–430 (2007). Article CAS ADS Google Scholar * Yang, F. K. & Zhao, B. Adhesion Properties of Self-Polymerized Dopamine
Thin Film. Open Surf. Sci. J. 3, 115–122 (2011). Article CAS ADS Google Scholar * Tsai, W. B., Chen, W. T., Chien, H. W., Kuo, W. H. & Wang, M. J. Poly(dopamine) coating of scaffolds
for articular cartilage tissue engineering. Acta. Biomater. 7, 4187–4194 (2011). Article CAS Google Scholar * Ko, E., Yang, K., Shin, J. & Cho, S. W. Polydopamine-assisted
osteoinductive peptide immobilization of polymer scaffolds for enhanced bone regeneration by human adipose-derived stem cells. Biomacromolecules 14, 3202–3213 (2013). Article CAS Google
Scholar * Tsai, W. B., Chen, W. T., Chien, H. W., Kuo, W. H. & Wang, M. J. Poly(dopamine) coating to biodegradable polymers for bone tissue engineering. J. Biomater. Appl. 28, 837–848
(2014). Article Google Scholar * Hong, S. et al. Attenuation of the _in vivo_ toxicity of biomaterials by polydopamine surface modification. Nanomedicine 6, 793–801 (2011). Article CAS
Google Scholar * Wei, X. et al. Mesenchymal stem cells: a new trend for cell therapy. Acta Pharmacol. Sin. 34, 747–754 (2013). Article CAS Google Scholar * van der Westen, R. et al.
Myoblast cell interaction with polydopamine coated liposomes. Biointerphases 7, 8 (2012). Article CAS Google Scholar * Lee, J. H., Lim, Y. W., Kwon, S. Y. & Kim, Y. S. _In vitro_
effects of mussel-inspired polydopamine coating on Ti6Al4V alloy. J. Tissue Eng. Regen. Med. 10, 273–278 (2013). Article Google Scholar * Low, W. C. et al. Nanofibrous scaffold-mediated
REST knockdown to enhance neuronal differentiation of stem cells. Biomaterials 34, 3581–3590 (2013). Article CAS Google Scholar * Luo, R. et al. _In vitro_ investigation of enhanced
hemocompatibility and endothelial cell proliferation associated with quinone-rich polydopamine coating. ACS Appl. Mater. Interfaces 5, 1704–1714 (2013). Article CAS Google Scholar *
Adams, J. C. & Watt, F. M. Regulation of development and differentiation by the extracellular matrix. Development 117, 1183–98 (1993). CAS PubMed Google Scholar * Kleinman, H. K.,
Philp, D. & Hoffman, M. P. Role of the extracellular matrix in morphogenesis. Curr. Opin. Biotechnol. 14, 526–532 (2003). Article CAS Google Scholar * Badylak, S. F. Regenerative
medicine and developmental biology: the role of the extracellular matrix. Anat. Rec. B New Anat. 287, 36–41 (2005). Article Google Scholar * Clyman, R. I., McDonald, K. A. & Kramer, R.
H. Integrin receptors on aortic smooth muscle cells mediate adhesion to fibronectin, laminin and collagen. Circ. Res. 67, 175–186 (1990). Article CAS Google Scholar * Heino, J. The
collagen family members as cell adhesion proteins. Bioessays 29, 1001–1010 (2007). Article CAS Google Scholar * Zhang, Y. et al. Tissue-specific extracellular matrix coatings for the
promotion of cell proliferation and maintenance of cell phenotype. Biomaterials 30, 4021–4028 (2009). Article CAS Google Scholar * Suzuki, S. et al. Effects of extracellular matrix on
differentiation of human bone marrow-derived mesenchymal stem cells into smooth muscle cell lineage: utility for cardiovascular tissue engineering. Cells Tissues Organs 191, 269–280 (2010).
Article CAS Google Scholar * Lee, S. J., Khang, G., Lee, Y. M. & Lee, H. B. The effect of surface wettability on induction and growth of neurites from the PC-12 cell on a polymer
surface. J. Colloid Interface Sci. 15, 259, 228–235 (2003). Article ADS Google Scholar * Curran, J. M., Chen, R. & Hunt, J. A. The guidance of human mesenchymal stem cell
differentiation _in vitro_ by controlled modifications to the cell substrate. Biomaterials 27, 4783–4793 (2006). Article CAS Google Scholar * Hao, L. et al. Directing the fate of human
and mouse mesenchymal stem cells by hydroxyl-methyl mixed self-assembled monolayers with varying wettability. J. Mater. Chem. B Mater. Biol. Med. 2, 4794–4801 (2014). Article CAS Google
Scholar * Jiang, X., Christopherson, G. T. & Mao, H. Q. The effect of nanofibre surface amine density and conjugate structure on the adhesion and proliferation of human haematopoietic
progenitor cells. Interface Focus 1, 725–733 (2011). Article Google Scholar * Lee, J. H., Jung, H. W., Kang, I. K. & Lee, H. B. Cell behaviour on polymer surfaces with different
functional groups. Biomaterials 15, 705–711 (1994). Article CAS Google Scholar * Spackman, D. H., Stein, W. H. & Moore, S. Automatic recording apparatus for use in chromatography of
amino acids. Anal. Chem. 30, 1190–1206 (1958). Article CAS Google Scholar * Moore, S. Amino acid analysis: Aqueous dimethyl sulfoxide as solvent for the ninhydrin reaction. J. Biol. Chem.
243, 6281–6283 (1968). CAS PubMed Google Scholar * Klemm, D. J. et al. Insulin-induced adipocyte differentiation. Activation of CREB rescues adipogenesis from the arrest caused by
inhibition of prenylation. J. Biol. Chem. 276, 28430–28435 (2001). Article CAS Google Scholar * Lennon, D. P., Edmison, J. M. & Caplan, A. I. Cultivation of Rat Marrow-Derived
Mesenchymal Stem Cells in Reduced Oxygen Tension: Effects on _In Vitro_ and _In Vivo_ Osteochondrogenesis. J. Cell Physio. 187, 345–355 (2001). Article CAS Google Scholar * Fu, Y., Luo,
N., Klein, R. L. & Garvey, W. T. Adiponectin promotes adipocyte differentiation, insulin sensitivity and lipid accumulation. J. Lipid Res. 46, 1369–1379 (2005). Article CAS Google
Scholar * Kyllönen, L. et al. Effects of different serum conditions on osteogenic differentiation of human adipose stem cells _in vitro_. Stem Cell Res. Ther. 4, 17 (2013). Article Google
Scholar * Akiyama, S. K. Integrins in cell adhesion and signaling. Hum Cell 9, 181–186 (1996). CAS PubMed Google Scholar * Lee, J. W. et al. Importance of integrin beta1-mediated cell
adhesion on biodegradable polymers under serum depletion in mesenchymal stem cells and chondrocytes. Biomaterials 25, 1901–9 (2004). Article CAS Google Scholar * Aubin, D., Gagnon, A.
& Sorisky, A. Phosphoinositide 3-kinase is required for human adipocyte differentiation in culture. Int. J Obes. (Lond.) 29, 1006–1009 (2005). Article CAS Google Scholar * Hamidouche,
Z. et al. Priming integrin alpha5 promotes human mesenchymal stromal cell osteoblast differentiation and osteogenesis. Proc. Natl. Acad. Sci. USA 106, 18587–18591 (2009). Article CAS ADS
Google Scholar * Lee, J. S., Yi, J. K., An, S. Y. & Heo, J. S. Increased osteogenic differentiation of periodontal ligament stem cells on polydopamine film occurs via activation of
integrin and PI3K signaling pathways. Cell Physiol. Biochem. 34, 1824–1834 (2014). Article CAS Google Scholar * Chen, Q. et al. An osteopontin-integrin interaction plays a critical role
in directing adipogenesis and osteogenesis by mesenchymal stem cells. Stem Cells 32, 327–337 (2014). Article CAS ADS Google Scholar * Zhang, W. et al. Upregulation of BMSCs Osteogenesis
by Positively-Charged Tertiary Amines on Polymeric Implants via Charge/iNOS Signaling Pathway. Sci. Rep. 5, 9369 (2015). Article CAS Google Scholar * Jansen, E. J. et al. Hydrophobicity
as a design criterion for polymer scaffolds in bone tissue engineering. Biomaterials 26, 4423–4431 (2005). Article CAS Google Scholar * Toepke, M. W. & Beebe, D. J. PDMS absorption of
small molecules and consequences in microfluidic applications. Lab Chip 6,1484–6 (2006). Article CAS Google Scholar * Wu, H. W., Lin, C. C. & Lee, G. B. Stem cells in microfluidics.
Biomicrofluidics 5, 013401 (2011). Article Google Scholar * Ertl, P., Sticker, D., Charwat, V., Kasper, C. & Lepperdinger, G. Lab-on-a-chip technologies for stem cell analysis. Trends
Biotechnol. 32, 245–253 (2014). Article CAS Google Scholar * Mehling, M. & Tay, S. Microfluidic cell culture. Curr. Opin. Biotech. 25, 95–102 (2014). Article CAS Google Scholar *
Shin, Y. et al. Microfluidic assay for simultaneous culture of multiple cell types on surfaces or within hydrogels. Nat. Protoc. 7, 1247–1259 (2012). Article CAS Google Scholar * Liu, X.
F., Yu, J. Q., Dalan, R., Liu, A. Q. & Luo, K. Q. Biological factors in plasma from diabetes mellitus patients enhance hyperglycaemia and pulsatile shear stress-induced endothelial cell
apoptosis. Integr. Biol. 6, 511–522 (2014). Article CAS Google Scholar * Yang, Z. et al. Improved mesenchymal stem cells attachment and _in vitro_ cartilage tissue formation on
chitosan-modified poly(L-lactide-co-epsilon-caprolactone) scaffold. Tissue. Eng. Part A 18, 242–251 (2012). Article CAS Google Scholar Download references ACKNOWLEDGEMENTS Y.K.
acknowledges the funding support by a Tier 2 Academic Research Fund (ARC 22/13) and a Tier 1 Academic Research Fund (RG 37/14) from the Ministry of Education of Singapore. The authors thank
Professor Lee Eng Hin from National University of Singapore for supplying the bone-marrow derived mesenchymal stem cells under an approved IRB protocol for this study. AUTHOR INFORMATION
AUTHORS AND AFFILIATIONS * School of Chemical and Biomedical Engineering, Nanyang Technological University, 62 Nanyang Drive, Singapore, 637459, Singapore Yon Jin Chuah, Yi Ting Koh, Kaiyang
Lim, Nishanth V. Menon, Yingnan Wu & Yuejun Kang Authors * Yon Jin Chuah View author publications You can also search for this author inPubMed Google Scholar * Yi Ting Koh View author
publications You can also search for this author inPubMed Google Scholar * Kaiyang Lim View author publications You can also search for this author inPubMed Google Scholar * Nishanth V.
Menon View author publications You can also search for this author inPubMed Google Scholar * Yingnan Wu View author publications You can also search for this author inPubMed Google Scholar *
Yuejun Kang View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS Y.J.C., Y.T.K. and Y.K. conceived the experiments, N.V.M. designed the
microfluidic experiments, K.L. conducted the EDS experiments and analysis, Y.J.C. and Y.T.K. conducted the experiments, Y.J.C., Y.T.K. and Y.W. analyzed the results, Y.J.C., Y.T.K. and Y.K.
wrote the manuscript. All authors reviewed and approved the manuscript. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing financial interests. ELECTRONIC SUPPLEMENTARY
MATERIAL SUPPLEMENTARY INFORMATION RIGHTS AND PERMISSIONS This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in
this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users
will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ Reprints and permissions
ABOUT THIS ARTICLE CITE THIS ARTICLE Chuah, Y., Koh, Y., Lim, K. _et al._ Simple surface engineering of polydimethylsiloxane with polydopamine for stabilized mesenchymal stem cell adhesion
and multipotency. _Sci Rep_ 5, 18162 (2016). https://doi.org/10.1038/srep18162 Download citation * Received: 14 May 2015 * Accepted: 13 November 2015 * Published: 09 December 2015 * DOI:
https://doi.org/10.1038/srep18162 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently
available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative