Computed tomographic angiography of anterior spinal artery in acute cervical spinal cord injury
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT BACKGROUND: In addition to spinal fracture and/or dislocation, acute traumatic spinal cord injury (SCI) includes neural tissue and vascular damage at the site of contusion. Vascular
damage results in spinal cord ischemia, which is a key factor that contributes to secondary pathogenesis. There is a need to identify spinal cord ischemia secondary to anterior spinal
artery (ASA) rupture in acute cervical SCI. METHODS: After admission, 20 patients with cervical SCI, including 10 cases of central cord syndrome (CCS), four cases of brown-sequard syndrome
(BSS) and six cases of tetraplegia (ASIA A), had computed tomographic angiography (CTA) of ASA performed on them. ASA rupture or occlusion was observed. RESULTS: The ASA was visualized in
all 20 patients. No ASA rupture was found in CCS and BSS patients, even for sever blunt cervical fracture and dislocation tetraplegia patients, except one stab-wound patient. CONCLUSION: CTA
provides the most detailed, highest resolution imaging of ASA in acute cervical SCI. ASA rupture is not commonly seen in acute blunt cervical SCI. SIMILAR CONTENT BEING VIEWED BY OTHERS
PEDIATRIC SPINAL CORD INFARCTION FOLLOWING A MINOR TRAUMA: A CASE REPORT Article 12 October 2020 VERY RARE INCIDENCE OF ASCENDING PARALYSIS IN A PATIENT OF TRAUMATIC SPINAL CORD INJURY: A
CASE REPORT Article 26 July 2022 VERTEBRAL ARTERY INJURY IN MAJOR TRAUMA PATIENTS IN SAUDI ARABIA: A RETROSPECTIVE COHORT STUDY Article Open access 01 October 2020 INTRODUCTION Acute
traumatic spinal cord injury (SCI), includes primary and secondary injuries. The primary mechanical injury results in axonal and vascular damage at the site of contusion and is generally
considered to be irreversible. In the minutes, weeks or even years following the initial injury, the secondary injury can include a cascade of a series of pathobiological events, such as
ischemia, vasospasm, delayed axonal loss, apoptosis, ion-mediated cell damage, excitotoxicity, neuroinflammation, mitochondrial dysfunction and oxidative cell damage.1 Among these
pathobiological events, ischemia is a key factor and key contributor to the secondary pathogenesis, which influences the chronic functional deficits in individuals who have experienced SCI.
The anterior spinal arteries (ASA) supply the anterior horn and the anterior part of the lateral column on the left or right side at each level of the spinal cord. However, whether spinal
cord ischemia is secondary to ASA rupture in SCI remains unknown. There are a number of papers that, for detecting spinal cord infarction of acute spinal cord ischemia syndrome, relied upon
autopsy, catheter angiography and magnetic resonance imaging (MRI) of ASA.2, 3 Compared with MR angiography (MRA), computed tomographic angiography (CTA) has higher spatial resolution and is
capable of covisualizing the anatomy (that is, spinal cord and vertebral bone) in addition to the spinal vessels. MRA is beneficial for the visualization of
submillimeter-to-millimeter-sized intradural vessels and helpful for indicating the location of the vascular lesion.4 We hypothesize that spinal cord ischemia may result in ASA rupture in
acute traumatic SCI. We also hypothesize that CTA of ASA is the ideal method of covisualizing both ASA and injured bones. This study aims to identify ASA rupture by CTA in acute cervical
SCI. MATERIALS AND METHODS PATIENTS Twenty patients with cervical SCI were admitted to our hospital between 2008 and 2009. The clinical characteristics of the patients are summarized in
Table 1. In these 20 patients(18 males and 2 females), the distribution of cervical trauma was from C2 to C7; the duration from injury until surgery ranged from 5 days to 6 months; follow-up
ranged from 8–13 months (mean, 10 months). All patients underwent plain cervical radiographs, CT scans and 1.5-Tesla MRI. T1-weighted and T2-weighted MRIs were performed to define spinal
cord compression and the extent of intramedullary signal intensity change. The mode of acute traumatic SCI was defined as central cord syndrome (CCS) (10 cases), brown-sequard syndrome (BSS)
(4 cases) and tetraplegia (ASIA A) (6 cases). All patients arriving within 8 h of trauma received high-dose methylprednisolone. Anterior decompression and fusion or/and posterior screw
fixation surgery was performed on the majority of patients. The timing of surgery depended on the severity of the neurological injury. Preoperative and postoperative neurological status was
evaluated using the American Spinal Injury Association (ASIA) impairment classification and the motor score.5 CTA OF ASA With the institutional review board approval of the research
protocol, CTA of ASA was performed on the patients. One or two days after patients underwent MRI, patients were scanned with 64-slice MDCT scanners (LightSpeed 64, GE Medical System,
Milwaukee, WI, USA). All CT scans were obtained with 0.5 s rotation, 0.625 mm nominal detector widths, pitch of 0.984, 120 kV and 480 mA. Transverse sections were reconstructed with 50%
overlap relative to the effective section thickness of 0.6 mm. Iohexol (Amersham Health, Princeton, NJ, USA) was administered through an antecubital vein with a dose of 120–150 ml (350 mgI
ml−1) at a rate of 5 ml s−1. The scan delay was determined by a preliminary 20 ml test injection at the level of basilar artery. All observations were made retrospectively by two
neuroradiologists. To examine the ASA transverse sections, multiplanar reformations, curved planar reformation and thin-slab (2–4 mm) maximum intensity projections were generated and
displayed on a workstation (Advantage Workstation 4.3, GE Medical Systems) with window and level settings selected to maximize arterial to background discrimination. The ASA was identified
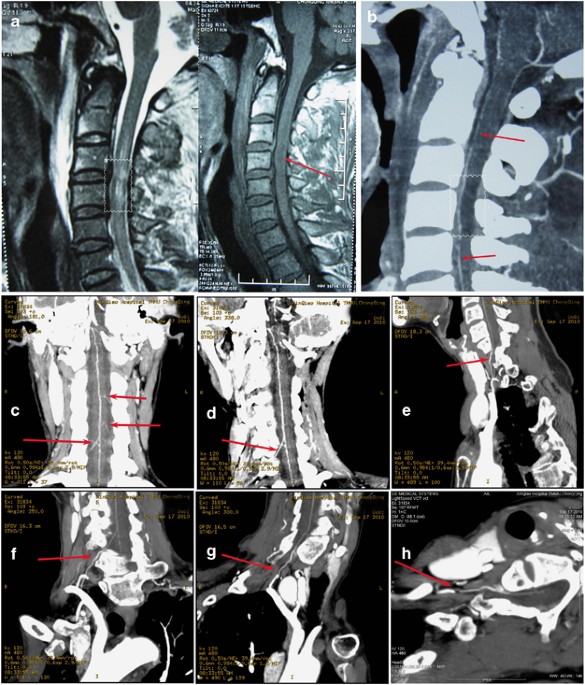
by maintaining the maximum intensity projection-slab parallel to the anterior surface of the spinal cord at each vertebral level assessed from C1 to C7. An enhanced artery on the midline
ventral surface of the spinal cord was interpreted as the ASA. At the same time, an artery originating from the aorta and coursing through the intervertebral foramen to join the ASA in a
hairpin configuration was interpreted as the adamkiewicz artery (Figures 1d, e). Covisualizations of ASA and adamkiewicz artery were used to identify ASA; covisualizations of veins were not
criteria of identifying ASA. Another criterion was that the ASA originated from vertebral artery in skull base. Inter-rater reliability, in the form of consensual identification of ASA by
two radiologists, was required. STATISTICAL ANALYSIS Comparisons were performed by using Student’s _t_ tests and linear regression analyses. We reported the results by using _P_<0.05 as
the criterion for a significant difference. The odds ratio was reported with 95% confidence intervals. RESULTS ASA VISUALIZATION The clinical data are summarized in Table 1. The ASA was
visualized in all 20 patients. The course of the ASA extended from C1 to C7 in the sagittal, coronal and transverse views. The ASA runs along the entire length of the anterior surface of the
spinal cord. Anterior radiculomedullary arteries (ARAs), which supply the spinal cord throughout its length by anastomoses branching upward and downward, were not visualized for the
angiography, because very little or even no blood flows from ARAs in either direction. However, arteries with iohexol in the transverse view were found, which may be part of ARAs. No ASA
rupture was found in CCS (Figure 1) and BSS, even for sever cervical fracture and dislocation patients with spinal cord compression (Figure 2), except one stab-wound patient (Figure 3).
Adamkiewicz artery was also visualized in most cases; no ruptures were found in any patients. CORRELATION BETWEEN SPINAL CORD ISCHEMIA AND ASA RUPTURE SCI includes edema, myelomalacia,
gliosis or inflammation, which present as irregular T2 hyperintensity in MRI and in the low signal intensity area in T1-weighted images. These intramedullary signal intensity changes can
also be seen as MRI-based predictors of spinal cord ischemia.6 Seeing as ARAs were not visualized and no ASA ruptures were found in any BSS, CCS and tetraplegia patients (except one
stab-wound patient), our evidence shows that spinal cord ischemia is not associated with ASA rupture(Figures 1, 2, 3). Predictors of SCI that can be determined through MRI (such as location
and extent of the cord edema) are correlated with primary SCI and, perhaps, the reduction of ARAs blood supply. ARAs maybe ruptured, occluded or compressed by swelling of the surrounding
tissue. NEUROLOGICAL PROGNOSIS There is a close correlation between spinal cord ischemia that can be determined through MRI and neurological prognosis. Subtle spinal cord ischemia BBS and
CCS patients usually had less severe neurological injuries and displayed faster improvement, while distinct spinal cord ischemia tetraplegia patients had severe neurological injuries and
displayed very slow or no improvement (Table 1)(_P_<0.05). No correlation was found between ASA and neurological prognosis, except in one stab-wound patient (_P_>0.05). DISCUSSION CTA
OF ASA The ASA (diameter, 0.2–0.8 mm) runs along the entire length of the anterior surface of the spinal cord and distributes blood to the anterior two thirds of the cord tissue by central
and pial branches.7 Numerous anterior ARAs supply the spinal cord throughout its length by anastomoses, which branch upward and downward. The posterior spinal arteries are usually paired,
course on the posterolateral surface of the spinal cord along its entire length, and may occasionally be discontinuous. ARAs and posterior spinal arteries are less than 0.5 mm in diameter,
have no blood flow in either direction,8 and, at present, can only occasionally be depicted _in vivo_ by catheter angiography. Thus, visualization of ASA is able to depict spinal cord blood
supply in normal spinal cord and, to some extent, spinal cord pathological ischemia. Catheter, CT and MRA are the ultimate imaging technique for diagnosing, localizing and classifying spinal
vascular lesions. Despite its superior spatial resolution and image quality, catheter angiography has several major drawbacks, including being an invasive technique, ionizing radiation
exposure, risk for major complications and can only be performed by experts.9, 10 MRA benefits include having a strong background of suppression techniques, which allow the depiction of
vessels smaller than the voxel size at acquisition.11 Moreover, reconstruction three-dimensional images of two-phase and time-resolved MRA is sufficient for the detection of the adamkiewicz
artery and vessels of interest.12, 13, 14 However, MRA does not allow for visualization of the spinal cord and bone anatomy. This is why we chose CTA for visualizing ASA, although its
disadvantages include inherent exposure to ionizing radiation and the requirement of administering a potentially nephrotoxic iodine contrast agent. The contrast injection parameters in this
paper are based on the preliminary experiments and related literature.15, 16 ASA RUPTURE IN SCI In addition to spinal fracture and/or dislocation, SCI includes neural tissue and vascular
damage at the site of contusion. However, to our knowledge, no studies have detected ASA rupture or injury in SCI patients. It is interesting that no ASA ruptures were found in 19 patients
of our 20 SCI patients (even in six sever cervical fracture and dislocation ASIA A patients). This indicates that the spinal cord is more easily injured than the ASA in its anterior surface.
Although ischemia is a key factor and key contributor to the secondary pathogenesis of SCI, ARAs injuries, including ruptured ARAs, occluded ARAs, or compressed ARAs (caused by the swelling
of the surrounding tissue of the ARAs), are one possible cause of spinal cord ischemia but not ASA ruptures. For the ASA rupture of the stab-wound patient, no neurological improvement was
found, which indicates that individuals who experience ASA-rupture SCI have an even worse prognosis than individuals who experience ARA injuries. For visualization of ARAs in SCI, we failed
with catheter, CT and MRA (data not show). Advancing imaging techniques will definitely further improve the possibility of non-invasively visualizing intradural spinal cord arteries and
veins of SCI patients. CORRELATION BETWEEN SPINAL CORD ISCHEMIA AND ASA RUPTURE Acute traumatic SCI includes ischemia, axonal damage, swelling, edema, and hemorrhaging. All of these
pathological changes demonstrate irregular T2 hyperintensity signals. The extent to which ischemia contributes to these SCI pathologies cannot be determined. However, spinal cord ischemia
signal intensity is correlated with pathological changes, such as location and extent of the cord edema, which can be observed through MRI. Subtle spinal cord ischemia was found in BSS and
CCS patients, while distinct spinal cord ischemia was found in tetraplegia patients. In our studies, no ASA ruptures were found in any BSS, CCS or tetraplegia patients (except one stab-wound
patient), which suggests that spinal cord ischemia is not associated with ASA rupture. Spinal cord ischemia maybe correlated with primary SCI and a reduction of ARAs blood supply.
Neurological recovery is, to some extent, related to the degree of ischemic neuronal damage for acute spinal cord ischemia syndrome.17 In animal models, the duration of the ischemia is
related to histological damage and motor recovery.18 We agree with Iseli _et al._19 that the severity of the initial neurological injury constitutes the main factor, which determines the
prognosis in traumatic spinal cord injuries. Waters _et al._20, 21 pointed out that less than 1% of paraplegics reach ambulatory ability in the community if they do not present motor
contraction in their lower limbs within a month after injury. If motor power develops within a month after injury, this percentage increases greatly for both paraplegia and incomplete
tetraplegia (47.0%, 42.1%, respectively). In our studies, we followed up with all 20 SCI and ischemia patients for over 6 months. The neurological deficit, classified according to the ASIA
impairment scale, improved in the subtle spinal cord ischemia BSS and CCS patients (70%). This is not only because of increased spinal cord blood supply but also because the original spinal
cord injuries were not severe. Thirty percent of tetraplegia patients with distinct spinal cord ischemia have an unfavorable neurological recovery prognosis. Our results show that, aside
from the initial neurological injury, ischemia determines the prognosis of neurological improvement, especially for incomplete tetraplegia patients. CONCLUSION Understanding the details of
blood supply to the spinal cord after trauma is crucial for the ongoing discussion about the secondary pathogenesis of SCI, which influences the chronic functional deficits and necessity of
early decompression. Although no ASA rupture was found, for blunt cervical fracture and dislocation patients, neurological prognosis after SCI has a close correlation with ischemia without
ASA rupture. The limitations of this preliminary study include, visualization of ARAs, blinded CT perfusion measurements, limited patient population and CTA and MRI taken during follow-up.
DATA ARCHIVING There were no data to deposit. REFERENCES * Young W . Secondary injury mechanisms in acute spinal cord injury. _J Emerg Med_ 1993; 11 (Suppl 1): 13–22. Google Scholar * Kuker
W, Weller M, Klose U, Krapf H, Dichgans J, Nagele T . Diffusion-weighted mri of spinal cord infarction—high resolution imaging and time course of diffusion abnormality. _J Neurol_ 2004;
251: 818–824. Article Google Scholar * Novy J, Carruzzo A, Maeder P, Bogousslavsky J . Spinal cord ischemia: clinical and imaging patterns, pathogenesis, and outcomes in 27 patients. _Arch
Neurol_ 2006; 63: 1113–1120. Article Google Scholar * Backes WH, Nijenhuis RJ . Advances in spinal cord MR angiography. _AJNR Am J Neuroradiol_ 2008; 29: 619–631. Article CAS Google
Scholar * DiTunno JF, Young W, Creasey G . International standards for neurological and functional classification of spinal cord injury: revised 1992. _Paraplegia_ 1994; 32: 70–80. PubMed
Google Scholar * Yagi M, Ninomiya K, Kihara M, Horiuchi Y . Long-term surgical outcome and risk factors in patients with cervical myelopathy and a change in signal intensity of
intramedullary spinal cord on magnetic resonance imaging. _J Neurosurg Spine_ 2010; 12: 59–65. Article Google Scholar * Thron A . _Vascular Anatomy of the Spine_. Oxford University Press:
Oxford. 2002. Google Scholar * Krauss WE . Vascular anatomy of the spinal cord. _Neurosurg Clin N Am_ 1999; 10: 9–15. Article CAS Google Scholar * Savader SJ, Williams GM, Trerotola SO,
Perler BA, Wang MC, Venbrux AC _et al_. Preoperative spinal artery localization and its relationship to postoperative neurologic complications. _Radiology_ 1993; 189: 165–171. Article CAS
Google Scholar * Kieffer E, Fukui S, Chiras J, Koskas F, Bahnini A, Cormier E . Spinal cord arteriography: a safe adjunct before descending thoracic or thoracoabdominal aortic
aneurysmectomy. _J Vasc Surg_ 2002; 35: 262–268. Article Google Scholar * Nijenhuis RJ, Leiner T, Cornips EM, Wilmink JT, Jacobs MJ, van Engelshoven JM _et al_. Spinal cord feeding
arteries at MR angiography for thoracoscopic spinal surgery: feasibility study and implications for surgical approach. _Radiology_ 2004; 233: 541–547. Article Google Scholar * Bley TA,
Duffek CC, François CJ, Schiebler ML, Acher CW, Mell M _et al_. Presurgical localization of the artery of Adamkiewicz with time-resolved 3.0-T MR angiography. _Radiology_ 2010; 255: 873–881.
Article Google Scholar * Sheehy NP, Boyle GE, Meaney JF . Normal anterior spinal arteries within the cervical region: high-spatial-resolution contrast-enhanced three-dimensional MR
angiography. _Radiology_ 2005; 236: 637–641. Article Google Scholar * Nijenhuis RJ, Mull M, Wilmink JT, Thron AK, Backes WH . MR angiography of the great anterior radiculomedullary artery
(Adamkiewicz artery) validated by digital subtraction angiography. _AJNR Am J Neuroradiol_ 2006; 27: 1565–1572. CAS PubMed Google Scholar * Ou P, Schmit P, Layouss W, Sidi D, Bonnet D,
Brunelle F . CT angiography of the artery of Adamkiewicz with 64-section technology: first experience in children. _AJNR Am J Neuroradiol_ 2007; 28: 216–219. CAS PubMed Google Scholar *
Zhao SH, Logan L, Schraedley P, Rubin GD . Assessment of the anterior spinal artery and the artery of Adamkiewicz using multi-detector CT angiography. _Chin Med J (Engl)_ 2009; 122: 145–149.
Google Scholar * Little JW, Goldstein B, Gitter A, Haselkorn JK . Spinal cord infarction: varying degrees of upper and lower motoneuron dysfunction. _J Spinal Cord Med_ 1996; 19: 242–248.
Article CAS Google Scholar * Gelfan S, Tarlov IM . Interneurons and rigidity of spinal cord origin. _J Physiol (London)_ 1959; 146: 594–617. Article CAS Google Scholar * Iseli E,
Cavigelli A, Dietz V, Curt A . Prognosis and recovery in ischaemic and traumatic spinal cord injury: clinical and electrophysiological evaluation. _J Neurol Neurosurg Psychiatry_ 1999; 67:
567–571. Article CAS Google Scholar * Waters RI . Functional prognosis of spinal cord injuries. _J Spinal Cord Med_ 1996; 19: 89–92. CAS PubMed Google Scholar * Waters RL, Adkins R,
Yakura J, Sie I . Donal Munro Lecture: functional and neurologic recovery following acute spinal cord injury. _J Spinal Cord Med_ 1998; 21: 195–199. Article CAS Google Scholar Download
references AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Orthopedics, Xinqiao Hospital, Third Military Medical University, Chongqing, China Z Zhang, H Wang, Y Zhou & J Wang
Authors * Z Zhang View author publications You can also search for this author inPubMed Google Scholar * H Wang View author publications You can also search for this author inPubMed Google
Scholar * Y Zhou View author publications You can also search for this author inPubMed Google Scholar * J Wang View author publications You can also search for this author inPubMed Google
Scholar CORRESPONDING AUTHOR Correspondence to Z Zhang. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no conflict of interest. RIGHTS AND PERMISSIONS Reprints and permissions
ABOUT THIS ARTICLE CITE THIS ARTICLE Zhang, Z., Wang, H., Zhou, Y. _et al._ Computed tomographic angiography of anterior spinal artery in acute cervical spinal cord injury. _Spinal Cord_ 51,
442–447 (2013). https://doi.org/10.1038/sc.2012.179 Download citation * Received: 28 August 2012 * Revised: 23 December 2012 * Accepted: 28 December 2012 * Published: 22 January 2013 *
Issue Date: June 2013 * DOI: https://doi.org/10.1038/sc.2012.179 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a
shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * Spinal cord injury * CT
angiography * anterior spinal artery * cervical spine