Natural killer t cells play a necessary role in modulating of immune-mediated liver injury by gut microbiota
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Gut microbiota are implicated in many liver diseases. Concanavalin A (ConA)-induced hepatitis is a well-characterized murine model of fulminant immunological hepatic injury. Oral
administration of pathogenic bacteria or gentamycin to the mice before ConA injection, liver injury and lymphocyte distribution in liver and intestine were assessed. Our data show that
administration of pathogenic bacteria exacerbated the liver damage. There was more downregulation of activation-induced natural killer T (NKT) cells in the liver of pathogenic
bacteria-treated ConA groups. Also, there was a negative correlation between the numbers of hepatic NKT cells and liver injury in our experiments. Moreover, intestinal dendritic cells (DCs)
were increased in pathogenic bacteria–treated ConA groups. The activation of DCs in Peyer's patches and the liver was similar to the intestine. However, depletion of gut gram-negative
bacteria alleviated ConA-induced liver injury, through suppressed hepatic NKT cells activation and DCs homing in liver and intestine. _In vitro_ experiments revealed that DCs promoted NKT
cell cytotoxicity against hepatocyte following stimulation with pathogenic bacteria. Our study suggests that increased intestinal pathogenic bacteria facilitate immune-mediated liver injury,
which may be due to the activation of NKT cells that mediated by intestinal bacterial antigens activated DCs. SIMILAR CONTENT BEING VIEWED BY OTHERS POLYMERIC IMMUNOGLOBULIN RECEPTOR
DEFICIENCY EXACERBATES AUTOIMMUNE HEPATITIS BY INDUCING INTESTINAL DYSBIOSIS AND BARRIER DYSFUNCTION Article Open access 28 January 2023 FECAL TRANSPLANTATION ALLEVIATES ACUTE LIVER INJURY
IN MICE THROUGH REGULATING TREG/TH17 CYTOKINES BALANCE Article Open access 15 January 2021 EXTRACELLULAR GP96 IS A CRUCIAL MEDIATOR FOR DRIVING IMMUNE HYPERACTIVATION AND LIVER DAMAGE
Article Open access 28 July 2020 INTRODUCTION Hepatitis, commonly induced by virus infection, autoimmune diseases, or alcohol abuse, can lead to liver fibrosis, cirrhosis and carcinoma.
Concanavalin A (ConA)-induced hepatitis is a well-characterized model of fulminant immunological hepatitis. Previous studies have shown that the role of natural killer T (NKT) cells was
critical in the process of ConA-induced hepatic injury1. In addition, NKT cell activation by ConA leads to a rapid reduction in NKT cell numbers due to profound downregulation of the NKT
cell receptor2. Liver plays a major role in metabolism and detoxification, it constantly exposed to microbial products from the enteric microflora and liver can metabolize the gut-derived
toxins; however, this ability is impaired when liver is injured. Many studies have reported that structural and microbiota disorders of the intestine are closely related to liver fibrosis3,4
and hepatocellular carcinoma (HCC)5. These studies have indicated that the intestinal microbiota might play an important role in the pathogenesis of liver disease. Large numbers of
microorganisms inhabit the gut symbiotically and are crucial for regulating intestinal motility, intestinal barrier homeostasis and nutrient absorption6. A balanced composition of gut
microflora confers a diversity of health benefits; however, dysbacteriosis of the intestinal microflora leads to altering immune responses and results in enhanced disease
susceptibility7,8,9. Breakdown of the gut microflora homeostasis might induce an inappropriate immune response, resulting in acute and chronic inflammatory liver diseases10. A recent report
demonstrated that intestinal dysbacteriosis induced intestinal inflammation, thereby promoting the release of pro-inflammatory cytokines such as tumor necrosis factor alpha (TNF-α) and
interleukin 6 (IL-6) by intestinal cells, which might contribute to the development of chronic inflammation in HCC patients11. In mice with non-alcoholic fatty liver disease (NAFLD),
dysbacteriosis induced TNF-α overexpression plays a pathogenic role in NAFLD progressing to fibrosis12. Elevated TNF-α production can directly induce hepatocyte necrosis, but also activate T
lymphocytes, dendritic cells (DCs), NK cells and Kupffer cells simultaneously. In addition, dysbacteriosis can lead to endotoxin accumulation in the portal vein, which promotes fibrosis and
HCC via activation of toll-like receptor four13. However, the correlation between intestinal microbial alteration and immunological hepatic injury, particularly the influence of intestinal
microbial alteration on immune cell activation and migration in the intestine and liver, remains obscure. Thus, we investigated whether changes of the gut microflora affect liver
inflammation and studied the relevant immune mechanism of liver inflammation influenced by the microbial variation. RESULTS PATHOGENIC BACTERIA EXACERBATED CONA- INDUCED LIVER INJURY
Previously, it was reported that depletion of the host microflora affects HCC13, therefore we conjectured that gut-derived bacteria might have a serious impact on liver injury. We
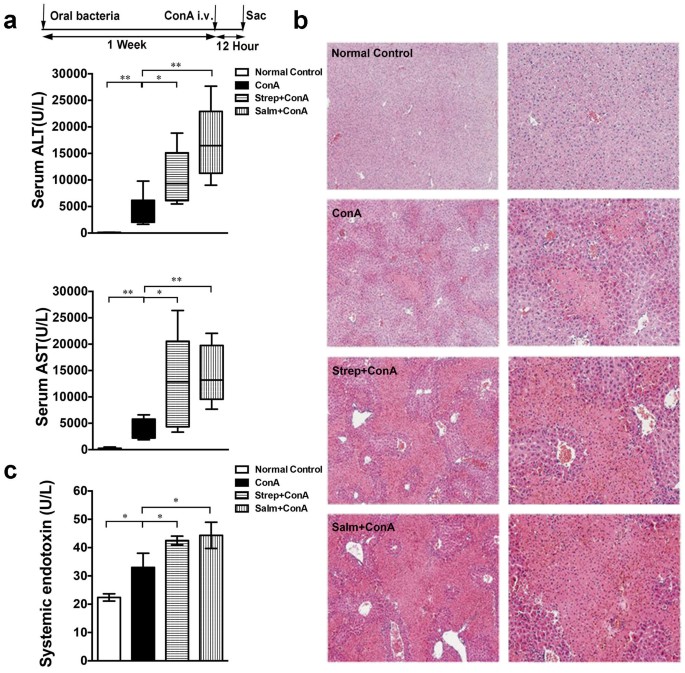
administered _Salmonella_ (gram-negative, G−) and _Streptococcus_ (gram-positive, G+) to the mice for one week prior to ConA injection, as expected, alanine aminotransferase (ALT) and
aspartate aminotransferase (AST) levels were higher in mice treated with _Salmonella_ or _Streptococcus_ before ConA injection than the mice that received ConA only (ConA group) (Fig. 1a).
Consistent with the ALT levels, histological examination showed diffuse and massive degenerative liver alterations after ConA injection, while the necrosis and lymphocyte infiltration in the
Salm + ConA and Strep + ConA groups were more severe (Fig. 1b). In addition, _Salmonella_ and _Streptococcus_ to the mice for one week prior to PBS injection did not cause marked liver
injury, which suggested that pathogenic bacteria did not cause significant liver damage independently (Supplementary Figure S1a–c). Mice were also treated with common intestinal bacteria,
_E.coli_ (G−) and _Lactobacillus_ (G+) before ConA injection to further investigate the effect of different bacteria. And we found such intestinal non-pathogenic bacteria treatment prior to
Con A injection did not aggravate the liver injury (Supplementary Fig S1d–g). Endotoxin is a crucial factor in bacteria-promoted liver injury5. We examined the level of systemic endotoxin,
which was significantly increased after ConA injection. Importantly, serum endotoxin levels were increased markedly in Salm + ConA and Strep + ConA groups compared to the ConA group (Fig.
1c). PATHOGENIC BACTERIA PROMOTED ACTIVATION-INDUCED NKT CELL DOWNREGULATION To investigate how gut-derived bacteria influenced ConA-induced liver injury, we analyzed the liver infiltrating
cells. NKT cell activation after stimulation with anti-CD3 or a specific ligand, α-GC, results in rapid NKT cell downregulation in mice14. In addition, NKT cell activation leads to a rapid
reduction in NKT cell numbers in ConA-induced hepatitis due to profound downregulation of NKT cell receptor15. Figure 2a shows that ConA led to significant reduction of the percentage of NKT
(NK1.1+, TCR-β+) cells in the liver, i.e., NKT cell activated and this was much more severe in the mice with pathogenic bacteria-treated ConA groups. The result also showed a similar change
in the percentage of invariant natural killer T (iNKT, CD1d tetramer+ TCR-β+) cells. In addition, the percentage of DCs (CD11c+) was markedly increased following ConA administration.
Moreover, the proportion of hepatic DCs was notably increased in the Salm + ConA and Strep + ConA groups compared with the ConA group, indicating that administration of pathogenic bacteria
promoted the Con A-induced DC augmentation and NKT cell activation. There was no difference in T (NK1.1−, TCR-β+) cells, NK (NK1.1+, TCR-β−) cells and the expression of CD44 on T cells
between the pathogenic bacteria-treated ConA groups and the ConA group (Fig 2a). The absolute number of the lymphocyte was also measured, the infiltrated lymphocytes in the liver were
increased after Con A injection, moreover the absolute numbers of NKT cells and DCs were in line with their percentages and the absolute numbers of NK and T cells were not significantly
increased after pathogenic bacteria administration (Supplementary Fig S2a). To investigate the significance of hepatic NKT cells activation in liver injury, we examined the association of
the hepatic NKT cell downregulation with liver injury. We found a negative correlation between the percentage of hepatic NKT cells and ALT level in ConA-induced hepatitis (Normal Control,
ConA, _Salmonella +_ ConA, _Streptococcus +_ ConA groups) (Fig. 2b). However, there was no statistically significant correlation between splenic NKT cell frequency and ALT (Supplementary Fig
S2b). Furthermore, IFN-γ production in NKT cells was increased after ConA treatment, which was also markedly higher in the Salm + ConA and Strep + ConA groups compared to the ConA group
(Fig. 2c). However, there was no significant difference in IFN-γ production in T cells after pathogenic administration (Supplementary Fig S2c). The presenting antigens CD1d+APCs, mainly
including DCs, promote NKT cell activation. These data suggest that liver DCs and NKT cells may play important roles in gut microflora-related ConA-induced liver injury. The activated immune
cells, following the ConA injection, synthesized and secreted a series of inflammatory cytokines that have a vital effect on ConA-induced liver injury. In the pathogenic bacteria-treated
ConA groups, TNF-α and IL-12 levels were significantly higher than that in the ConA group (Fig. 2d). However, there was no significant difference in IL-4 levels between the pathogenic
bacteria-treated ConA groups and the ConA group. INTESTINAL DCS MAY INFLUENCE CONA-INDUCED LIVER INJURY To explore the effect of gut microbiota on immune cells, we next investigated the
change in the intestines following oral pathogenic bacteria. Figure 3a shows that, compared with the normal control, the intestinal mucosa microvilli were shorter and there was edema
following ConA injection, moreover, the intestines of mice that received the administration of _Streptococcus_ or _Salmonella_ with ConA treatment were more severely damaged, characterized
by disappearance of the mucosa, thinned seromuscular layer, ruptured microvilli and lymphocyte infiltration; however, the difference between the two pathogenic bacteria treatments was not
significant. Interestingly, there was a marked increase in the percentage of intestinal DCs and NK cells after ConA administration. Meanwhile, the percentage of DCs was markedly increased in
Strep + ConA or Salm + ConA groups compared to the ConA group (Fig. 3b). Nevertheless, the percent of γδT cells, the main T cell subset in the intestine16, was similar between the mice with
or without pathogenic bacteria treatment prior to Con A injection. There was also no significant difference between the other cells in the four groups (Fig. 3b, Supplementary Fig S3a).
IL-12 levels were significantly increased after ConA injection. Compared to the ConA group, the levels of inflammatory cytokines such as TNF-α and IL-12 in the intestinal tissue were
significantly elevated following _Salmonella_ or _Streptococcus_ with ConA treatment. However, there was no significant difference on IL-4 production in the pathogenic bacteria-treated ConA
groups compared to the ConA group (Fig. 3c). As the immune sensors of the intestine17, the distribution of mononuclear cells from Peyer's Patches (PPs) were detected. There was no
distinction between the percentage of T lymphocytes and the expression of CD44 on T cells in all four groups, while the percentage of DCs was higher in the pathogenic bacteria-treated ConA
groups compared to the ConA group (Fig. 3d). The change of DCs was similar to the intestine. DEPLETION OF GRAM-NEGATIVE GUT-DERIVED BACTERIA ALLEVIATED CONA-INDUCED LIVER INJURY AND
SUPPRESSED NKT ACTIVATION AND DCS HOMING IN LIVER AND INTESTINE To verify the mechanism of intestinal pathogenic bacteria in liver injury, we treated mice with gentamycin, which is
bactericidal mainly for gram-negative (G−) organisms in the gut, for one week before ConA injection. Figure 4a shows that ALT and AST levels in gentamycin-treated mice (Genta + ConA group)
were significantly lower than that in ConA group. Also, there was only focal and mild liver injury (Fig. 4a) as well as significantly reduced endotoxin level (Fig. 4b) in Genta + ConA mice
in consistent with the ALT levels. Then, we analyzed the liver infiltrating cells. The percentage of NKT cells in the liver was significantly upregulated in the Genta + ConA group compared
to the ConA group. Moreover, the proportion of DCs was notably decreased in the Genta + ConA group compared with the ConA group, but we detected with no marked change in other cells (Fig.
4c). Besides, TNF-α and IL-12 levels were significantly lower in the Genta + ConA group (Fig. 4d). The results indicated that depletion of intestinal G− bacteria suppressed DC augmentation
and NKT cell activation. We next investigated the change in the intestines following the depletion of G− bacteria and ConA treatment. Figure 4e shows that the shorter, edema intestinal
mucosa microvilli were remitted following gentamycin treatment and ConA injection. Interestingly, the marked increase in the percentage of intestinal DCs after ConA administration was
obviously inhibited in the Genta + ConA group (Fig. 4f). There was also no significant difference between the other cells between the two groups (Fig. 4f). IL-12 levels were significantly
lower in the Genta + ConA group than ConA group. In contrast, there was no significant difference in TNF-α level in the two groups (Fig. 4g). The change in the percentage of DCs in PPs was
the same as that in the intestine (Fig. 4h). CHANGES IN INTESTINAL MICROBIOTA AFTER PATHOGENIC BACTERIA AND CONA TREATMENT To investigate the effect of intestinal microbial alteration on the
gut-liver axis, we measured the bacterial population in stool obtained from the cecum in the five groups. Table 1 lists the major differences in the numbers of 16S rRNA gene copies of the
predominant bacterial groups in the five groups. Compared with the normal controls, there were higher 16S rRNA gene copy numbers for _Enterobacteriaceae_ and fewer copy numbers for
_Bifidobacterium_ and _Lactobacillus_in in the ConA group. The copy numbers for _Enterobacteriaceae_ and _Bacteroides-Prevotella_ were significantly increased in the pathogenic
bacteria-treated ConA groups but reduced in the Genta + ConA group compared to the ConA group, whereas _Bifidobacterium_ and _Lactobacillus_ were both decreased in the pathogenic
bacteria-treated ConA groups but increased in the Genta + ConA group (Table 1). NKT CELLS PLAY A CRITICAL ROLE IN THE PROCESS THAT PATHOGENIC BACTERIA AGGRAVATE THE CON A-INDUCED LIVER
INJURY To further confirm the role of NKT cells in pathogenic bacteria aggravating the Con A-induced liver injury, wild type (WT) and NKT cell-deficient CD1d-/- mice received _Salmonella_
treatment before Con A. Figure 5a showed that ALT and AST levels in CD1d-/- mice were significantly lower than WT mice, but the percentages of T, NK and DC cells were similar between WT mice
and CD1d-/- mice (Fig. 5b). In addition, IL-12 level was not significantly different between WT mice and CD1d-/- mice (Fig. 5c). Besides, we treated the WT mice with anti-IL-12 antibody
before ConA injection. The liver injury was markedly reduced in the anti-IL-12 antibody treated mice (Fig. 5d), moreover, the downregulation of NKT cells (Fig. 5e) and IFN-γ production by
NKT cells (Fig. 5f) were ameliorated. In addition, IL-12 neutralization antibody did not influence IFN-γ production in T cell (Supplementary Fig S3b). Collectively, the NKT cells play a
critical role in the mechanism of pathogenic bacteria aggravate the Con A-induced liver injury and the activation of NKT cells was partly depend on IL-12, mainly produced by DCs. PATHOGENIC
BACTERIA ENHANCED NKT CELL CYTOTOXICITY AGAINST HEPATOCYTES VIA DC-MEDIATED ANTIGEN PRESENTATION To investigate the mechanism that pathogenic bacteria aggravated the ConA-induced liver
injury, we tested whether pathogenic bacterial antigens would increase NKT cell cytotoxic activity. We analyzed the effects of pathogenic bacteria on NKT cell cytotoxic activity against a
murine hepatocyte cell line (TLR2). The cytotoxicity of NKT cells stimulated with DCs in the presence of inactivated _Streptococcus_ or _Salmonella_ was significantly increased, which
suggested that pathogenic bacterial antigens improve the cytotoxicity of NKT cells (Fig. 6a). We measured the TNF-α production by NK cells, NKT cells and T cells in pathogenic bacteria
treated groups. We found that TNF-α production by NKT cells was significantly higher than NK cells and T cells (Supplementary Fig S3c) and TNF-α secretion in the supernatant incubated with
pathogenic bacteria was markedly higher than without bacteria treated (Fig. 6a). TNF-α production in NKT cells was increased after ConA treatment, which was also markedly higher in the Salm
+ ConA and Strep + ConA groups compared to the ConA group, while TNF-α produced by NK and T cells was not significantly changed. (Supplementary Fig S3c) Furthermore, TNF-α neutralization
antibody inhibited the cytotoxicity of NKT cells against hepatocytes (Fig. 6b). In consequence, the cytotoxicity of NKT cell against hepatocytes may depend on TNF-α. BACTERIAL TRANSLOCATION
AFTER LIVER INJURY The number of translocated bacteria to the liver was significantly increased in the ConA group compared to the normal controls (Table 2). Compared to the ConA group, the
number of translocated bacteria was significantly reduced in the Genta + ConA group, but increased in the pathogenic bacteria-treated ConA groups (Table 2). The change in the percentage of
translocated bacteria to the mesenteric lymph nodes was consistent with the liver. These data imply that bacteria translocated to the liver might supply antigens for hepatic NKT cell
activation. DISCUSSION The liver receives portal blood which enriched with nutrients absorbed by the intestine and is exposed to gut-derived factors, including bacterial endotoxins and
cytokines11,18. Intestinal dysbacteriosis can lead to the accumulation of liver damage in fibrosis and HCC5,19,20. Excessive numbers of _Salmonella_ promote the development of liver
damage21; in addition, the ratio of _Streptococcaceae_ in gut microflora is markedly increased in patients with cirrhosis22. Thus, we studied the influence of intestinal dysbacteriosis on
ConA-induced liver injury via gavage with pathogenic bacteria prior to ConA administration. Intestinal microbiota play a crucial role in the process of liver damage, however, whether
intestinal microbiota modulate the liver damage involved immune cell is still unknown. We found that administration of exogenous pathogenic bacteria aggravated liver damage. Previous studies
have shown that NKT cells play a critical role in the process of ConA-induced hepatic injury1,23. In addition, hepatic NKT cell activation in ConA-induced hepatitis was characterized by
rapid NKT cell receptor downregulation. NKT cells are specialized T cells of the immune system that express markers of the NK cell lineage, such as NK1.1, which recognize glycolipid
antigens, presented by the MHC class I-related protein CD1d. In mice, these cells are sometimes referred to invariant NKT (_i_NKT) cells24. It was reported that the microbiota regulated
susceptibility to hepatic injury by Fas/FasL pathway9 and NKT mediated hepatic damage greatly involved to Fas/FasL pathway1, these suggested the influence of microbiota on liver injury was
associated with NKT cells. Our results showed the downregulation of both NKT and iNKT cells were more pronounced in the pathogenic bacteria-treated ConA group. In contrast, the depletion of
gut G− bacteria by gentamycin alleviated the ConA-induced liver injury and was accompanied by reduced liver immune cell infiltration and NKT cell downregulation was impaired in these mice.
Therefore, we speculated that NKT cells might play an important role in the impact of intestinal microbial alteration on immunological hepatic injury. Moreover, NKT cell activation is
associated with DCs. In this study, intestinal microbial alteration led to variation of intestinal DC distribution. Compared to DCs in the ConA group, there were more DCs in the pathogenic
bacteria-treated ConA groups, which was similar to the trend of DCs in the PPs and liver, whereas intestinal DCs in the gentamycin-treated ConA group were decreased. This indicates that
intestinal microbial alteration might contribute to the activation of gut-derived DCs and then induce the activation of hepatic DCs, consequently leading to hepatic NKT cell activation and
liver injury. The _in vitro_ experiments determined that DCs, stimulated with pathogenic bacteria, promoted NKT cell cytotoxicity against liver parenchyma cells. These results suggest that
intestinal pathogenic bacteria aggravate liver damage may originate from DCs activation, which subsequently augment hepatic NKT cell cytotoxicity against liver parenchyma cells. As powerful
professional APCs, DCs are the center of immune response regulation and have strong migration ability25,26. Intestinal DCs can also induce helper T (Th) cell differentiation into Th1 or Th2
cells, which secrete IFN-γ or IL-4, IL-10 and IL-1327,28. As the key initiators and regulators of mucosal adaptive immune responses, DCs also play an important role in maintaining intestinal
homeostasis, particularly the response to intestinal bacterial antigens29,30. When we studied the number and function of DCs, we found that pathogenic bacteria significantly increased the
proportion of DCs in the intestine and liver. Compared to the ConA group, the tendency for variation of the hepatic DCs was similar to that of the intestine following the depletion of G-
bacteria or pathogenic bacteria treatment, suggesting the importance of DCs in the gut-liver axis. DCs may migrate from the gut to the liver, but the pathway is unclear. In rats that had
undergone liver transplantation, DCs migrated to the spleen and PPs31. Therefore, we detected lymphocyte distribution in the spleen (Supplementary Fig. S4, 5) and PPs. The change of splenic
DCs was not consistent with the intestinal and hepatic DCs. Interestingly, the trend of DCs in the PPs was very similar to the intestine and liver in ConA-induced liver injury, suggesting
that DCs may first migrate to PPs and then to liver. IL-12 derived from DCs induces Th1 differentiation and produces IFN-γ, exacerbating liver damage32. The change trend of the IL-12 level
was consistent with the DCs, supporting the changes of DCs in the liver. Previous research has reported obvious downregulation of NKT cells following ConA administration1,23. In the immune
response process where dysbacteriosis influences the severity of hepatitis, we found that the severity of liver damage was related to NKT cell activation. Previous studies have found that
NKT cells recognized glycolipids from pathogenic bacteria during microbial infections through antigens presented by glycolipid-activated DCs33,34. _In vitro_ experiments revealed that NKT
cells activated by DCs stimulated with pathogenic bacteria had greater cytotoxicity against liver parenchyma cells than cells that had not been stimulated with bacteria. Moreover, NK cells
were activated following treatment with exogenous pathogenic bacteria, indicating that the exogenous pathogenic bacteria aggravated the inflammation. However, NK cell deletion does not
inhibit the development of ConA-induced hepatic injury35, suggesting that NK cell is not critical in ConA-induced hepatic injury. Besides, Lin et al. found that the gut-derived endotoxin
promotes the liver injury through TLR-4, mediated by T cell activation36. NKT cell was an unconventional T cell subset, thus in the study of Lin et al. T cells might also include the NKT
cells. Quantitative 16S rRNA analysis of intestinal bacteria in the feces revealed intestinal dysbacteriosis. _Salmonella_ or _Streptococcus_ administration decreased the copy numbers of
_Bifidobacterium_ and _Lactobacillus,_ but increased _Enterobacteriaceae_ and _Bacteroides-Prevotella_. Although _Salmonella_ is gram-negative and _Streptococcus_ is gram-positive, serum
endotoxin levels were increased in both _Salmonella_ and _Streptococcus_ treated mice. The increased endotoxin levels might be associated with increased gut permeability and more
_Enterobacteriaceae_ and _Bacteroides-Prevotella_. In conclusion, during the course of ConA-induced hepatitis, intestinal pathogenic bacterial antigens induced DC activation in the intestine
and intestinal DCs may migrated into the liver, passing through the PPs, which could led to NKT cell activation in the liver. Another pathway might be that the pathogenic bacteria increased
gut permeability, thus large amounts of bacterial antigen from the intestine entered the liver, subsequently activated the hepatic DCs and in turn result in the activation of NKT cells.
Upon activation, NKT cells caused liver injury (Fig. 6c). Our data suggest that modulation of the gut microbiota can affect liver injury via DCs induced NKT cells activation. Capitalizing on
the preponderant intestinal flora and DC activation may represent a new avenue for treating or preventing immune-mediated hepatitis. METHODS All experimental procedures were carried out in
accordance with the 1996 National Institutes of Health Guide for the Care and use of Laboratory Animals and the experimental procedures were approved by the Local Committee of Animal Use and
Protection. MICE C57BL/6 female mice were purchased from the Zhejiang Chinese Medical University Medical Experiment Animal Research Center (Hangzhou, China). CD1d-/- female mice were
purchased from the Jackson Laboratory (Bar Harbor, ME) and bred and maintained at University of Science and Technology of China, Hefei, China. All mice were maintained under specific
pathogen-free conditions and used six to eight weeks old. Mice were under daily intragastric administration with a dosage of pathogenic bacteria or gentamycin (Genta). Group A Streptococcus
and Salmonella enteritidis was 5 × 108 colony-forming units [CFU]/200 µl/mouse and gentamycin (Genta) was 2 × 104 U/200 µl/mouse. Such treatment was once per day and maintained for a week
prior to ConA administration (n = 8). Group A Streptococcus and Salmonella enteritidis were isolated from a patient and maintained in our laboratory as previously described21,37.
Streptococcus was grown in brain heart infusion broth (Oxoid, Basingstoke, UK) supplemented with 5% (v/v) defibrinated goat blood; Salmonella was grown on a blood agar base (bioMérieux,
Durham, North Carolina, USA) at 37°C overnight, then harvested and diluted in phosphate-buffered saline (PBS). ConA was purchased from Vector Laboratories (Burlingame, California, USA).
Intravenous injections of Con A (10 mg/kg) were administered via the tail vein 12 h before examination. The normal group mice were treated with 200 µl of PBS by daily gavage or tail vein
injection 12 h before examination. To block IL-12, 250 μg purified anti–IL-12 MAb (Biolegend San Diego, California, USA) or isotype-control rat IgG were injected intravenously to the mice 2
hour before ConA treatment. MEASUREMENT OF LIVER INJURY Serum alanine aminotransferase (ALT) and aspartate transaminase (AST) levels were measured using a standard clinical automated
analyzer (SRL, Tokyo, Japan). Livers were fixed in 10% formalin and embedded in paraffin. Sections were stained with hematoxylin and eosin (H&E) and examined. INTRACELLULAR CYTOKINE
STAINING Cells (1 × 106/ml) from the liver were stimulated for 4 h with 50 ng/ml PMA and 500 ng/ml ionomycin (Sigma-Aldrich). Cells were harvested, washed, stained with anti-NK1.1-PE mAb
anti-T cell receptor (TCR)-β-FITC mAb Surface-stained cells and fixed by Cytofix/CytopermFixation/Permeabilization Kit (BD, Bioscience). Permeabilized cells were incubated for 30 min at room
temperature with anti-IFN-γ-APC-mAb or anti-TNF-α-APC-mAb. Stained cells were washed twice in permeabilization buffer and resuspended in PBS supplemented with 0.3% w/v BSA and 0.1% w/v
sodium azide. The number of cytokine-expressing NKT cells per 105 NKT cells was determined by flow cytometry. FLOW CYTOMETRY ANALYSIS Hepatic and intestinal mononuclear cells or splenic
cells were isolated and labeled with anti-CD11c-PE mAb, anti-NK1.1-PE mAb, anti-CD44-FITC mAb, anti-T cell receptor (TCR)-β-FITC mAb, anti-TCR-β-Allophycocyanin (APC) mAb, anti-TCR-γδ-APC
mAb, anti-IFN-γ-APC-mAb (all from eBioscience, San Diego, California, USA), anti-TNF-α-APC-mAb (Biolegend San Diego, California, USA). Stained cells were assessed using a FC500 System
(Beckman Coulter, Brea, California, USA) equipped with Summit 5.1 software. For the cytotoxic assay, target cells were labeled in advance with carboxyfluorescein diacetate succinimidylester
(CFSE; Molecular Probes, Eugene, Oregon, USA) as indicated and analyzed using flow cytometry. CYTOTOXIC ASSAY NKT cells and DCs (CD11c+) were isolated from the liver and spleen,
respectively, using a FACS Aria III instrument (Becton Dickinson); the purity of the sorted cells was > 95% DCs were first cultured with inactivated _Streptococcus_ or _Salmonella_ at a
1:10 ratio. Bacterial sonicates were generated by adding 70% ethanol then washing the bacteria thrice with PBS, followed by resuspension in PBS at a concentration equivalent to 109 CFU/mL
before use. NKT cells were cultured overnight with recombinant IL-2 stimulus in the presence of 100-Gy irradiated DCs. The target cells (TLR2 hepatocyte cell line; RIKEN Cell Bank, Tsukuba,
Japan) were labeled in advance with CFSE (Molecular Probes) as previously described. The cells were harvested and seeded at the indicated effector/target cell (E/T) ratio of 10:1. To block
TNF-α, 20 ng/ml purified TNF-α MAb (Biolegend San Diego, California, USA) or isotype-control rat IgG were incubated with TLR2 for 6 h before cytotoxic assay. NKT cell cytotoxic activity was
assayed using flow cytometry. ISOLATION AND QUANTITATIVE PCR OF INTESTINAL MICROBIOTA DNA DNA was extracted from feces and bacterial precipitates using a Qiagen Stool Kit (Hilden, Germany)
and a modified cell lysis protocol. Qualitative procedures and the method for detecting bacterial population were performed as described previously38. And the copy number of target DNA was
calculated by comparison with serially diluting standards, (101 to 108 copies of plasmid DNA containing the respective amplicon for each set of primers) running on the same plate. Bacterial
quantity was expressed as log10 bacteria per gram of stool. The specific primers used were as follows: _Bacteroides-Prevotella_: 5′-GAAGGTCCCCCACATTG-3′ (sense), 5′-CAATCGGAGTTCTTCGTG-3′
(anti-sense); _Bifidobacteria_: 5′-GGGTGGTAATGCCGGATG-3′ (sense), 5′-TAAGCCATGGACTTTCACACC-3′ (anti-sense); _Enterobacteriacea_: 5′-CATTGACGTTACCCGCAGAAGAAGC-3′ (sense),
5′-CTCTACGAGACTCAAGCTTGC-3′ (anti-sense); _Lactobacillus_: 5′-AGCAGTAGGGAATCTTCCA-3′ (sense), 5′-ATTTCACCGCTACACATG-3′ (anti-sense)39. ENDOTOXIN ANALYSIS Blood samples (100 μL) were
centrifuged at 3000 g for 10 min at room temperature to separate the serum for analysis; the amount of endotoxin in the separated serum was determined using a quantitative chromogenic
Limulus amebocyte lysate assay as instructed by the manufacturer (Eihua Medical, Shanghai, China). BACTERIAL TRANSLOCATION Liver and mesenteric lymph node samples were weighed and placed in
a sterile glass homogenizer. The homogenate (50 μL) was then placed on a blood agar base and an anaerobic blood agar base (bioMérieux) within 30 min of sample collection and incubated at
37°C for 24 h. The number of CFU per plate was counted and corrected for the original weight of the sample as described previously. ENZYME-LINKED IMMUNOSORBENT ASSAY TNF-α, IL-4 and IL-12
(p70) levels were measured using mouse enzyme-linked immunosorbent assay (ELISA) Ready-Set-Go Kits (eBioscience) as specified by the manufacturer. Cytokine content in the liver, spleen and
intestine extracts was expressed as amounts per gram of tissue. STATISTICS Comparisons between groups were made using Mann-Whitney U test, or ANOVA tests of GraphPad Prism software for two
or multigroup comparisons (GraphPad Software, Inc.). _P_ < 0.05 were considered significant. NS, no significance. REFERENCES * Diao, H. et al. Osteopontin as a mediator of NKT cell
function in T cell-mediated liver diseases. Immunity 21, 539–550 (2004). Article CAS Google Scholar * Wilson, M. T. et al. The response of natural killer T cells to glycolipid antigens is
characterized by surface receptor down-modulation and expansion. Proc Natl Acad Sci U S A 100, 10913–10918 (2003). Article CAS ADS Google Scholar * Miele, L. et al. Increased intestinal
permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology 49, 1877–1887 (2009). Article CAS Google Scholar * Hartmann, P., Haimerl, M., Mazagova, M.,
Brenner, D. A. & Schnabl, B. Toll-like receptor 2-mediated intestinal injury and enteric tumor necrosis factor receptor I contribute to liver fibrosis in mice. Gastroenterology 143,
1330–1340 e1331 (2012). Article CAS Google Scholar * De Minicis, S. et al. Dysbiosis contributes to fibrogenesis in the course of chronic liver injury in mice. Hepatology 59, 1738–1749
(2014). Article CAS Google Scholar * Backhed, F. et al. The gut microbiota as an environmental factor that regulates fat storage. P Natl Acad Sci USA 101, 15718–15723 (2004). Article ADS
Google Scholar * Cryan, J. F. & O'Mahony, S. M. The microbiome-gut-brain axis: from bowel to behavior. Neurogastroenterol Motil 23, 187–192 (2011). Article CAS Google Scholar
* Round, J. L., O'Connell, R. M. & Mazmanian, S. K. Coordination of tolerogenic immune responses by the commensal microbiota. Journal of Autoimmunity 34, J220–J225 (2010). Article
CAS Google Scholar * Celaj, S. et al. The microbiota regulates susceptibility to Fas-mediated acute hepatic injury. Laboratory investigation; a journal of technical methods and pathology
94, 938–949 (2014). Article CAS Google Scholar * Schnabl, B. & Brenner, D. A. Interactions between the intestinal microbiome and liver diseases. Gastroenterology 146, 1513–1524
(2014). Article CAS Google Scholar * Zhang, H. L. et al. Profound impact of gut homeostasis on chemically-induced pro-tumorigenic inflammation and hepatocarcinogenesis in rats. J Hepatol
57, 803–812 (2012). Article Google Scholar * Li, Z. et al. Probiotics and antibodies to TNF inhibit inflammatory activity and improve nonalcoholic fatty liver disease. Hepatology 37,
343–350 (2003). Article CAS ADS Google Scholar * Dapito, D. H. et al. Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4. Cancer Cell 21, 504–516 (2012). Article
CAS Google Scholar * Harada, M. et al. Down-regulation of the invariant Valpha14 antigen receptor in NKT cells upon activation. Int Immunol 16, 241–247 (2004). Article CAS Google
Scholar * Takeda, K. et al. Critical contribution of liver natural killer T cells to a murine model of hepatitis. Proc Natl Acad Sci U S A 97, 5498–5503 (2000). Article CAS ADS Google
Scholar * Park, S. G. et al. T regulatory cells maintain intestinal homeostasis by suppressing gammadelta T cells. Immunity 33, 791–803 (2010). Article CAS Google Scholar * Jung, C.,
Hugot, J. P. & Barreau, F. Peyer's Patches: The Immune Sensors of the Intestine. International journal of inflammation 2010, 823710 (2010). Article Google Scholar * Imajo, K.,
Yoneda, M., Ogawa, Y., Wada, K. & Nakajima, A. Microbiota and nonalcoholic steatohepatitis. Seminars in immunopathology 36, 115–132 (2014). Article CAS Google Scholar * Bajaj, J. S.
et al. Altered profile of human gut microbiome is associated with cirrhosis and its complications. Journal of hepatology 60, 940–947 (2014). Article CAS Google Scholar * Yu, L. X. et al.
Endotoxin accumulation prevents carcinogen-induced apoptosis and promotes liver tumorigenesis in rodents. Hepatology 52, 1322–1333 (2010). Article CAS Google Scholar * Li, Y. T. et al.
Effects of gut microflora on hepatic damage after acute liver injury in rats. J Trauma 68, 76–83 (2010). Article Google Scholar * Zhang, Z. et al. Large-scale survey of gut microbiota
associated with MHE Via 16S rRNA-based pyrosequencing. Am J Gastroenterol 108, 1601–1611 (2013). Article CAS ADS Google Scholar * Diao, H. et al. Osteopontin regulates development and
function of invariant natural killer T cells. Proc Natl Acad Sci U S A 105, 15884–15889 (2008). Article CAS ADS Google Scholar * Sullivan, B. A. & Kronenberg, M. Activation or
anergy: NKT cells are stunned by alpha-galactosylceramide. The Journal of clinical investigation 115, 2328–2329 (2005). Article CAS Google Scholar * Crispe, I. N. The Liver as a Lymphoid
Organ. Annu Rev Immunol 27, 147–163 (2009). Article CAS Google Scholar * Gilboa, E. DC-based cancer vaccines. J Clin Invest 117, 1195–1203 (2007). Article CAS Google Scholar *
Banchereau, J. et al. Immunobiology of dendritic cells. Annu Rev Immunol 18, 767–+ (2000). Article CAS Google Scholar * Huang, H., Dawicki, W., Zhang, X., Town, J. & Gordon, J. R.
Tolerogenic dendritic cells induce CD4+CD25hiFoxp3+ regulatory T cell differentiation from CD4+CD25-/loFoxp3- effector T cells. J Immunol 185, 5003–5010 (2010). Article CAS Google Scholar
* Kinnebrew, M. A. et al. Interleukin 23 production by intestinal CD103(+)CD11b(+) dendritic cells in response to bacterial flagellin enhances mucosal innate immune defense. Immunity 36,
276–287 (2012). Article CAS Google Scholar * Tezuka, H. & Ohteki, T. Regulation of intestinal homeostasis by dendritic cells. Immunol Rev 234, 247–258 (2010). Article CAS Google
Scholar * Ueta, H. et al. Systemic transmigration of allosensitizing donor dendritic cells to host secondary lymphoid organs after rat liver transplantation. Hepatology 47, 1352–1362
(2008). Article Google Scholar * Sallusto, F. DCs: a dual bridge to protective immunity. Nat Immunol 14, 890–891 (2013). Article CAS Google Scholar * Mattner, J. et al. Exogenous and
endogenous glycolipid antigens activate NKT cells during microbial infections. Nature 434, 525–529 (2005). Article CAS ADS Google Scholar * Kinjo, Y. et al. Invariant natural killer T
cells recognize glycolipids from pathogenic Gram-positive bacteria. Nat Immunol 12, 966–U972 (2011). Article CAS Google Scholar * Toyabe, S. et al. Requirement of IL-4 and liver NK1+ T
cells for concanavalin A-induced hepatic injury in mice. J Immunol 159, 1537–1542 (1997). CAS PubMed Google Scholar * Lin, Y. et al. Gut-derived lipopolysaccharide promotes
T-cell-mediated hepatitis in mice through Toll-like receptor 4. Cancer prevention research (Philadelphia, Pa.) 5, 1090–1102 (2012). Article CAS Google Scholar * Diao, H. & Kohanawa,
M. Endogenous interleukin-6 plays a crucial protective role in streptococcal toxic shock syndrome via suppression of tumor necrosis factor alpha production. Infection and immunity 73,
3745–3748 (2005). Article CAS Google Scholar * Chen, Y. et al. Characterization of fecal microbial communities in patients with liver cirrhosis. Hepatology 54, 562–572 (2011). Article
Google Scholar * Bartosch, S., Fite, A., Macfarlane, G. T. & McMurdo, M. E. Characterization of bacterial communities in feces from healthy elderly volunteers and hospitalized elderly
patients by using real-time PCR and effects of antibiotic treatment on the fecal microbiota. Applied and environmental microbiology 70, 3575–3581 (2004). Article CAS Google Scholar
Download references ACKNOWLEDGEMENTS This study was supported by grants from the National Natural Science Foundation of China (No. 81271810), the National Basic Research Program
(2013CB531405) and Doctoral Fund of Ministry of Education of China (20120101110009). AUTHOR INFORMATION Author notes * Chen Jianing and Wei Yingfeng contributed equally to this work AUTHORS
AND AFFILIATIONS * State Key Laboratory for Diagnosis and Treatment of Infectious Diseases, The First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou, Zhejiang,
310003, China Jianing Chen, Yingfeng Wei, Jianqin He, Guangying Cui, Yunan Zhu, Chong Lu, Yulong Ding, Lanjuan Li & Hongyan Diao * Collaborative Innovation Center for Diagnosis and
Treatment of Infectious Diseases, Hangzhou, China Jianing Chen, Yingfeng Wei, Jianqin He, Guangying Cui, Yunan Zhu, Chong Lu, Yulong Ding, Lanjuan Li & Hongyan Diao * Institute of
Immunology and Key Laboratory of Innate Immunity and Chronic Disease of CAS, School of Life Sciences and Medical Center, University of Science and Technology of China, Hefei, 230027, China
Rufeng Xue & Li Bai * Innovation Center for Cell Biology, Hefei National Laboratory for Physical Sciences at Microscale, 230027, Hefei, China Rufeng Xue & Li Bai * Molecular
Immunology, Institute for Genetic Medicine, Hokkaido University, Sapporo, 0600815, Japan Toshimitsu Uede Authors * Jianing Chen View author publications You can also search for this author
inPubMed Google Scholar * Yingfeng Wei View author publications You can also search for this author inPubMed Google Scholar * Jianqin He View author publications You can also search for this
author inPubMed Google Scholar * Guangying Cui View author publications You can also search for this author inPubMed Google Scholar * Yunan Zhu View author publications You can also search
for this author inPubMed Google Scholar * Chong Lu View author publications You can also search for this author inPubMed Google Scholar * Yulong Ding View author publications You can also
search for this author inPubMed Google Scholar * Rufeng Xue View author publications You can also search for this author inPubMed Google Scholar * Li Bai View author publications You can
also search for this author inPubMed Google Scholar * Toshimitsu Uede View author publications You can also search for this author inPubMed Google Scholar * Lanjuan Li View author
publications You can also search for this author inPubMed Google Scholar * Hongyan Diao View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS
H.D. designed the experiments; J.C., Y.W., G.C., Y.D., Y.Z. and C.L. performed the experiments; Y.W. and J.H. analyzed the data; L.L., T.U., R.X. and L.B. provided technical and material
support. H.D., J.C. and Y.W. wrote the manuscript; all authors reviewed the manuscript. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing financial interests.
ELECTRONIC SUPPLEMENTARY MATERIAL SUPPLEMENTARY INFORMATION Supporting Information RIGHTS AND PERMISSIONS This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs
4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit
line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of
this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/ Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Chen, J., Wei, Y., He, J. _et al._ Natural killer T cells
play a necessary role in modulating of immune-mediated liver injury by gut microbiota. _Sci Rep_ 4, 7259 (2014). https://doi.org/10.1038/srep07259 Download citation * Received: 26 August
2014 * Accepted: 10 November 2014 * Published: 01 December 2014 * DOI: https://doi.org/10.1038/srep07259 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this
content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative