A unified framework for diagnostic test development and evaluation during outbreaks of emerging infections
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Evaluating diagnostic test accuracy during epidemics is difficult due to an urgent need for test availability, changing disease prevalence and pathogen characteristics, and
constantly evolving testing aims and applications. Based on lessons learned during the SARS-CoV-2 pandemic, we introduce a framework for rapid diagnostic test development, evaluation, and
validation during outbreaks of emerging infections. The framework is based on the feedback loop between test accuracy evaluation, modelling studies for public health decision-making, and
impact of public health interventions. We suggest that building on this feedback loop can help future diagnostic test evaluation platforms better address the requirements of both patient
care and public health. SIMILAR CONTENT BEING VIEWED BY OTHERS A NOVEL BENCHMARK FOR COVID-19 PANDEMIC TESTING EFFECTIVENESS ENABLES THE ACCURATE PREDICTION OF NEW INTENSIVE CARE UNIT
ADMISSIONS Article Open access 13 October 2021 MACHINE LEARNING IN POINT-OF-CARE TESTING: INNOVATIONS, CHALLENGES, AND OPPORTUNITIES Article Open access 02 April 2025 PREDICTION OF
INDIVIDUAL COVID-19 DIAGNOSIS USING BASELINE DEMOGRAPHICS AND LAB DATA Article Open access 06 July 2021 INTRODUCTION Newly emerging infectious agents present a particular challenge for
diagnostic test development and evaluation. These agents often surface in the form of an outbreak, an epidemic or a pandemic with high urgency for targeted infection control, but minimal
knowledge about the infectious agents themselves. Rapid availability of diagnostic tests, along with information on their accuracy, however limited, is critical in these situations.
Traditional diagnostic study designs and quality assessment tools developed for individual patient care, as proposed in existing guidelines for diagnostic tests1,2,3, are difficult to apply
in a volatile environment in which there are continuously evolving research questions, infectious agents, and intervention options. These challenges were particularly apparent during the
SARS-CoV-2 pandemic, where the quality of diagnostic studies available in the field was generally limited. The Cochrane review on rapid, point-of-care (POC) antigen and molecular-based tests
for diagnosing SARS-CoV-2 infection found a high risk of bias in different domains in 66 of the 78 studies considered (85%)4. The most frequent potential source of bias was identified in
the reference standard domain, including potential of imperfect gold/reference standard bias, incorporation bias, and diagnostic review bias (an explanation of these biases is given in Table
1). In the Cochrane review on antibody tests for identification of current and past infection with SARS-CoV-25, the most frequent potential source of bias was identified in the patient
selection domain, due to selection or spectrum bias (48 of 54 studies, 89%). A particular issue is that biases with regard to the reference standard or the index test can lead to an
overestimation or underestimation of sensitivity and specificity. In both application fields, the differences between the diagnostic test accuracy estimates reported by the manufacturers and
those estimated later in the Cochrane meta-analyses were enormous. The mean sensitivity reported by manufacturers for antigen tests was 89% (as of 22/06/2022)6. In comparison, the
sensitivity estimated in the meta-analysis on antigen tests4 was 72% in symptomatic and 58% in asymptomatic individuals. This discrepancy shows that the timely evaluation of newly developed
laboratory tests under real-life conditions is crucial and should be planned and started before market launch. This Perspective article is the result of an interdisciplinary workshop which
we conducted as part of a research project funded by the German Research Foundation. This brought together expertise from all disciplines relevant to diagnostic test development and
evaluation, ranging from molecular test development to public-health decision-making. Firstly, the project members gave presentations on the respective sub-areas of the project to create a
common basis for the following moderated panel discussions, integrating the expertise and experience from the individual workshop participants. Subsequently, a previously created draft of
the framework was further developed and the next steps were planned. We describe the challenges and potential solutions that were discussed for implementing state-of–the-art diagnostic test
development and evaluation processes, based on accuracy studies performed at different phases of an epidemic or pandemic. This Perspective is divided into three key sections. First, we
discuss the relevance of diagnostic studies for public health decision-making based on mathematical models. Second, we describe the challenges in developing diagnostic tests and propose
study designs to accelerate the evaluation of their diagnostic accuracy. Third, considering the challenges mentioned above, we propose a unified framework for rapid diagnostic test
development and clinical evaluation. This highlights that multiple and perhaps different study designs will be necessary to build a convincing portfolio of evidence for various stakeholders
during outbreaks of emerging infections. DIAGNOSTIC TESTS AND TESTING STRATEGIES DURING THE COVID-19 PANDEMIC For SARS-CoV-2, three types of tests can be distinguished according to their
target: the polymerase chain reaction (PCR) test, the antigen test and the antibody test. The PCR test detects viral particles, the antigen test viral surface proteins and the antibody test
SARS-CoV-2 specific antibodies. POC tests refer to those that can be evaluated directly on site. They are available for all three test types. While PCR and antibody tests are usually only
performed by trained staff in hospitals and testing centers or similar, there are antigen tests for trained staff (rapid antigen test) but also as a home testing kit (antigen self-test,
freely accessible)7. The cost of testing varied widely across phases of the pandemic, countries, type of test, and manufacturer. Rapid antigen tests now cost $1 in the United States of
America (USA), PCR tests cost $5, and antibody tests cost $50 (test kit only with no personnel costs or similar; average approximate based on internet research and Du et al.8). PCR and
antigen tests use nasal or throat swabs as specimen material, antibody tests use a blood sample. The PCR test takes up to 48 h to give results, whilst the antigen and the antibody test give
results within 15 min. All tests are performed once, and a second test is often performed to confirm the test result (e.g., a PCR test to confirm a positive antigen test). A recently
published network meta-analysis showed a mean sensitivity of 93% and specificity of 98% for PCR tests, 75% sensitivity and 99% specificity for antigen tests9, and a Cochrane review reported
a sensitivity and specificity of 94.3% and 99.8% for total antibody tests10. Throughout the pandemic, these tests were used in different combinations as part of various population-level
testing strategies. Rapid antigen tests were used as part of screening and isolation programmes to detect asymptomatic infections in the community, and especially in key workers and
workplaces. This was often combined with follow-up testing with PCR tests to minimise unnecessary isolation due to false positives. Different testing strategies were used to fulfil various
aims, e.g. full population screening programmes to break infection chains and studies such as the Real-time Assessment of Community Transmission (REACT) study for high-quality, real-time
surveillance. Their strategies characteristics and costs differed accordingly. No public data on costs is available for most of these strategies, but modelling studies have been used to
assess their cost-effectiveness under certain assumptions, taking the context in which the testing strategies were used into account and synthesizing all available evidence8,11,12,13.
POPULATION-LEVEL INFORMATION AS A KEY INPUT FOR PUBLIC HEALTH DECISION-MAKING While diagnostic tests are usually developed for individual diagnosis and patient care, their results also play
a crucial role in public health decision-making. Population-level case data, collected based on the number of positive diagnostic tests in surveillance systems worldwide, are a central input
parameter for decision-making processes in public health policy. Cases might in this situation represent different outcomes of contact with an infectious agent (e.g., infections or deaths),
and also different types of measures of this contact (e.g., incident or cumulative cases derived from seroprevalence studies). Surveillance systems for infectious diseases provide reports
on the number of cases associated with specific pathogens using standardized case definitions based on pre-defined rules (including diagnostic test results) and legal obligations. These
surveillance systems run constantly for notifiable diseases associated with high public health risks14. Surveillance-related case data (based on diagnostic test results) are directly used
for public health decision-making. They enable the development and parameterization of infectious disease models (e.g., for early warning and monitoring) and for decision-analytic models
(e.g., for assessing the benefit-harm, cost-effectiveness or other trade-offs when guiding public health interventions). This is especially true in epidemic or pandemic situations when
reducing harm at a population level becomes a crucial aspect of the decision-making philosophy15,16, and high consequence decisions must be made under uncertainty and time pressure. In such
scenarios, two fundamental and extremely relevant quantities are some measure of the presence of the infection in the population (e.g., prevalence or incidence data) and a measure of
existing immunity to the infection in the population, i.e. seroprevalence data. An important decision supported by dynamic infectious disease modelling studies focusing on predicting
infection dynamics is the timing of interventions. Interventions are most effective when deployed in time17 and may cease to be effective if implemented too late18. Therefore, it is
imperative that decisions about implementing interventions are made in a timely manner and sometimes with incomplete evidence, but with all relevant information being collected and reported
appropriately. Monitoring population-level data from as soon as possible is essential since it can be used to set thresholds for starting interventions19 and determine when intervention
measures are no longer necessary and can be ended20. Due to reporting delays and the fact that the decision-making process is not instantaneous, decisions can come too late when relying
solely on current population-level data. This is where infectious disease modelling comes in. Models help decision-makers obtain reasonable estimates of how the epidemic is likely to
progress and what impact different interventions may have. This enables timely and informed decision-making21,22,23. Combined with benefit-harm and health economic models to account for
unintended effects and costs of interventions, infectious disease models enable decision-makers to make optimal decisions given the available evidence and resources24,25,26. The points
discussed above are exemplified by decision-making during the SARS-CoV-2 pandemic. Even during the early phases of the pandemic, decisions about interventions were made from population-level
data. In the United Kingdom (UK), the timing of the first nationwide lockdown was determined based on the predicted number of people treated for SARS-CoV-2 in intensive care units (ICUs)19.
In Australia, more targeted lockdowns were implemented based on regional prevalence data27,28, and local lockdowns were also implemented in the UK during later phases of the pandemic29.
Prevalence data became even more important when contact tracing and test-intervention strategies were implemented, because the predictive value of diagnostic tests depends on the infection
prevalence. As vaccines became available, subpopulations most at risk of severe COVID-19 were prioritised and given the opportunity to be vaccinated first30,31. In Germany, vaccination and
testing control rules for access to parts of public life varied from region to region. Again, the region-specific thresholds were based on the number of hospitalised patients testing
positive for SARS-CoV-2 in the respective region32. Mathematical models were used throughout to support the decision-making process. The threshold for applying the first nationwide lockdown
in the UK was set based on the number of people estimated to be potentially needing ICU treatment based on different modelling scenarios19. In Austria, the decision to prioritise vaccinating
elderly and vulnerable groups was based on decision-analytic modelling aiming to minimise hospitalisations and deaths33. In general, infectious disease and decision-analytic models
contributed substantially to the type and intensity of interventions implemented34,35,36. Once tests became widely available, they were also used to devise effective mass testing and
isolating strategies37,38. The current pandemic has thus demonstrated the need for accurate and timely population-level case data and clinical case data (requiring different diagnostic tests
and testing strategies), to allow public health policy decisions to be as well-informed as possible. Diagnostic tests, as the primary tool to obtain these population-level data, are
therefore at the heart of all modelling efforts during an epidemic or pandemic, and early and precise knowledge about their accuracy is crucial for interpreting and further applying these
case data. CHALLENGES FOR DIAGNOSTIC TEST EVALUATION IN AN EPIDEMIC SETTING Diagnostic tests developed for emerging infections should serve various purposes, including individual clinical
diagnosis, screening, and surveillance. These purposes demand distinct strategies and, in theory, require separate approval mechanisms39. However, test development, evaluation of technical
validity, clinical validity and utility, as well as test validation currently do not account for the different uses in a generalized way. The challenges and potential solutions in this
article and the framework proposed therein have been described with all these purposes in mind and are summarised in Box 1. In the initial phase of an outbreak of an emerging infection, the
main focus of diagnostic test development is providing a diagnostic test that can identify infected individuals with high sensitivity, so that they can be isolated and treated as soon as
possible. This is especially important because the effectiveness of contact tracing depends directly on the quality and timeliness of case identification. Of course, a high specificity is
also important, to prevent unnecessary isolation or treatment. This is usually achieved by direct detection of the pathogen, e.g., by molecular genetic tools such as PCR, microscopy, antigen
tests or cultivation of the microorganisms involved. Later, a better understanding of the immune protection caused by contact with the agent is required, leading to the development of
indirect pathogen detection tools such as antibody tests. Here, sensitivity and specificity are equally important to evaluate proxies of long-term immune protection and to detect past low
severity infections which would have been missed otherwise. However, from the perspective of population-level modelling, some accuracy may be sacrificed if the true diagnostic accuracy of
the test is known so that aggregate correction methods can be applied. Knowledge of the specificity of the direct detection tools developed earlier can also come into play in the case of
reported reinfections, when it becomes important to understand whether these are true reinfections or due to false positives in a time of intensified testing. High specificity is also
important once treatment options are available, but possibly come with relevant side effects, high costs or limited availability. Different population-level uses also require different
diagnostic characteristics. Although PCR tests with a noticeable delay between testing and communication of results were used for population-level testing during the early phases of the
COVID-19 pandemic, tests used for population screening generally need to be easily and quickly administered as POC tests, and lower accuracies, especially in specificity, are accepted as a
trade-off for this. However, relatively high sensitivity is still important to make testing-and-isolation strategies effective. Deficiencies in specificity may be compensated for by
confirmatory follow-up testing with highly specific tests to minimise unnecessary isolation. Furthermore, target populations, testing aims and prioritised estimators (e.g. sensitivity or
specificity) can change rapidly, necessitating constant test evaluation and re-evaluation. During an epidemic or pandemic, direct and indirect tests are thus used for different purposes and
require different study designs, with different sample size calculations and study populations, to provide critical information with high precision and validity. During epidemics with
emerging infections, all new tests must, in general, quickly go through three steps: the test must be developed, its clinical performance assessed, and then information on its performance
incorporated into infectious disease modelling to inform public health decision-making. Each step has potential sources of various biases that must be considered. Next we describe potential
challenges during these steps and how these challenges might affect the submission process to regulatory agencies, also considering the perspective of test developers from industry. BOX 1
SUMMARY OF DISCUSSED CHALLENGES AND PROPOSED SOLUTIONS THERE IS A LACK OF SAMPLES FROM INFECTED INDIVIDUALS FOR PHASE I TEST DEVELOPMENT STUDIES DURING THE EARLY PHASES OF AN EPIDEMIC OR
PANDEMIC. * Joint data collection and sharing infrastructure can be used to make infected samples available to test developers. THRESHOLD SELECTION FOR DIAGNOSTIC TESTS MUST BE DONE IN
ADVANCE, BUT THE OPTIMAL THRESHOLD DEPENDS ON EVER-CHANGING DISEASE PREVALENCES AND CONSEQUENCES OF MISCLASSIFICATION. * A limited pool of promising thresholds can be evaluated
simultaneously. * Mixture modelling without defining a threshold can be used. * Prevalence-specific thresholds can be developed and defined a-priori. OFTEN-USED TWO-GATE DESIGNS FOR PHASE II
STUDIES LIKELY LEAD TO OVERESTIMATION OF DIAGNOSTIC TEST ACCURACY. * Seamless enrichment designs, whereby proof-of-concept and confirmation are performed together as one study, can be used.
TESTS USED AS REFERENCE STANDARDS ARE THEMSELVES IMPERFECT. * The use of follow-up data or composite reference standards that use all tests or clinical criteria available for diagnosis can
alleviate this issue. However, one should be mindful of incorporation bias if the test under evaluation is part of the composite reference standard. THE NEED FOR TESTS TO BE DEVELOPED AND
EVALUATED RAPIDLY TO BE USED AS PART OF PUBLIC HEALTH INTERVENTIONS CONFLICTS WITH THE THOROUGH STUDY PROCESSES REQUIRED TO ENSURE TRANSPARENCY, REPRODUCIBILITY, AND PRIVACY. * Adaptive
study designs can shorten time-to-market while maintaining the rigour of a high-quality study. RAPIDLY CHANGING DISEASE PREVALENCES DURING RECRUITMENT MAY MAKE A PRIORI SAMPLE SIZE
CALCULATIONS INAPPROPRIATE. * Estimates of changing prevalence obtained via predictive modelling can be incorporated into the design of the study. REQUIREMENTS SUCH AS SAMPLE SIZE,
PROPERTIES OF REFERENCE TEST, AND INCLUSION CRITERIA DIFFER BY COUNTRY, INCREASING STUDY COMPLEXITY. * Careful upfront planning of multi-centre studies within a network such as the European
Centre for Disease Control or a European Society of Clinical Microbiology and Infectious Diseases study group can keep complexity to a minimum. DIAGNOSTIC TESTS HAVE DIFFERENT ROLES AND
TARGET POPULATIONS DURING DIFFERENT STAGES OF AN EPIDEMIC, REQUIRING DIFFERENT PERFORMANCE CHARACTERISTICS AND NECESSITATING CONSTANT EVALUATION AND RE-EVALUATION. * Adaptive designs allow
for sample size re-estimation and additional recruitment during the study to handle changing target populations. * A longitudinal panel to be tested regularly using the test under evaluation
can facilitate and expedite recruitment into diagnostic studies. * A platform comparable to the REACT study or the ONS panel in the UK can be extended to make use of data from hospitals,
health insurance companies, or public health agencies in diagnostic studies. * Value-of-information analyses based on infectious disease modelling can help guide selection of optimal
performance characteristics taking into account the purpose of the test being evaluated. DIAGNOSTIC TEST DEVELOPMENT Diagnostic tests for emerging infections typically are in the in vitro
diagnostic (IVD) test category, as they examine human body specimens (e.g., nasopharyngeal swabs, nasal swabs, blood or saliva39). IVDs are generally considered medical devices40.
Consequently, their development has to adhere to the rules of regulatory agencies and a pre-defined complex legal framework. Currently, the European Union (EU) IVD Regulation 2017/746 covers
IVD medical devices, and focuses on a legislative process that prioritises individual safety, which means that different types of clinical data must be collected before submission. If a
test is deemed capable of distinguishing infected individuals from non-infected ones, it has to be shown not to produce a one-off result41. There are several phase models for the development
of diagnostic tests described in the literature. We discuss using the frequently used four-phase model2,42,43. The four phases for this are: I, evaluation of analytical performance; II,
diagnostic accuracy estimation and determination of threshold; III, clinical performance estimation; and IV, evaluation together with diagnostic and/or therapeutic measures with regard to a
patient-relevant endpoint. Inter-rater agreement, analytical sensitivity (minimally detectable levels)41 and cross-reactivity have to be investigated in the phase I studies to verify the
technical validity, repeatability and reproducibility of laboratory tests (on a lot-to-lot, instrument group, and day-to-day basis). However, in the early phase of an epidemic or pandemic,
there are often not enough samples from infected individuals. Sharing data and using a common infrastructure by, for instance, collecting samples at national reference centres, could solve
this problem, if they are made accessible to IVD developers. A possible limitation of this approach is the risk of spectrum bias due to the particular mix of individuals, e.g. there may be
more severe cases in the samples than in the population. Furthermore, regulatory agencies do not allow the use of (frozen) biobank samples for approval. After having shown good technical
performance, clinical performance in phase II and III studies must be demonstrated. An integral part of assessing the sensitivity and specificity of a continuous diagnostic test is the
determination of the threshold at which it should be used41. This must be fixed before moving on to diagnostic test evaluation, to avoid bias caused by a data-driven threshold
selection44,45. The optimal threshold for a diagnostic test depends on the prevalence and consequences associated with misclassification38,46, which may both change over time. Thus a new
study is needed every time the threshold changes, requiring extensive resources (particularly time and money). Phase II studies are initial, so-called proof-of-concept studies covering
clinical performance and are often carried out in a two-gate design47, where sensitivity is estimated in diseased individuals and specificity in healthy samples from a different source.
However, this design can lead to spectrum bias (Table 1). Sensitivity and specificity have been shown to be generally overestimated in such studies47. Likewise, a meta-analysis showed that a
two-gate case-control design can lead to an overestimation of diagnostic accuracy48. In most situations outside an epidemic or pandemic, individuals tested are symptomatic and suspect they
have the infection of interest, if the test is to be used to guide therapy or decide about isolation. However, during epidemics or pandemics, tested individuals can also be asymptomatic if
the test is intended as a contact tracing or screening test41. In both cases, real-world samples may not be as perfect as in a laboratory situation41 because testing can also be performed at
POC, in the community, at the workplace, school, or home39. A test may require different performance characteristics if it is the first test in line, used to triage who will be tested
further, compared to when the test is used to confirm infection. For instance, in a confirmation setting, most individuals who clearly do not have the infection of interest will be
excluded41. DIAGNOSTIC TEST EVALUATION IVDs must be evaluated in phase III diagnostic accuracy studies that ideally start by including all individuals who will be tested in clinical practice
to avoid selection bias (all-comer studies). Individuals fulfilling the inclusion criteria should be enrolled consecutively, without judging how likely this person is to test positive or
negative41. In such prospective diagnostic studies, to minimise variability and thus increase statistical power, all study participants ideally undergo all tests under investigation (index
tests) as well as the reference standard to assign their final diagnosis. The reference standard must be sufficiently reliable to differentiate between people with and without the target
condition, but it is usually not perfect41. This imperfectness has to be taken into account when interpreting the results. Suppose a POC antigen test for SARS-CoV-2 is evaluated with a PCR
test as reference standard resulting in a sensitivity of 90%. This does not mean that 90% of people with SARS-CoV-2 will be detected but that the POC test will be positive in 90% of cases
with a positive PCR test. Solutions to this may include follow-up data or composite reference standards, which use all tests or clinical criteria available for a diagnosis. However, if the
test under evaluation is part of this composite reference standard, this may lead to incorporation bias49. Depending on the phase of the epidemic or pandemic, recruitment speed can vary
considerably due to changes in incidence. The guideline on clinical evaluation of diagnostic agents of the European Medicine Agency2 demands sample size specification in a confirmatory
diagnostic accuracy study in the study protocol. The required sample size is highly dependent on the prevalence of the target condition, which may change during the recruitment phase, making
a priori sample size calculations inappropriate at the time of recruitment. SUBMISSION TO REGULATORY AGENCIES Studies for industry face rigorous regulatory and ethics requirements as
clinical trials follow strict processes and regulatory guidelines which are assessed in the regulatory submission process and are potentially controlled by audits. Clinical studies must be
transparent, traceable and reproducible. Special attention must be paid to data quality and privacy. This leads to very detailed study preparation, documentation, quality control, and long
and less flexible study processes. When the SARS-CoV-2 pandemic began, the need for diagnostic tests grew with the rising number of cases. Regulatory bodies (like the U.S. Food & Drug
Administration, FDA) established country-specific emergency use authorization guidelines50,51to make it easier and faster to bring a test for SARS-CoV-2 to the market and make it accessible
during the pandemic. The WHO declared the end of the COVID-19 pandemic as a Public Health Emergency on 5 May 202352. However, the FDA did not set a specific end date for the use of
diagnostic tests authorized under Emergency Use Authorization (EUA), and they still remain valid under section 564 of the Federal Food, Drug, and Cosmetic Act, enabling uninterrupted use of
EUA-authorized COVID-19 tests until further notice of regulatory transition requirements53,54. Submission process requirements such as sample size, inclusion criteria for subjects, and
properties of the reference test differ between countries and may change during an epidemic or pandemic. Therefore, it is not always possible for a single study to be the basis for
submissions to different countries or certificates, and several studies must be planned. The different and changing requirements are not the only challenges submission teams face. The
changing prevalence of infection makes adequate project management and timeline planning difficult. Recruitment of positive cases fulfilling the recruitment requirements can be slow which
leads to a longer study duration and, subsequently, longer time to market. New mutations make re-evaluations of statistical properties necessary. Considering regulatory changes during
pandemics and possible mutations, (pre)planning such a study is complicated and time-consuming. POTENTIAL SOLUTIONS FOR THE CHALLENGES PRESENTED The challenges discussed previously are
multidimensional but can be addressed by three countermeasures in several areas. First, test developers should use methodological approaches to address study designs and statistical
analyses, increasing study efficiency and reducing the risk of bias. Second, strategic approaches and regulatory guidance for the industry should be deployed to clearly define opportunities
but also limitations in the development and approval process. Third, results and feedback from population-level mathematical modelling should inform test development and validation for
deriving optimal study designs based on formal value-of-information analyses. METHODOLOGICAL SOLUTIONS Methodological solutions fall into two categories; statistical methods to control bias,
and those to increase speed and efficiency. The different biases in diagnostic studies have been described extensively, both in general55,56,57 and also specifically in the context of the
SARS-CoV-2 pandemic58 and POC tests for respiratory pathogens59. From a methodological standpoint, the problem of bias can be addressed in two ways: either by choosing a study design in the
planning stage that minimises the risk of bias, or by using analytical methods that correct for potential bias. An excellent overview of how to avoid bias through an appropriate design can
be found in Pavlou et al.60. Important for the planning phase is the work of Shan et al.61, who present an approach to calculate the sample size in the presence of verification bias (i.e.,
partial or differential verification bias). In terms of bias reduction methods during the analysis phase, most studies focus on the correction of verification bias. Bayesian approaches are
mainly proposed for differential verification bias62,63, while there are a variety of methods for partial verification bias64. Time to market has to be reduced significantly in pandemics to
find an optimal trade-off between misclassification and missed opportunities for action. From a statistical point of view, the methods and processes must be reconsidered. One possibility to
improve study designs and statistical analysis is adaptive designs, that can increase efficiency. These approaches have been long established in therapeutic studies and are also anchored in
guidelines3,65. With adaptive designs, it is possible to make pre-specified modifications during the study. For example, inclusion and exclusion criteria can be changed, the trial can be
terminated early due to futility or efficacy, or the sample size can be recalculated. The characteristics and typical adaptive designs have been very clearly summarised66. A review of
published studies with adaptive designs showed that the pharmaceutical industry in particular increasingly uses simple adaptive designs, with more complex adaptive designs still being
rare67. In diagnostic studies, however, experience in using adaptive designs in diagnostic clinical trials for submissions is limited. A summary of the current state of research is available
for diagnostic accuracy studies68, for randomised test-treatment studies69 and for adaptive seamless designs70. Methods for blinded and unblinded sample size re-calculations for diagnostic
accuracy studies have been published recently71,72,73, as well as adaptive designs for test-treatment studies74 and adaptive seamless designs. The diagnostic industry heavily depends on
regulatory guidelines worldwide. If regulatory bodies emphasise more efficient diagnostic trials that include, e.g., adaptive designs, the implementation of modern study designs will be
incentivised. In the following, concrete possible solutions to the above-mentioned challenges are explained as examples. For details, please refer to the corresponding articles. Firstly, the
problem of setting a threshold in an early study that may later turn out not to be optimal can be addressed by selecting a limited pool of promising thresholds75. These are then evaluated
simultaneously in the validation study, with the type I error adjusted accordingly. Another approach is to use mixture modelling without defining a threshold76. Prevalence-specific cut-offs
might be developed and defined a priori. Secondly, if the testing strategy and thus the target population change during the study, adaptive designs offer the possibility to re-estimate the
sample size in a blinded manner based on the prevalence estimated in the interim analysis71. Thirdly, a seamless enrichment design can be chosen to address the problem of biased diagnostic
accuracy in two-gate designs, in which proof-of-concept and confirmation are performed together in one study. However, it is apparent that regulatory authorities are cautious of the possible
shortcomings of these innovative designs, and a lot of work needs to be done to get them approved77. This, in turn, results in the manufacturers of diagnostic tests being conservative in
their study designs. SOLUTIONS FOR POLITICAL DECISION-MAKING BASED ON MATHEMATICAL MODELLING The accuracy, accessibility, and costs of diagnostic tests all play a role in decisions about
testing programmes, and the decision on whether, for instance, a more sensitive test with lower accessibility and higher costs (in the context of SARS-CoV-2, e.g. a real-time PCR test)
should be administered with low frequency or a less sensitive test with better accessibility and lower costs (in the context of SARS-CoV-2, e.g. a rapid antigen test) should be administered
with higher frequency is context-specific. Mathematical models can and should support these decisions in real-time, as was the case during the COVID-19 pandemic78. When considering model
input data, one key aspect that has to be taken into account by modelling studies is the deliberate parameterization of accuracy for case numbers based directly or indirectly on the results
of a diagnostic test79. This typically includes incidence rates as well as seroprevalence estimates. Knowledge about the diagnostic accuracy of the tests is critical as biased estimates of
sensitivity and specificity can lead to biased estimates in modelling results used for health decision making. The textbook example for this is an overestimation of the specificity of an
antibody test used for seroprevalence studies in low-prevalence settings which leads to an overestimation of the proportion of the population which has had already contact with the emerging
infectious agent. As a consequence, population immunity would be overestimated, underestimating the risk associated with an uncontrolled spread in the population. If true diagnostic accuracy
is known, population-level estimates on e.g. seroprevalence can be corrected, either before the modelling study or within the modelling framework80. If it is not known that the proposed
diagnostic accuracy is biased, modelling can help in detecting implausibilities, especially if parameter fitting is carried out regularly. Here, modelling can inform diagnostic test
evaluation with respect to potential biases, but also in the context of value of information analyses. During the first three months of the SARS-CoV-2 pandemic, only a minority of modelling
studies in the field accounted for test accuracy estimates; the remaining used incidence and later seroprevalence data as if they represented the ground truth. This approach would be
appropriate if incidence or seroprevalence data were already corrected for imperfect test accuracy estimates. However, in this case, the correction procedure should still be reported in the
modelling study to enable a transparent evaluation of model parameterization, and the model(s) should be reparametrized once updated information on diagnostic test accuracy is available.
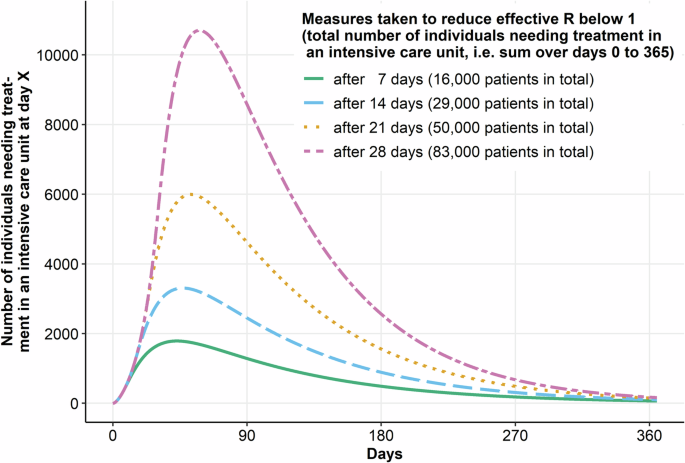
Earlier decision making based on updated information increases the impact of these decisions on population health (Fig. 1). Decisions just a few weeks or even a couple of days earlier can
make a huge difference, offering a critical time window for accelerated diagnostic studies. Fig. 2 shows the sensitivity of model-based assessments of interventions to diagnostic test
accuracy parameters. The results show that even relatively small biases in the estimation of test accuracy (much smaller than those found in the Cochrane reviews) for an antibody test used
to derive the proportion of undetected cases in a population have an enormous effect on the predicted further course of the epidemic (the mechanism for this impact is that the proportion of
undetected cases is used to correct reported case numbers before they are used to calibrate transmissibility estimates and other parameters). The results are enough to change public health
decision-making from, for example, not implementing population-level contact reduction measures to introducing a hard lockdown if the defined outcome of interest crosses a set
decision-analytic threshold. LONGITUDINAL PANELS AS A PLATFORM FOR DIAGNOSTIC ACCURACY STUDIES Given the rapidly changing research questions during an epidemic or pandemic, there is a huge
practical challenge in setting up diagnostic studies even with the modern study designs described above, because the acceptable time spans for recruiting study participants and for
conducting the actual studies are very short. The availability of a study platform that allows immediate initiation of diagnostic studies reflecting the current research question and
infection dynamics is indispensable for timely studies in the field. One way to ensure this is the sustainable implementation of a longitudinal panel within existing cohorts (e.g., as the
NAKO Health Study81) that is tested regularly for the presence or absence of the pathogen by a defined test (or several) under evaluation. Another approach is to use data from hospitals,
health insurance or public health agencies. For example, a platform comparable to the UK Office for National Statistics (ONS) panel82 or the REACT study83 can be built and used to evaluate
the tests or testing strategies under study, and for real-time communication of the results of the respective tests representing current or past infection dynamics. In this setting, flexible
and fast study designs can fulfil both, equally important, purposes at the same time. FEEDBACK TRIANGLE AT THE CENTRE OF A UNIFIED FRAMEWORK As discussed above, the development and
evaluation of diagnostic tests in an epidemic or pandemic setting is closely linked to modelling studies used to inform political and public health decision-making. This link is at the
centre of the unified framework we propose based on experiences during the SARS-CoV-2 pandemic (Fig. 3). The execution of diagnostic studies for new tests or new application areas of
existing tests depends heavily on current test strategies and those potentially applied in the future. Results from diagnostic studies are a direct input in mathematical modelling studies,
and in turn results from these models are used for decision-making based on a defined decision-making framework. However, modelling studies can also give crucial feedback to those
responsible for planning and analysing diagnostic accuracy studies. Here, so-called value-of-information analyses can help identify those gaps in knowledge regarding diagnostic test accuracy
that need to be tackled first or require the greatest attention84. This can directly affect sample size estimations, for instance if more precision is needed to estimate the test’s
specificity (as is often the case with antibody tests). Therefore, the optimal strategy to deal with these constant feedback loops is to establish continuous collaboration between the
disciplines representing the three parts of this loop (in green in Fig. 3). This collaboration platform can use the longitudinal panel with complementary perspectives described above to
create a unified diagnostic test development and evaluation framework during an epidemic or pandemic. The modern study designs and bias reduction methods described above can be applied to
obtain the best potentially available evidence about diagnostic test accuracy in different settings. When creating such a framework for developing and evaluating diagnostic tests and
considering the corresponding results in modelling studies, both infection-specific (e.g. transmission rates, case fatality ratios) and test-specific characteristics (e.g. test type, costs,
availability) must be considered, especially for the collection of population-level data from testing programmes. DIAGNOSTIC TEST-INTERVENTION STUDIES USING A CLUSTER-RANDOMISED APPROACH In
many situations, diagnostic test accuracy estimates should only be seen as surrogate information since the actual outcome of interest during an ever-changing pandemic, especially in the
later phases, is the effect of an application of this test on clinical or population-level outcomes. Here, it is possible, as has been discussed during the SARS-CoV-2 pandemic, to take a
step further and move test evaluation to phase IV or diagnostic test-intervention studies. In this phase, individuals or clusters of individuals are randomised to a diagnostic strategy
(e.g., regular testing of the entire population or testing only in case of symptoms). The relevant clinical endpoint is then compared between randomised groups42. Thus, the test strategy is
treated as an intervention evaluated for its effectiveness and safety. Diagnostic test accuracy helps to reach this endpoint but is not the only factor under evaluation. The practicability
of the strategy, as well as real-world effectiveness and interaction with other interventions (e.g., the case isolation and quarantine of close contacts), are also assessed indirectly in
this approach. In a dynamic infectious disease setting where an intervention can have indirect effects on people other than the target population, only cluster-randomised approaches allow
for a reasonable estimation of population-level effects of the intervention under study. In infectious disease epidemiology, similar designs are applied when assessing the effectiveness of
vaccination programs on a population level, often combined with a staggered entry approach to allow all clusters to benefit from the intervention over time (so-called stepped-wedge design).
During the pandemic, small-scale pilot studies were discussed, trying to mirror such an approach in a non-randomised way, often claiming to be a natural experiment. However, most of them did
not follow guidelines and recommendations available for diagnostic test-intervention studies that would have improved the quality of the results and their usefulness for evidence-based
public health. Rigorous application of cluster-randomised diagnostic test-intervention studies to implement testing strategies can support decision-making processes in the later stages of an
epidemic or pandemic. CONCLUSION The development and evaluation of diagnostic tests for emerging infectious agents during an epidemic or pandemic come with many serious challenges. We
propose integrating diagnostic studies in a unified framework representing the triangle of diagnostic test evaluation, predictive or decision-analytic public health modelling and the testing
strategy applied in this population. This framework can use modern, flexible and fast study designs and should incorporate a longitudinal panel as a continuous study platform. Diagnostic
test-intervention studies need to be planned early and should be used for evidence-based public health during later phases of an epidemic or pandemic, when research questions become more
complicated and testing strategies serve as interventions to counteract infectious disease dynamics. REFERENCES * Regulation (EU) 2017/746 of the European Parliament and of the Council of 5
April 2017 on in vitro diagnostic medical devices and repealing Directive 98/79/EC and Commission Decision 2010/227/EU. http://data.europa.eu/eli/reg/2017/746/oj (2017). * European Medicines
Agency. _Guideline on clinical evaluation of diagnostic agents_ (European Medicines Agency, 2009). * FDA. Statistical Guidance on Reporting Results from Studies Evaluating Diagnostic Tests
- Guidance for Industry and FDA Staff.
https://www.fda.gov/regulatory-information/search-fda-guidance-documents/statistical-guidance-reporting-results-studies-evaluating-diagnostic-tests-guidance-industry-and-fda (2007). *
Dinnes, J. et al. Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection. _Cochrane Database Syst. Rev._ 2021, CD013705 (2021). PubMed Central Google
Scholar * Deeks, J. J. et al. Antibody tests for identification of current and past infection with SARS-CoV-2. _Cochrane Database Syst. Rev._ 2020, CD013652 (2020). PubMed Central Google
Scholar * DxConnect Test Directory. https://finddx.shinyapps.io/testdirexplorer_beta/ (2022). * Röhrig, B. The Diagnostic Test: Goodness, Characteristics, and Interpretation: Under the
Impact of the Corona Pandemic and Different SARS-CoV-2 Tests. _Gesundheitswesen_ 85, 578–594 (2023). Article PubMed PubMed Central Google Scholar * Du, Z. et al. Comparative
cost-effectiveness of SARS-CoV-2 testing strategies in the USA: a modelling study. _Lancet Public Health_ 6, e184–e191 (2021). Article PubMed PubMed Central Google Scholar * Veroniki, A.
A. et al. Rapid antigen-based and rapid molecular tests for the detection of SARS-CoV-2: a rapid review with network meta-analysis of diagnostic test accuracy studies. _BMC Med_ 21, 110
(2023). Article PubMed PubMed Central Google Scholar * Fox, T. et al. Antibody tests for identification of current and past infection with SARS-CoV-2. _Cochrane Database Syst. Rev._
2022, CD013652 (2022). PubMed Central Google Scholar * Hurtado, A. V. et al. The economic cost of implementing antigen-based rapid diagnostic tests for COVID-19 screening in high-risk
transmission settings: evidence from Germany. _Health Econ. Rev._ 12, 15 (2022). Article PubMed PubMed Central Google Scholar * Pighi, L. et al. Cost-effectiveness analysis of different
COVID-19 screening strategies based on rapid or laboratory-based SARS-CoV-2 antigen testing. _Clin. Chem. Lab. Med._ 61, E168–E171 (2023). Article PubMed CAS Google Scholar * Nguyen, H.
T. et al. Cost and cost-effectiveness of four different SARS-CoV-2 active surveillance strategies: evidence from a randomised control trial in Germany. _Eur. J. Health Econ._ 24, 1545–1559
(2023). Article PubMed PubMed Central Google Scholar * ECDC. Surveillance Atlas of Infectious Diseases. http://atlas.ecdc.europa.eu/public/index.aspx (2024). * Daugherty, B. L. et al.
Ethical considerations: care of the critically ill and injured during pandemics and disasters. _Chest Consens. Statement Chest_ 146, e145S–e155S (2014). Google Scholar * Emanuel, E. J. et
al. Fair Allocation of Scarce Medical Resources in the Time of Covid-19. _N. Engl. J. Med._ 382, 2049–2055 (2020). Article PubMed Google Scholar * Kelso, J. K., Milne, G. J. & Kelly,
H. Simulation suggests that rapid activation of social distancing can arrest epidemic development due to a novel strain of influenza. _BMC Public Health_ 9, 117 (2009). Article PubMed
PubMed Central Google Scholar * Faes, C., Hens, N. & Gilbert, M. On the timing of interventions to preserve hospital capacity: lessons to be learned from the Belgian SARS-CoV-2
pandemic in 2020. _Arch_. _Public Health_ 79, 164 (2021). Google Scholar * Ferguson, N. M. et al. Report 9: Impact of non-pharmaceutical interventions (NPIs) to reduce COVID19 mortality and
healthcare demand. https://doi.org/10.25561/77482 (2020). * Ngonghala, C. N. et al. Mathematical assessment of the impact of non-pharmaceutical interventions on curtailing the 2019 novel
Coronavirus. _Math. Biosci._ 325, 108364 (2020). Article PubMed PubMed Central CAS Google Scholar * van Kerkhove, M. D. & Ferguson, N. M. Epidemic and intervention modelling – a
scientific rationale for policy decisions? Lessons from the 2009 influenza pandemic. _Bull. World Health Organ_ 90, 306 (2012). Article PubMed PubMed Central Google Scholar *
Heesterbeek, H. et al. Modeling infectious disease dynamics in the complex landscape of global health. _Science_ 347, aaa4339 (2015). Article PubMed PubMed Central Google Scholar *
Kretzschmar, M. E. et al. Challenges for modelling interventions for future pandemics. _Epidemics_ 38, 100546 (2022). Article PubMed PubMed Central CAS Google Scholar * Mauskopf, J. et
al. Economic Analysis of Vaccination Programs: An ISPOR Good Practices for Outcomes Research Task Force Report. _Value Health_ 21, 1133–1149 (2018). Article PubMed Google Scholar *
Siebert, U. When should decision-analytic modeling be used in the economic evaluation of health care? _Eur. J. Health Econ._ 4, 143–150 (2003). Article Google Scholar * Ultsch, B. et al.
Methods for Health Economic Evaluation of Vaccines and Immunization Decision Frameworks: A Consensus Framework from a European Vaccine Economics Community. _Pharmacoeconomics_ 34, 227–244
(2016). Article PubMed Google Scholar * ABC News. Melbourne lockdown extended by seven days as Victoria records 20 local COVID-19 cases.
https://www.abc.net.au/news/2021-08-11/victoria-covid-cases-melbourne-lockdown-extension/100366822 (2021). * Al Jazeera. Australia’s Canberra extends COVID-19 lockdown.
https://www.aljazeera.com/news/2021/8/31/australias-canberra-extends-covid-19-lockdown (2021). * The Institute for Government. Coronavirus: local lockdowns.
https://www.instituteforgovernment.org.uk/explainers/coronavirus-local-lockdowns (2020). * Federal Government: Federal and Länder Governments consult on the coronavirus situation.
https://www.bundeskanzler.de/bk-en/news/corona-state-premier-conference-1983156 (2021). * World Health Organisation. WHO _SAGE Roadmap for Prioritizing Uses of COVID-19 Vaccines in the
Context of Limited Supply: An Approach to Inform Planning and Subsequent Recommendations Based on Epidemiological Setting and Vaccine Supply Scenarios, First Issued 20 October 2020, Latest_
(WHO, 2021). * Gesetz zur Verhütung und Bekämpfung von Infektionskrankheiten beim Menschen (IfSG). https://www.gesetze-im-internet.de/ifsg/ (2000). * Jahn, B. et al. Targeted COVID-19
Vaccination (TAV-COVID) Considering Limited Vaccination Capacities—An Agent-Based Modeling Evaluation. _Vaccines_ 9, 434 (2021). Article PubMed PubMed Central CAS Google Scholar *
Moore, S., Hill, E. M., Tildesley, M. J., Dyson, L. & Keeling, M. J. Vaccination and non-pharmaceutical interventions for COVID-19: a mathematical modelling study. _Lancet Infect. Dis._
21, 793–802 (2021). Article PubMed PubMed Central CAS Google Scholar * Chowdhury, R. et al. Dynamic interventions to control COVID-19 pandemic: a multivariate prediction modelling study
comparing 16 worldwide countries. _Eur. J. Epidemiol._ 35, 389–399 (2020). Article PubMed PubMed Central CAS Google Scholar * Giordano, G. et al. Modelling the COVID-19 epidemic and
implementation of population-wide interventions in Italy. _Nat. Med._ 26, 855–860 (2020). Article PubMed PubMed Central CAS Google Scholar * Nussbaumer-Streit, B. et al. Quarantine
alone or in combination with other public health measures to control COVID-19: a rapid review. _Cochrane Database Syst. Rev_ 4, CD013574 (2020). PubMed Google Scholar * Nussbaumer-Streit,
B. et al. Quarantine alone or in combination with other public health measures to control COVID-19: a rapid review [Update]. _Cochrane Database Syst. Rev._ 9, CD013574 (2020). PubMed Google
Scholar * Mina, M. J. & Andersen, K. G. COVID-19 testing: One size does not fit all. _Science_ 371, 126–127 (2021). Article PubMed CAS Google Scholar * Commission Implementing
Regulation (EU) 2022/1107 of 4 July 2022 laying down common specifications for certain class D in vitro diagnostic medical devices in accordance with Regulation (EU) 2017/746 of the European
Parliament and of the Council. http://data.europa.eu/eli/reg_impl/2022/1107/oj (2022). * Leeflang, M. M. G. & Allerberger, F. How to: evaluate a diagnostic test. _Clin. Microbiol.
Infect._ 25, 54–59 (2019). Article PubMed CAS Google Scholar * Sackett, D. L. & Haynes, R. B. The architecture of diagnostic research. _BMJ_ 324, 539–541 (2002). Article PubMed
PubMed Central CAS Google Scholar * Koebberling, J., Trampisch, H. & Windeler, J. Memorandum: Evaluation of diagnostic measures. _J. Clin. Chem. Clin. Biochem._ 28, 873–880 (1990).
Google Scholar * Leeflang, M. M. G., Moons, K. G. M., Reitsma, J. B. & Zwinderman, A. H. Bias in sensitivity and specificity caused by data-driven selection of optimal cutoff values:
mechanisms, magnitude, and solutions. _Clin. Chem._ 54, 729–737 (2008). Article PubMed CAS Google Scholar * Ewald, B. Post hoc choice of cut points introduced bias to diagnostic
research. _J. Clin. Epidemiol._ 59, 798–801 (2006). Article PubMed Google Scholar * Jahn, B. et al. On the role of data, statistics and decisions in a pandemic. _AStA Adv. Statistical
Anal_ 106, 349–382 (2022). Article Google Scholar * Rutjes, A. W. S., Reitsma, J. B., Vandenbroucke, J. P., Glas, A. S. & Bossuyt, P. M. M. Case-control and two-gate designs in
diagnostic accuracy studies. _Clin. Chem._ 51, 1335–1341 (2005). Article PubMed CAS Google Scholar * Lijmer, J. G. et al. Empirical evidence of design-related bias in studies of
diagnostic tests. _JAMA_ 282, 1061–1066 (1999). Article PubMed CAS Google Scholar * Karch, A., Koch, A., Zapf, A., Zerr, I. & Karch, A. Partial verification bias and incorporation
bias affected accuracy estimates of diagnostic studies for biomarkers that were part of an existing composite gold standard. _J. Clin. Epidemiol._ 78, 73–82 (2016). Article PubMed Google
Scholar * Regulation (EU) 2017/746 of the European Parliament and of the Council of 5 April 2017 on in vitro diagnostic medical devices and repealing Directive 98/79/EC and Commission
Decision 2010/227/EU. http://data.europa.eu/eli/reg/2017/746/2022-01-28 (2022). * FDA. In Vitro Diagnostics EUAs.
https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/in-vitro-diagnostics-euas (2023). * WHO. Coronavirus disease (COVID-19)
pandemic. https://www.who.int/europe/emergencies/situations/covid-19 (2024). * Roche Diagnostics. COVID-19. https://diagnostics.roche.com/us/en/landing-pages/roche-covid-19-updates.html
(2024). * FDA. COVID-19 Emergency Use Authorizations for Medical Devices.
https://www.fda.gov/medical-devices/emergency-use-authorizations-medical-devices/covid-19-emergency-use-authorizations-medical-devices (2023). * Hall, M. K., Kea, B. & Wang, R.
Recognising bias in studies of diagnostic tests part 1: patient selection. _Emerg. Med. J._ 36, 431 (2019). Article PubMed Google Scholar * Kea, B., Hall, M. K. & Wang, R. Recognising
bias in studies of diagnostic tests part 2: interpreting and verifying the index test. _Emerg. Med. J._ 36, 501–505 (2019). Article PubMed Google Scholar * Kohn, M. A., Carpenter, C. R.
& Newman, T. B. Understanding the direction of bias in studies of diagnostic test accuracy. _Acad. Emerg. Med._ 20, 1194–1206 (2013). Article PubMed Google Scholar * Suchá, D., van
Hamersvelt, R. W., van den Hoven, A. F., de Jong, P. A. & Verkooijen, H. M. Suboptimal quality and high risk of bias in diagnostic test accuracy studies at chest radiography and CT in
the acute setting of the COVID-19 pandemic: a systematic review. _Radiol. Cardiothorac. Imaging_ 2, e200342 (2020). Article PubMed PubMed Central Google Scholar * Hughes, J. M., Penney,
C., Boyd, S. & Daley, P. Risk of bias and limits of reporting in diagnostic accuracy studies for commercial point-of-care tests for respiratory pathogens. _Epidemiol. Infect._ 146,
747–756 (2018). Article PubMed CAS Google Scholar * Pavlou, A., Kurtz, R. M. & Song, J. W. Diagnostic accuracy studies in radiology: how to recognize and address potential sources of
bias. _Radio. Res. Pr._ 2021, 5801662 (2021). Google Scholar * Shan, G., Zhang, H. & Jiang, T. Determining sample size for a binary diagnostic test in the presence of verification
bias. _J. Biopharm. Stat._ 28, 1193–1202 (2018). Article PubMed Google Scholar * De Groot, J. A. H. et al. Adjusting for differential-verification bias in diagnostic-accuracy studies: a
Bayesian approach. _Epidemiology_ 22, 234–241 (2011). Article PubMed Google Scholar * Lu, Y., Dendukuri, N., Schiller, I. & Joseph, L. A Bayesian approach to simultaneously adjusting
for verification and reference standard bias in diagnostic test studies. _Stat. Med._ 29, 2532–2543 (2010). Article PubMed Google Scholar * de Groot, J. A. H. et al. Correcting for
partial verification bias: a comparison of methods. _Ann. Epidemiol._ 21, 139–148 (2011). Article PubMed Google Scholar * European Medicines Agency. Adaptive Pathways.
https://www.ema.europa.eu/en/human-regulatory-overview/research-development/adaptive-pathways (2016). * Thorlund, K., Haggstrom, J., Park, J. J. & Mills, E. J. Key design considerations
for adaptive clinical trials: a primer for clinicians. _BMJ_ 360, k698 (2018). Article PubMed PubMed Central Google Scholar * Cerqueira, F. P., Jesus, A. M. C. & Cotrim, M. D.
Adaptive design: a review of the technical, statistical, and regulatory aspects of implementation in a clinical trial. _Ther. Innov. Regul. Sci._ 54, 246–258 (2020). Article PubMed Google
Scholar * Zapf, A. et al. Adaptive trial designs in diagnostic accuracy research. _Stat. Med._ 39, 591–601 (2020). Article PubMed Google Scholar * Hot, A. et al. Randomized
test-treatment studies with an outlook on adaptive designs. _BMC Med. Res. Methodol._ 21, 110 (2021). Article PubMed PubMed Central Google Scholar * Vach, W. et al. A potential for
seamless designs in diagnostic research could be identified. _J. Clin. Epidemiol._ 129, 51–59 (2021). Article PubMed Google Scholar * Stark, M. & Zapf, A. Sample size calculation and
re-estimation based on the prevalence in a single-arm confirmatory diagnostic accuracy study. _Stat. Methods Med. Res._ 29, 2958–2971 (2020). Article PubMed Google Scholar * Stark, M. et
al. Blinded sample size re-estimation in a comparative diagnostic accuracy study. _BMC Med Res Methodol_ 22, 115 (2022). Article PubMed PubMed Central Google Scholar * Köster, D., Hoyer,
A. & Zapf, A. Adaptive designs with unblinded sample size re-estimation for diagnostic accuracy studies. 66. Jahrestagung der Deutschen Gesellschaft für Medizinische Informatik,
Biometrie und Epidemiologie e. V. (GMDS), 12. Jahreskongress der Technologie- und Methodenplattform für die vernetzte medizinische Forschung e.V. (TMF) (2021)
https://doi.org/10.3205/21GMDS079. * Hot, A. et al. Sample size recalculation based on the prevalence in a randomized test-treatment study. _BMC Med Res Methodol_ 22, 205 (2022). Article
PubMed PubMed Central Google Scholar * Westphal, M., Zapf, A. & Brannath, W. A multiple testing framework for diagnostic accuracy studies with co-primary endpoints. _Stat. Med._ 41,
891–909 (2022). Article PubMed Google Scholar * Bouman, J. A., Riou, J., Bonhoeffer, S. & Regoes, R. R. Estimating the cumulative incidence of SARS-CoV-2 with imperfect serological
tests: Exploiting cutoff-free approaches. _PLoS Comput. Biol._ 17, e1008728 (2021). Article PubMed PubMed Central CAS Google Scholar * Pepić, A. et al. A diagnostic phase III/IV
seamless design to investigate the diagnostic accuracy and clinical effectiveness using the example of HEDOS and HEDOS II. _Stat. Methods Med. Res._ 33, 433–448 (2024). Article PubMed
PubMed Central Google Scholar * Krumkamp, R. et al. Negative SARS-CoV-2 PCR or rapid antigen test result and the subsequent risk of being infectious: a mathematical simulation study. _BMC
Med. Res. Methodol._ 21, 165 (2021). Article PubMed PubMed Central CAS Google Scholar * Trikalinos, T. A., Siebert, U. & Lau, J. Decision-analytic modeling to evaluate benefits and
harms of medical tests: Uses and limitations. _Med. Decision Making_ 29, E22–9 (2009). Article Google Scholar * Rogan, W. J. & Gladen, B. Estimating prevalence from the results of a
screening test. _Am. J. Epidemiol._ 107, 71–76 (1978). Article PubMed CAS Google Scholar * Peters, A. et al. Framework and baseline examination of the German National Cohort (NAKO).
_Eur. J. Epidemiol._ 37, 1107–1124 (2022). Article PubMed PubMed Central CAS Google Scholar * Office for National Statistics. COVID-19 Infection Survey.
https://www.ons.gov.uk/surveys/informationforhouseholdsandindividuals/householdandindividualsurveys/covid19infectionsurvey (2023). * Imperial College London. Real-time Assessment of
Community Transmission (REACT) Study. https://www.imperial.ac.uk/medicine/research-and-impact/groups/react-study/ (2024). * Siebert, U., Rochau, U. & Claxton, K. When is enough evidence
enough? - Using systematic decision analysis and value-of-information analysis to determine the need for further evidence. _Z. Evid. Fortbild. Qual. Gesundhwes_ 107, 575–584 (2013). Article
PubMed Google Scholar * Leeflang, M. M. G. Systematic reviews and meta-analyses of diagnostic test accuracy. _Clin. Microbiol. Infect._ 20, 105–113 (2014). Article PubMed CAS Google
Scholar * Streeck, H. et al. Infection fatality rate of SARS-CoV2 in a super-spreading event in Germany. _Nat. Commun._ 11, 5829 (2020). Article PubMed PubMed Central CAS Google Scholar
Download references ACKNOWLEDGEMENTS This work was supported by unrestricted grants from the Deutsche Forschungsgemeinschaft (German Research Council, grant numbers KA 5361/1-1 and ZA
687/3-1, project number 458526380). FUNDING Open Access funding enabled and organized by Projekt DEAL. AUTHOR INFORMATION Author notes * These authors contributed equally: Madhav Chaturvedi,
Denise Köster. * These authors jointly supervised this work: Nicole Rübsamen, André Karch, Antonia Zapf. AUTHORS AND AFFILIATIONS * Institute of Epidemiology and Social Medicine, University
of Münster, Münster, Germany Madhav Chaturvedi, Nicole Rübsamen & André Karch * Institute of Medical Biometry and Epidemiology, University Medical Center Hamburg-Eppendorf, Hamburg,
Germany Denise Köster & Antonia Zapf * Amsterdam University Medical Centers, University of Amsterdam, Epidemiology and Data Science, Amsterdam, The Netherlands Patrick M. Bossuyt *
Department of Clinical Research, University of Southern Denmark, Odense, Denmark Oke Gerke * Department of Infectious Disease Epidemiology, NRW Centre for Health, Bochum, Germany Annette
Jurke * Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht University, Utrecht, The Netherlands Mirjam E. Kretzschmar & Johannes B. Reitsma *
Institute of Medical Microbiology, Virology and Hygiene, University Medical Center Hamburg-Eppendorf, Hamburg, Germany Marc Lütgehetmann * Institute for Medical Epidemiology, Biometrics and
Informatics, Interdisciplinary Center for Health Sciences, Medical Faculty of the Martin Luther University Halle-Wittenberg, Halle, Germany Rafael Mikolajczyk * NMI Natural and Medical
Sciences Institute at the University of Tübingen, Reutlingen, Germany Nicole Schneiderhan-Marra * Department of Public Health, Health Services Research and Health Technology Assessment,
Institute of Public Health, Medical Decision Making and Health Technology Assessment, UMIT- University for Health Sciences, Medical Informatics and Technology, Hall in Tirol, Austria Uwe
Siebert * Division of Health Technology Assessment and Bioinformatics, ONCOTYROL - Center for Personalized Cancer Medicine, Innsbruck, Austria Uwe Siebert * Center for Health Decision
Science, Departments of Epidemiology and Health Policy & Management, Harvard T.H. Chan School of Public Health, Boston, MA, USA Uwe Siebert * Program on Cardiovascular Research,
Institute for Technology Assessment and Department of Radiology, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA Uwe Siebert * Roche Diagnostics GmbH, Penzberg,
Germany Carina Stekly & Christoph Ehret Authors * Madhav Chaturvedi View author publications You can also search for this author inPubMed Google Scholar * Denise Köster View author
publications You can also search for this author inPubMed Google Scholar * Patrick M. Bossuyt View author publications You can also search for this author inPubMed Google Scholar * Oke Gerke
View author publications You can also search for this author inPubMed Google Scholar * Annette Jurke View author publications You can also search for this author inPubMed Google Scholar *
Mirjam E. Kretzschmar View author publications You can also search for this author inPubMed Google Scholar * Marc Lütgehetmann View author publications You can also search for this author
inPubMed Google Scholar * Rafael Mikolajczyk View author publications You can also search for this author inPubMed Google Scholar * Johannes B. Reitsma View author publications You can also
search for this author inPubMed Google Scholar * Nicole Schneiderhan-Marra View author publications You can also search for this author inPubMed Google Scholar * Uwe Siebert View author
publications You can also search for this author inPubMed Google Scholar * Carina Stekly View author publications You can also search for this author inPubMed Google Scholar * Christoph
Ehret View author publications You can also search for this author inPubMed Google Scholar * Nicole Rübsamen View author publications You can also search for this author inPubMed Google
Scholar * André Karch View author publications You can also search for this author inPubMed Google Scholar * Antonia Zapf View author publications You can also search for this author
inPubMed Google Scholar CONTRIBUTIONS M.C. contributed to writing the first draft, attended the workshop and participated in the discussions, and contributed to and approved the final
version of the manuscript. D.K. contributed to writing the first draft, attended the workshop and participated in the discussions, and contributed to and approved the final version of the
manuscript. P.B. attended the workshop, participated in the discussions, and contributed to and approved the final version of the manuscript. O.G. attended the workshop, participated in the
discussions, and contributed to and approved the final version of the manuscript. A.J. attended the workshop, participated in the discussions, and contributed to and approved the final
version of the manuscript. M.K. attended the workshop, participated in the discussions, and contributed to and approved the final version of the manuscript. M.L. participated in the
discussions and contributed to and approved the final version of the manuscript. R.M. attended the workshop, participated in the discussions, and contributed to and approved the final
version of the manuscript. J.R. attended the workshop, participated in the discussions, and contributed to and approved the final version of the manuscript. N.S.-M. attended the workshop,
participated in the discussions, and contributed to and approved the final version of the manuscript. U.S. participated in the discussions and contributed to and approved the final version
of the manuscript. C.S. participated in the discussions and contributed to and approved the final version of the manuscript. C.E. participated in the discussions and contributed to and
approved the final version of the manuscript. N.R. contributed to writing the first draft, attended the workshop and participated in the discussions, and contributed to and approved the
final version of the manuscript. A.K. contributed to writing the first draft, attended the workshop and participated in the discussions, and contributed to and approved the final version of
the manuscript. A.Z. contributed to writing the first draft, attended the workshop and participated in the discussions, and contributed to and approved the final version of the manuscript.
CORRESPONDING AUTHOR Correspondence to André Karch. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. PEER REVIEW PEER REVIEW INFORMATION _Communications
Medicine_ thanks Kathy Leung and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available. ADDITIONAL INFORMATION PUBLISHER’S
NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION TRANSPARENT PEER REVIEW FILE RIGHTS AND
PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any
medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The
images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not
included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly
from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Chaturvedi, M.,
Köster, D., Bossuyt, P.M. _et al._ A unified framework for diagnostic test development and evaluation during outbreaks of emerging infections. _Commun Med_ 4, 263 (2024).
https://doi.org/10.1038/s43856-024-00691-9 Download citation * Received: 17 April 2023 * Accepted: 28 November 2024 * Published: 10 December 2024 * DOI:
https://doi.org/10.1038/s43856-024-00691-9 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative