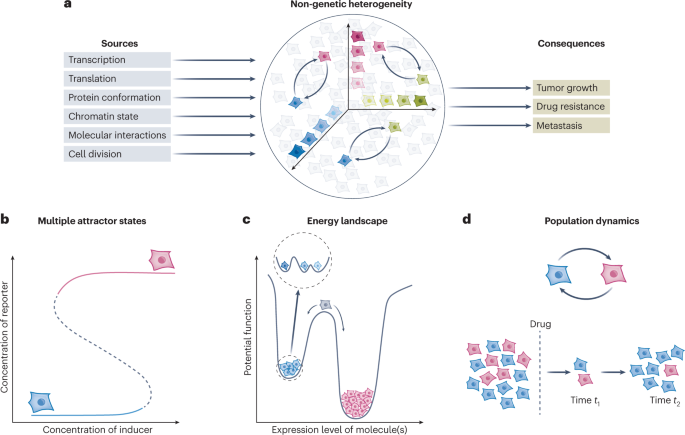
Unraveling non-genetic heterogeneity in cancer with dynamical models and computational tools
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Individual cells within an otherwise genetically homogenous population constantly undergo fluctuations in their molecular state, giving rise to non-genetic heterogeneity. Such
diversity is being increasingly implicated in cancer therapy resistance and metastasis. Identifying the origins of non-genetic heterogeneity is therefore crucial for making clinical
breakthroughs. We discuss with examples how dynamical models and computational tools have provided critical multiscale insights into the nature and consequences of non-genetic heterogeneity
in cancer. We demonstrate how mechanistic modeling has been pivotal in establishing key concepts underlying non-genetic diversity at various biological scales, from population dynamics to
gene regulatory networks. We discuss advances in single-cell longitudinal profiling techniques to reveal patterns of non-genetic heterogeneity, highlighting the ongoing efforts and
challenges in statistical frameworks to robustly interpret such multimodal datasets. Moving forward, we stress the need for data-driven statistical and mechanistically motivated dynamical
frameworks to come together to develop predictive cancer models and inform therapeutic strategies. Access through your institution Buy or subscribe This is a preview of subscription content,
access via your institution ACCESS OPTIONS Access through your institution Access Nature and 54 other Nature Portfolio journals Get Nature+, our best-value online-access subscription $32.99
/ 30 days cancel any time Learn more Subscribe to this journal Receive 12 digital issues and online access to articles $99.00 per year only $8.25 per issue Learn more Buy this article *
Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn
about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS SINGLE-CELL TRANSCRIPTOMICS IN CANCER: COMPUTATIONAL CHALLENGES AND
OPPORTUNITIES Article Open access 15 September 2020 INTEGRATING GENETIC AND NON-GENETIC DETERMINANTS OF CANCER EVOLUTION BY SINGLE-CELL MULTI-OMICS Article 17 August 2020 DELINEATING THE
EVOLUTIONARY DYNAMICS OF CANCER FROM THEORY TO REALITY Article 22 June 2020 REFERENCES * ICGC/TCGA Pan-Cancer Analysis of Whole Genomes Consortium Pan-cancer analysis of whole genomes.
_Nature_ 578, 82–93 (2020). Google Scholar * Welch, D. R. & Hurst, D. R. Defining the hallmarks of metastasis. _Cancer Res._ 79, 3011–3027 (2019). Google Scholar * Bell, C. C. &
Gilan, O. Principles and mechanisms of non-genetic resistance in cancer. _Br. J. Cancer_ 122, 465–472 (2020). Google Scholar * Ramirez, M. et al. Diverse drug-resistance mechanisms can
emerge from drug-tolerant cancer persister cells. _Nat. Commun._ 7, 10690 (2016). Google Scholar * Marine, J.-C., Dawson, S.-J. & Dawson, M. A. Non-genetic mechanisms of therapeutic
resistance in cancer. _Nat. Rev. Cancer_ 20, 743–756 (2020). Google Scholar * Rambow, F. et al. Toward minimal residual disease-directed therapy in melanoma. _Cell_ 174, 843–855.e19 (2018).
Google Scholar * Sharma, S. V. et al. A chromatin-mediated reversible drug-tolerant state in cancer cell subpopulations. _Cell_ 141, 69–80 (2010). Google Scholar * Gupta, P. B. et al.
Stochastic state transitions give rise to phenotypic equilibrium in populations of cancer cells. _Cell_ 146, 633–644 (2011). Google Scholar * Shaffer, S. M. et al. Rare cell variability and
drug-induced reprogramming as a mode of cancer drug resistance. _Nature_ 546, 431–435 (2017). Google Scholar * Su, Y. et al. Single-cell analysis resolves the cell state transition and
signaling dynamics associated with melanoma drug-induced resistance. _Proc. Natl Acad. Sci. USA_ 114, 13679–13684 (2017). Google Scholar * Quinn, J. J. et al. Single-cell lineages reveal
the rates, routes, and drivers of metastasis in cancer xenografts. _Science_ 371, eabc1944 (2021). Google Scholar * Fennell, K. A. et al. Non-genetic determinants of malignant clonal
fitness at single-cell resolution. _Nature_ 601, 125–131 (2022). Google Scholar * Wouters, J. et al. Robust gene expression programs underlie recurrent cell states and phenotype switching
in melanoma. _Nat. Cell Biol._ 22, 986–998 (2020). Google Scholar * Marin-Bejar, O. et al. Evolutionary predictability of genetic versus nongenetic resistance to anticancer drugs in
melanoma. _Cancer Cell_ 39, 1135–1149.e8 (2021). Google Scholar * Roesch, A. et al. Overcoming intrinsic multidrug resistance in melanoma by blocking the mitochondrial respiratory chain of
slow-cycling JARID1B(high) cells. _Cancer Cell_ 23, 811–825 (2013). Google Scholar * Goyal, Y. et al. Pre-determined diversity in resistant fates emerges from homogenous cells after
anti-cancer drug treatment. Preprint at _bioRxiv_ https://doi.org/10.1101/2021.12.08.471833 (2021). * Emert, B. L. et al. Variability within rare cell states enables multiple paths toward
drug resistance. _Nat. Biotechnol._ 39, 865–876 (2021). Google Scholar * Hanahan, D. Hallmarks of cancer: new dimensions. _Cancer Discov._ 12, 31–46 (2022). Google Scholar * Nevozhay, D.,
Adams, R. M., Van Itallie, E., Bennett, M. R. & Balázsi, G. Mapping the environmental fitness landscape of a synthetic gene circuit. _PLoS Comput. Biol._ 8, e1002480 (2012). Google
Scholar * Mooney, S. M., Jolly, M. K., Levine, H. & Kulkarni, P. Phenotypic plasticity in prostate cancer: role of intrinsically disordered proteins. _Asian J. Androl._ 18, 704–710
(2016). Google Scholar * Raj, A. & van Oudenaarden, A. Nature, nurture, or chance: stochastic gene expression and its consequences. _Cell_ 135, 216–226 (2008). Google Scholar * Schuh,
L. et al. Gene networks with transcriptional bursting recapitulate rare transient coordinated high expression states in cancer. _Cell Syst._ 10, 363–378.e12 (2020). Google Scholar * Kim, J.
& DeBerardinis, R. J. Mechanisms and implications of metabolic heterogeneity in cancer. _Cell Metab._ 30, 434–446 (2019). Google Scholar * Huh, D. & Paulsson, J. Non-genetic
heterogeneity from stochastic partitioning at cell division. _Nat. Genet._ 43, 95–100 (2011). Google Scholar * Su, Y. et al. Phenotypic heterogeneity and evolution of melanoma cells
associated with targeted therapy resistance. _PLoS Comput. Biol._ 15, e1007034 (2019). Google Scholar * Roux, J. et al. Fractional killing arises from cell-to-cell variability in overcoming
a caspase activity threshold. _Mol. Syst. Biol._ 11, 803 (2015). Google Scholar * Huang, B. et al. Modeling the transitions between collective and solitary migration phenotypes in cancer
metastasis. _Sci. Rep._ 5, 17379 (2015). Google Scholar * Huang, S., Eichler, G., Bar-Yam, Y. & Ingber, D. E. Cell fates as high-dimensional attractor states of a complex gene
regulatory network. _Phys. Rev. Lett._ 94, 128701 (2005). Google Scholar * Li, Q. et al. Dynamics inside the cancer cell attractor reveal cell heterogeneity, limits of stability, and
escape. _Proc. Natl Acad. Sci. USA_ 113, 2672–2677 (2016). Google Scholar * Blake, W. J. et al. Phenotypic consequences of promoter-mediated transcriptional noise. _Mol. Cell_ 24, 853–865
(2006). Google Scholar * Pillai, M. & Jolly, M. K. Systems-level network modeling deciphers the master regulators of phenotypic plasticity and heterogeneity in melanoma. _iScience_ 24,
103111 (2021). Google Scholar * Paudel, B. B. et al. A nonquiescent ‘idling’ population state in drug-treated, BRAF-mutated melanoma. _Biophys. J._ 114, 1499–1511 (2018). Google Scholar *
Udyavar, A. R. et al. Novel hybrid phenotype revealed in small cell lung cancer by a transcription factor network model that can explain tumor heterogeneity. _Cancer Res._ 77, 1063–1074
(2017). Google Scholar * Wooten, D. J. et al. Systems-level network modeling of small cell lung cancer subtypes identifies master regulators and destabilizers. _PLoS Comput. Biol._ 15,
e1007343 (2019). Google Scholar * Chauhan, L., Ram, U., Hari, K. & Jolly, M. K. Topological signatures in regulatory network enable phenotypic heterogeneity in small cell lung cancer.
_Elife_ 10, e64522 (2021). Google Scholar * Kalluri, R. & Weinberg, R. A. The basics of epithelial–mesenchymal transition. _J. Clin. Invest._ 119, 1420–1428 (2009). Google Scholar *
Hari, K., Ullanat, V., Balasubramanian, A., Gopalan, A. & Jolly, M. K. Landscape of epithelial-mesenchymal plasticity as an emergent property of coordinated teams in regulatory networks.
_Elife_ https://doi.org/10.7554/eLife.76535 (2022). * Celià-Terrassa, T. et al. Hysteresis control of epithelial-mesenchymal transition dynamics conveys a distinct program with enhanced
metastatic ability. _Nat. Commun._ 9, 5005 (2018). Google Scholar * Pastushenko, I. & Blanpain, C. EMT transition states during tumor progression and metastasis. _Trends Cell Biol._ 29,
212–226 (2019). Google Scholar * Lee, J. et al. Network of mutually repressive metastasis regulators can promote cell heterogeneity and metastatic transitions. _Proc. Natl Acad. Sci. USA_
111, E364–E373 (2014). Google Scholar * Bhang, H.-E. C. et al. Studying clonal dynamics in response to cancer therapy using high-complexity barcoding. _Nat. Med._ 21, 440–448 (2015). Google
Scholar * Turke, A. B. et al. Preexistence and clonal selection of MET amplification in EGFR mutant NSCLC. _Cancer Cell_ 17, 77–88 (2010). Google Scholar * Oren, Y. et al. Cycling cancer
persister cells arise from lineages with distinct programs. _Nature_ 596, 576–582 (2021). Google Scholar * Russo, M. et al. A modified fluctuation-test framework characterizes the
population dynamics and mutation rate of colorectal cancer persister cells. _Nat. Genet._ 54, 976–984 (2022). Google Scholar * Fröhlich, F., Gerosa, L., Muhlich, J. & Sorger, P. K.
Mechanistic model of MAPK signaling reveals how allostery and rewiring contribute to drug resistance. _Mol. Syst. Biol._ 19, e10988 (2023). * Goldman, A. et al. Temporally sequenced
anticancer drugs overcome adaptive resistance by targeting a vulnerable chemotherapy-induced phenotypic transition. _Nat. Commun._ 6, 6139 (2015). Google Scholar * Risom, T. et al.
Differentiation-state plasticity is a targetable resistance mechanism in basal-like breast cancer. _Nat. Commun._ 9, 3815 (2018). Google Scholar * Sahoo, S. et al. A mechanistic model
captures the emergence and implications of non-genetic heterogeneity and reversible drug resistance in ER+ breast cancer cells. _NAR Cancer_ 3, zcab027 (2021). Google Scholar * Rehman, S.
K. et al. Colorectal cancer cells enter a diapause-like DTP state to survive chemotherapy. _Cell_ 184, 226–242.e21 (2021). Google Scholar * Pisco, A. O. et al. Non-Darwinian dynamics in
therapy-induced cancer drug resistance. _Nat. Commun._ 4, 2467 (2013). Google Scholar * Farquhar, K. S. et al. Role of network-mediated stochasticity in mammalian drug resistance. _Nat.
Commun._ 10, 2766 (2019). Google Scholar * Hayford, C. E. et al. An in vitro model of tumor heterogeneity resolves genetic, epigenetic, and stochastic sources of cell state variability.
_PLoS Biol._ 19, e3000797 (2021). Google Scholar * Vipparthi, K. et al. Emergence of hybrid states of stem-like cancer cells correlates with poor prognosis in oral cancer. _iScience_ 25,
104317 (2022). * Gillespie, D. T. Exact stochastic simulation of coupled chemical reactions. _J. Phys. Chem._ 81, 2340–2361 (1977). Google Scholar * Hari, K. et al. Identifying inhibitors
of epithelial-mesenchymal plasticity using a network topology-based approach. _npj Syst. Biol. Appl._ 6, 15 (2020). Google Scholar * Nevozhay, D., Adams, R. M., Murphy, K. F., Josic, K.
& Balázsi, G. Negative autoregulation linearizes the dose–response and suppresses the heterogeneity of gene expression. _Proc. Natl Acad. Sci. USA_ 106, 5123–5128 (2009). Google Scholar
* Tirosh, I. et al. Dissecting the multicellular ecosystem of metastatic melanoma by single-cell RNA-seq. _Science_ 352, 189–196 (2016). Google Scholar * Tirosh, I. et al. Single-cell
RNA-seq supports a developmental hierarchy in human oligodendroglioma. _Nature_ 539, 309–313 (2016). Google Scholar * Spencer, S. L., Gaudet, S., Albeck, J. G., Burke, J. M. & Sorger,
P. K. Non-genetic origins of cell-to-cell variability in TRAIL-induced apoptosis. _Nature_ 459, 428–432 (2009). Google Scholar * Hafemeister, C. & Satija, R. Normalization and variance
stabilization of single-cell RNA-seq data using regularized negative binomial regression. _Genome Biol._ 20, 296 (2019). Google Scholar * Linderman, G. C. et al. Zero-preserving imputation
of single-cell RNA-seq data. _Nat. Commun._ 13, 192 (2022). Google Scholar * Hie, B., Bryson, B. & Berger, B. Efficient integration of heterogeneous single-cell transcriptomes using
Scanorama. _Nat. Biotechnol._ 37, 685–691 (2019). Google Scholar * Korsunsky, I. et al. Fast, sensitive and accurate integration of single-cell data with Harmony. _Nat. Methods_ 16,
1289–1296 (2019). Google Scholar * Stuart, T. et al. Comprehensive integration of single-cell data. _Cell_ 177, 1888–1902.e21 (2019). Google Scholar * Aibar, S. et al. SCENIC: single-cell
regulatory network inference and clustering. _Nat. Methods_ 14, 1083–1086 (2017). Google Scholar * Chen, W. S. et al. Uncovering axes of variation among single-cell cancer specimens. _Nat.
Methods_ 17, 302–310 (2020). Google Scholar * Wolock, S. L., Lopez, R. & Klein, A. M. Scrublet: computational identification of cell doublets in single-cell transcriptomic data. _Cell
Syst._ 8, 281–291.e9 (2019). Google Scholar * Xi, N. M. & Li, J. J. Benchmarking computational doublet-detection methods for single-cell RNA sequencing data. _Cell Syst._ 12, 176–194.e6
(2021). Google Scholar * van Dijk, D. et al. Recovering gene interactions from single-cell data using data diffusion. _Cell_ 174, 716–729.e27 (2018). Google Scholar * Azizi, E. et al.
Single-cell map of diverse immune phenotypes in the breast tumor microenvironment. _Cell_ 174, 1293–1308.e36 (2018). Google Scholar * Shaffer, S. M. et al. Memory sequencing reveals
heritable single-cell gene expression programs associated with distinct cellular behaviors. _Cell_ 182, 947–959.e17 (2020). Google Scholar * Karacosta, L. G. et al. Mapping lung cancer
epithelial–mesenchymal transition states and trajectories with single-cell resolution. _Nat. Commun._ 10, 5587 (2019). Google Scholar * Wagner, J. et al. A single-cell atlas of the tumor
and immune ecosystem of human breast cancer. _Cell_ 177, 1330–1345.e18 (2019). Google Scholar * Luria, S. E. & Delbrück, M. Mutations of bacteria from virus sensitivity to virus
resistance. _Genetics_ 28, 491–511 (1943). Google Scholar * Kaur, A. et al. Metastatic potential in clonal melanoma cells is driven by a rare, early-invading subpopulation. Preprint at
_bioRxiv_ https://doi.org/10.1101/2022.04.17.488591 (2022). * Mold, J. E. et al. Clonally heritable gene expression imparts a layer of diversity within cell types. Preprint at _bioRxiv_
https://doi.org/10.1101/2022.02.14.480352 (2022). * Banerji, C. R. S. et al. Cellular network entropy as the energy potential in Waddington’s differentiation landscape. _Sci. Rep._ 3, 3039
(2013). Google Scholar * Park, Y., Lim, S., Nam, J.-W. & Kim, S. Measuring intratumor heterogeneity by network entropy using RNA-seq data. _Sci. Rep._ 6, 37767 (2016). Google Scholar *
Rigau, J., Feixas, M., Sbert, M., Bardera, A. & Boada, I. Medical image segmentation based on mutual information maximization. In _Medical Image Computing and Computer-Assisted
Intervention_ 135–142 (Springer, 2004). * Margolin, A. A. et al. ARACNE: an algorithm for the reconstruction of gene regulatory networks in a mammalian cellular context. _BMC Bioinformatics_
7, S7 (2006). Google Scholar * Trapnell, C. et al. The dynamics and regulators of cell fate decisions are revealed by pseudotemporal ordering of single cells. _Nat. Biotechnol._ 32,
381–386 (2014). Google Scholar * La Manno, G. et al. RNA velocity of single cells. _Nature_ 560, 494–498 (2018). Google Scholar * Qiu, X. et al. Mapping transcriptomic vector fields of
single cells. _Cell_ 185, 690–711.e45 (2022). Google Scholar * Couturier, C. P. et al. Single-cell RNA-seq reveals that glioblastoma recapitulates a normal neurodevelopmental hierarchy.
_Nat. Commun._ 11, 3406 (2020). Google Scholar * Weinreb, C., Wolock, S., Tusi, B. K., Socolovsky, M. & Klein, A. M. Fundamental limits on dynamic inference from single-cell snapshots.
_Proc. Natl Acad. Sci. USA_ 115, E2467–E2476 (2018). Google Scholar * Chaligne, R. et al. Epigenetic encoding, heritability and plasticity of glioma transcriptional cell states. _Nat.
Genet._ https://doi.org/10.1038/s41588-021-00927-7 (2021). * Bergen, V., Soldatov, R. A., Kharchenko, P. V. & Theis, F. J. RNA velocity—current challenges and future perspectives. _Mol.
Syst. Biol._ 17, e10282 (2021). Google Scholar * Gorin, G., Fang, M., Chari, T. & Pachter, L. RNA velocity unraveled. _PLoS Comput. Biol._ 18, e1010492 (2022). * Zheng, S. C.,
Stein-O’Brien, G., Boukas, L., Goff, L. A. & Hansen, K. D. Pumping the brakes on RNA velocity—understanding and interpreting RNA velocity estimates. Preprint at _bioRxiv_
https://doi.org/10.1101/2022.06.19.494717 (2022). * Biddy, B. A. et al. Single-cell mapping of lineage and identity in direct reprogramming. _Nature_ 564, 219–224 (2018). Google Scholar *
Gutierrez, C. et al. Multifunctional barcoding with ClonMapper enables high-resolution study of clonal dynamics during tumor evolution and treatment. _Nat. Cancer_ 2, 758–772 (2021). Google
Scholar * Umkehrer, C. et al. Isolating live cell clones from barcoded populations using CRISPRa-inducible reporters. _Nat. Biotechnol._ 39, 174–178 (2021). Google Scholar * Leighton, J.,
Hu, M., Sei, E., Meric-Bernstam, F. & Navin, N. E. Reconstructing mutational lineages in breast cancer by multi-patient-targeted single-cell DNA sequencing. _Cell Genom._ 3, 100215
(2023). * Weinreb, C., Rodriguez-Fraticelli, A., Camargo, F. D. & Klein, A. M. Lineage tracing on transcriptional landscapes links state to fate during differentiation. _Science_ 367,
eaaw3381 (2020). Google Scholar * McKenna, A. et al. Whole-organism lineage tracing by combinatorial and cumulative genome editing. _Science_ 353, aaf7907 (2016). Google Scholar * Jiang,
C. L. et al. Cell type determination for cardiac differentiation occurs soon after seeding of human-induced pluripotent stem cells. _Genome Biol._ 23, 90 (2022). * Weinreb, C. & Klein,
A. M. Lineage reconstruction from clonal correlations. _Proc. Natl Acad. Sci. USA_ 117, 17041–17048 (2020). MathSciNet MATH Google Scholar * Richman, L. P., Goyal, Y., Jiang, C. L. &
Raj, A. ClonoCluster: a method for using clonal origin to inform transcriptome clustering. _Cell Genom._ 3, 100247 (2023). * Wang, S.-W., Herriges, M. J., Hurley, K., Kotton, D. N. &
Klein, A. M. CoSpar identifies early cell fate biases from single-cell transcriptomic and lineage information. _Nat. Biotechnol_. https://doi.org/10.1038/s41587-022-01209-1 (2022). * Wagner,
D. E. & Klein, A. M. Lineage tracing meets single-cell omics: opportunities and challenges. _Nat. Rev. Genet._ 21, 410–427 (2020). Google Scholar * Gerosa, L. et al. Receptor-driven
ERK pulses reconfigure MAPK signaling and enable persistence of drug-adapted BRAF-mutant melanoma cells. _Cell Syst._ 11, 478–494.e9 (2020). Google Scholar * Yang, C., Tian, C., Hoffman, T.
E., Jacobsen, N. K. & Spencer, S. L. Melanoma subpopulations that rapidly escape MAPK pathway inhibition incur DNA damage and rely on stress signalling. _Nat. Commun._ 12, 1747 (2021).
Google Scholar * Stichel, D. et al. An individual-based model for collective cancer cell migration explains speed dynamics and phenotype variability in response to growth factors. _npj
Syst. Biol. Appl._ 3, 5 (2017). Google Scholar * Meyer, M. et al. Profiling the non-genetic origins of cancer drug resistance with a single-cell functional genomics approach using
predictive cell dynamics. _Cell Syst._ 11, 367–374.e5 (2020). Google Scholar * You, L. et al. Linking the genotypes and phenotypes of cancer cells in heterogenous populations via real-time
optical tagging and image analysis. _Nat. Biomed. Eng._ https://doi.org/10.1038/s41551-022-00853-x (2022). * Bao, F. et al. Integrative spatial analysis of cell morphologies and
transcriptional states with MUSE. _Nat. Biotechnol._ https://doi.org/10.1038/s41587-022-01251-z (2022). * Tian, C., Yang, C. & Spencer, S. L. EllipTrack: a global-local cell-tracking
pipeline for 2D fluorescence time-lapse microscopy. _Cell Rep._ 32, 107984 (2020). Google Scholar * Bagheri, N., Carpenter, A. E., Lundberg, E., Plant, A. L. & Horwitz, R. The new era
of quantitative cell imaging-challenges and opportunities. _Mol. Cell_ 82, 241–247 (2022). Google Scholar * Stringer, C., Wang, T., Michaelos, M. & Pachitariu, M. Cellpose: a generalist
algorithm for cellular segmentation. _Nat. Methods_ 18, 100–106 (2021). Google Scholar * Satija, R., Farrell, J. A., Gennert, D., Schier, A. F. & Regev, A. Spatial reconstruction of
single-cell gene expression data. _Nat. Biotechnol._ 33, 495–502 (2015). Google Scholar * Su, Y. et al. Multi-omic single-cell snapshots reveal multiple independent trajectories to drug
tolerance in a melanoma cell line. _Nat. Commun._ 11, 2345 (2020). Google Scholar * Sammut, S.-J. et al. Multi-omic machine learning predictor of breast cancer therapy response. _Nature_
601, 623–629 (2022). Google Scholar * Rao, A., Barkley, D., França, G. S. & Yanai, I. Exploring tissue architecture using spatial transcriptomics. _Nature_ 596, 211–220 (2021). Google
Scholar * Atta, L. & Fan, J. Computational challenges and opportunities in spatially resolved transcriptomic data analysis. _Nat. Commun._ 12, 5283 (2021). Google Scholar * Nam, A. S.,
Chaligne, R. & Landau, D. A. Integrating genetic and non-genetic determinants of cancer evolution by single-cell multi-omics. _Nat. Rev. Genet._ 22, 3–18 (2021). Google Scholar *
Salgia, R. & Kulkarni, P. The genetic/non-genetic duality of drug ‘resistance’ in cancer. _Trends Cancer Res._ 4, 110–118 (2018). Google Scholar * Liang, W. et al. Development and
validation of a nomogram for predicting survival in patients with resected non-small-cell lung cancer. _J. Clin. Oncol._ 33, 861–869 (2015). Google Scholar * Karakiewicz, P. I. et al.
Multi-institutional validation of a new renal cancer-specific survival nomogram. _J. Clin. Oncol._ 25, 1316–1322 (2007). Google Scholar * Huang, C., Zhao, J. & Zhu, Z. Prognostic
nomogram of prognosis-related genes and clinicopathological characteristics to predict the 5-year survival rate of colon cancer patients. _Front Surg._ 8, 681721 (2021). Google Scholar *
Phillips, R. Theory in biology: Fig. 1 or Figure 7? _Trends Cell Biol._ 25, 723–729 (2015). Google Scholar * Theorists and experimentalists must join forces. _Nat. Comput. Sci._ 1, 299–299
(2021). * Cassidy, T., Nichol, D., Robertson-Tessi, M., Craig, M. & Anderson, A. R. A. The role of memory in non-genetic inheritance and its impact on cancer treatment resistance. _PLoS
Comput. Biol._ 17, e1009348 (2021). Google Scholar * Mellis, I. A. & Raj, A. Half dozen of one, six billion of the other: what can small- and large-scale molecular systems biology learn
from one another? _Genome Res._ 25, 1466–1472 (2015). Google Scholar * Bauer, A. L., Jackson, T. L. & Jiang, Y. A cell-based model exhibiting branching and anastomosis during
tumor-induced angiogenesis. _Biophys. J._ 92, 3105–3121 (2007). Google Scholar * Zhang, L., Strouthos, C. G., Wang, Z. & Deisboeck, T. S. Simulating brain tumor heterogeneity with a
multiscale agent-based model: linking molecular signatures, phenotypes and expansion rate. _Math. Comput. Model._ 49, 307–319 (2009). MathSciNet MATH Google Scholar * Saez-Rodriguez, J.
et al. Comparing signaling networks between normal and transformed hepatocytes using discrete logical models. _Cancer Res._ 71, 5400–5411 (2011). Google Scholar * Macklin, P., Edgerton, M.
E., Thompson, A. M. & Cristini, V. Patient-calibrated agent-based modelling of ductal carcinoma in situ (DCIS): from microscopic measurements to macroscopic predictions of clinical
progression. _J. Theor. Biol._ 301, 122–140 (2012). MathSciNet MATH Google Scholar * Fumiã, H. F. & Martins, M. L. Boolean network model for cancer pathways: predicting carcinogenesis
and targeted therapy outcomes. _PLoS ONE_ 8, e69008 (2013). Google Scholar * Steinway, S. N. et al. Network modeling of TGFβ signaling in hepatocellular carcinoma epithelial-to-mesenchymal
transition reveals joint sonic hedgehog and Wnt pathway activation. _Cancer Res._ 74, 5963–5977 (2014). Google Scholar * Wang, Z., Zhang, L., Sagotsky, J. & Deisboeck, T. S. Simulating
non-small cell lung cancer with a multiscale agent-based model. _Theor. Biol. Med. Model._ 4, 50 (2007). Google Scholar * Andasari, V., Roper, R. T., Swat, M. H. & Chaplain, M. A. J.
Integrating intracellular dynamics using CompuCell3D and Bionetsolver: applications to multiscale modelling of cancer cell growth and invasion. _PLoS ONE_ 7, e33726 (2012). Google Scholar *
Swat, M. H. et al. in _Methods in Cell Biology_ Vol. 110 (eds Asthagiri, A. R. & Arkin, A. P.) 325–366 (Academic Press, 2012). * Lu, M., Jolly, M. K., Levine, H., Onuchic, J. N. &
Ben-Jacob, E. MicroRNA-based regulation of epithelial-hybrid-mesenchymal fate determination. _Proc. Natl Acad. Sci. USA_ 110, 18144–18149 (2013). Google Scholar * Wang, W. et al. Dynamics
between cancer cell subpopulations reveals a model coordinating with both hierarchical and stochastic concepts. _PLoS ONE_ 9, e84654 (2014). Google Scholar * Jain, P., Bhatia, S., Thompson,
E. W. & Jolly, M. K. Population dynamics of epithelial–mesenchymal heterogeneity in cancer cells. _Biomolecules_ 12, 348 (2022). Google Scholar * Di Filippo, M. et al. Zooming-in on
cancer metabolic rewiring with tissue specific constraint-based models. _Comput. Biol. Chem._ 62, 60–69 (2016). Google Scholar * Ghaffarizadeh, A., Heiland, R., Friedman, S. H.,
Mumenthaler, S. M. & Macklin, P. PhysiCell: an open source physics-based cell simulator for 3-D multicellular systems. _PLoS Comput. Biol._ 14, e1005991 (2018). Google Scholar *
Damiani, C. et al. A metabolic core model elucidates how enhanced utilization of glucose and glutamine, with enhanced glutamine-dependent lactate production, promotes cancer cell growth: the
WarburQ effect. _PLoS Comput. Biol._ 13, e1005758 (2017). Google Scholar * Gatto, F., Miess, H., Schulze, A. & Nielsen, J. Flux balance analysis predicts essential genes in clear cell
renal cell carcinoma metabolism. _Sci. Rep._ 5, 10738 (2015). Google Scholar * Zhou, J. X., Pisco, A. O., Qian, H. & Huang, S. Nonequilibrium population dynamics of phenotype conversion
of cancer cells. _PLoS ONE_ 9, e110714 (2014). Google Scholar * Angel, P., Hattori, K., Smeal, T. & Karin, M. The _jun_ proto-oncogene is positively autoregulated by its product,
Jun/AP-1. _Cell_ 55, 875–885 (1988). Google Scholar * Castles, C. G., Oesterreich, S., Hansen, R. & Fuqua, S. A. Auto-regulation of the estrogen receptor promoter. _J. Steroid Biochem.
Mol. Biol._ 62, 155–163 (1997). Google Scholar * Gardner, T. S., Cantor, C. R. & Collins, J. J. Construction of a genetic toggle switch in _Escherichia coli_. _Nature_ 403, 339–342
(2000). Google Scholar * Cherry, J. L. & Adler, F. R. How to make a biological switch. _J. Theor. Biol._ 203, 117–133 (2000). Google Scholar * Burk, U. et al. A reciprocal repression
between ZEB1 and members of the miR-200 family promotes EMT and invasion in cancer cells. _EMBO Rep._ 9, 582–589 (2008). Google Scholar * Kim, Y., Roh, S., Lawler, S. & Friedman, A.
miR451 and AMPK mutual antagonism in glioma cell migration and proliferation: a mathematical model. _PLoS ONE_ 6, e28293 (2011). Google Scholar * Lu, M. et al. Tristability in
cancer-associated microRNA-TF chimera toggle switch. _J. Phys. Chem. B_ 117, 13164–13174 (2013). Google Scholar * Novák, B. & Tyson, J. J. Design principles of biochemical oscillators.
_Nat. Rev. Mol. Cell Biol._ 9, 981–991 (2008). Google Scholar * Ma, L. et al. A plausible model for the digital response of p53 to DNA damage. _Proc. Natl Acad. Sci. USA_ 102, 14266–14271
(2005). Google Scholar * Purvis, J. E. et al. p53 dynamics control cell fate. _Science_ 336, 1440–1444 (2012). Google Scholar Download references ACKNOWLEDGEMENTS We thank the members of
the Goyal and Jolly labs for helpful discussions. We thank K. Kiani and I. Mellis for their critical reading of the manuscript. The Goyal lab thanks R. Valadka at Northwestern University for
his prompt and unwavering support in setting up the lab space. M.P. was supported by grants to Y.G. including the Career Award at the Scientific Interface from BWF (1020614.01) and start-up
funds from Northwestern University, and KVPY Fellowship (Department of Science and Technology, Government of India). E.H. acknowledges support from the Career Award at the Scientific
Interface from BWF (1020614.01) and Northwestern University’s Biomedical Engineering Department for the BME Summer Undergraduate Research Grant Award (SURA). E.H. thanks E. Hojel and M.
Hojel for the support and encouragement to pursue her passions. M.K.J. acknowledges support from Ramanujan Fellowship awarded by the Science and Engineering Research Board, Department of
Science and Technology, Government of India (SB/S2/RJN-049/2018), and from the InfoSys Foundation, Bangalore. Y.G. acknowledges support from the Career Award at the Scientific Interface from
BWF (1020614.01), start-up funds from Northwestern University, and a grant (10063150.01) from Research Catalyst Program from the McCormick School of Engineering at Northwestern University.
AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Cell and Developmental Biology, Feinberg School of Medicine, Northwestern University, Chicago, IL, USA Maalavika Pillai, Emilia
Hojel & Yogesh Goyal * Center for Synthetic Biology, Northwestern University, Chicago, IL, USA Maalavika Pillai, Emilia Hojel & Yogesh Goyal * Robert H. Lurie Comprehensive Cancer
Center, Northwestern University Feinberg School of Medicine, Chicago, IL, USA Maalavika Pillai & Yogesh Goyal * Centre for BioSystems Science and Engineering, Indian Institute of
Science, Bangalore, India Maalavika Pillai & Mohit Kumar Jolly * Department of Biomedical Engineering, Northwestern University McCormick School of Engineering, Evanston, IL, USA Emilia
Hojel & Yogesh Goyal Authors * Maalavika Pillai View author publications You can also search for this author inPubMed Google Scholar * Emilia Hojel View author publications You can also
search for this author inPubMed Google Scholar * Mohit Kumar Jolly View author publications You can also search for this author inPubMed Google Scholar * Yogesh Goyal View author
publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS M.K.J. and Y.G. conceived the project, wrote the first draft of the manuscript and contributed to
figure design. M.P. revised the initial draft, contributed to figure design, and prepared the boxes and the table. E.H. contributed to the figure, box and table design and literature survey.
All authors contributed to revisions. CORRESPONDING AUTHORS Correspondence to Mohit Kumar Jolly or Yogesh Goyal. ETHICS DECLARATIONS COMPETING INTERESTS Y.G. received consultancy fee from
the Schmidt Science Fellows and the Rhodes Trust. The other authors declare no competing interests. PEER REVIEW PEER REVIEW INFORMATION _Nature Computational Science_ thanks Gábor Balázsi
and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Ananya Rastogi and Kaitlin McCardle, in collaboration with the _Nature
Computational Science_ team. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
RIGHTS AND PERMISSIONS Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other
rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law. Reprints and
permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Pillai, M., Hojel, E., Jolly, M.K. _et al._ Unraveling non-genetic heterogeneity in cancer with dynamical models and computational tools.
_Nat Comput Sci_ 3, 301–313 (2023). https://doi.org/10.1038/s43588-023-00427-0 Download citation * Received: 30 March 2022 * Accepted: 03 March 2023 * Published: 24 April 2023 * Issue Date:
April 2023 * DOI: https://doi.org/10.1038/s43588-023-00427-0 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a
shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative