Modeling how antibody responses may determine the efficacy of covid-19 vaccines
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Predicting the efficacy of COVID-19 vaccines would aid vaccine development and usage strategies, which is of importance given their limited supplies. Here we develop a multiscale
mathematical model that proposes mechanistic links between COVID-19 vaccine efficacies and the neutralizing antibody (NAb) responses they elicit. We hypothesized that the collection of all
NAbs would constitute a shape space and that responses of individuals are random samples from this space. We constructed the shape space by analyzing reported in vitro dose–response curves
of ~80 NAbs. Sampling NAb subsets from the space, we recapitulated the responses of convalescent patients. We assumed that vaccination would elicit similar NAb responses. We developed a
model of within-host SARS-CoV-2 dynamics, applied it to virtual patient populations and, invoking the NAb responses above, predicted vaccine efficacies. Our predictions quantitatively
captured the efficacies from clinical trials. Our study thus suggests plausible mechanistic underpinnings of COVID-19 vaccines and generates testable hypotheses for establishing them.
SIMILAR CONTENT BEING VIEWED BY OTHERS CHARACTERIZING SARS-COV-2 NEUTRALIZATION PROFILES AFTER BIVALENT BOOSTING USING ANTIGENIC CARTOGRAPHY Article Open access 26 August 2023 CORRELATES OF
PROTECTION FOR BOOSTER DOSES OF THE SARS-COV-2 VACCINE BNT162B2 Article Open access 29 July 2023 MODELING OF WANING IMMUNITY AFTER SARS-COV-2 VACCINATION AND INFLUENCING FACTORS Article Open
access 28 March 2022 MAIN The coronavirus disease 2019 (COVID-19) pandemic has triggered the fastest vaccine development efforts so far, as well as urgent global vaccination rollouts. Yet,
limited vaccine supplies are hindering our ability to fight the pandemic1,2. Enormous efforts are ongoing to develop new vaccines. As of 14 October 2021, there were 332 vaccine candidates
under development, of which 113 were in clinical testing3. The ability to predict vaccine efficacies may expedite vaccine development by helping to shortlist promising candidates and/or
minimize the subsequent reliance on expensive and time-consuming clinical trials for assessing their efficacies4. Simultaneously, it may help identify optimal vaccination strategies5,6. For
example, although several approved vaccines are administered in two doses separated by a few weeks, it would be useful to know the protection conferred by a single dose, by lower dosages or
by doses separated by longer intervals, especially in less vulnerable populations, so as to ease demand7,8,9. Approved COVID-19 vaccines have shown remarkable but varying efficacies in
clinical trials, reducing the incidence of ‘symptomatic’ infections with the wild-type severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) strain by 62–96% (refs.
7,10,11,12,13,14,15,16). The protection has been argued to be due to neutralizing antibodies (NAbs) elicited by the vaccines. Cellular immunity appears to play a less important role,
especially after the recommended doses of the vaccine are administered10,11,17,18,19,20,21,22. The protection due to NAbs is consistent with independent observations, in which higher levels
of pre-existing NAbs were correlated with protection and lower risk of infection, respectively, in an early outbreak in a fishery vessel23 and in a longitudinal study of healthcare
workers24. Protection from seasonal coronaviruses too has been associated with pre-existing NAbs25. Strong statistical correlations have been identified between COVID-19 vaccine efficacies
and the NAb responses they elicit17,18. An important question that arises is how the NAb responses confer the protection observed. An understanding of the dependence of the level of
protection on the NAb titers and their neutralization efficiencies is lacking. In this Article, we develop a theoretical framework that describes and integrates several key phenomena
associated with SARS-CoV-2 infection and vaccination, across length and time scales spanning the range from the workings of individual NAbs to the responses of clinical cohorts to
vaccination. The framework employs testable, mechanistic hypotheses and quantitatively predicts the population-level protection conferred by vaccines as a function of the NAb titers they
elicit. RESULTS CONSTRUCTION OF THE SARS-COV-2 NAB LANDSCAPE A major challenge to describing the effects of vaccination is the diversity of the NAb responses elicited. The NAb response to
primary SARS-CoV-2 infection in unvaccinated individuals is diverse, spanning >1,000-fold variation in Ab titers and in vitro neutralization efficiencies across individuals26,27. NAb
titers following vaccination have been found to be comparable to those from convalescent patients10,11,28. No formalism exists to predict this diversity or its effects on protection. We
addressed this challenge by adapting the classic idea of ‘shape space’, which has aided quantification of the immune repertoire29, for characterizing NAbs. Accordingly, we sought features
(also termed shape parameters) of the NAbs that would predict their neutralization efficiencies. Numerous studies have isolated individual NAbs from patients and assessed their
neutralization efficiencies in vitro. We compiled dose–response curves (DRCs) of ~80 NAbs26,27,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44 thus isolated and fit them using the median-effect
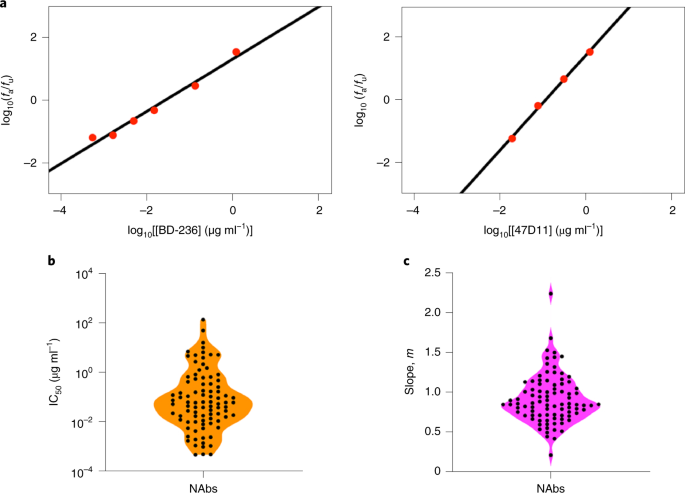
equation45 (Methods, Supplementary Fig. 1 and Supplementary Data 1). The equation fit the data well (Fig. 1a and Supplementary Fig. 2), indicating that two parameters, the 50% inhibitory
concentration (IC50) and the slope (_m_) of the DRC, were sufficient to characterize the neutralization efficiency of individual NAbs (Fig. 1a and Supplementary Data 1). The best-fit IC50
and _m_ varied widely across NAbs (Fig. 1b). IC50 values ranged from ~10−3 to ~140 µg ml−1 (Fig. 1b), in close agreement with reported estimates (Supplementary Fig. 3a and Supplementary Data
1). The values of _m_, the importance of which has been recognized with HIV-1 and hepatitis C45,46 but has not typically been reported for SARS-CoV-2, ranged from ~0.2 to 2 (Fig. 1c). This
variability in IC50 and _m_ was not restricted to a particular pseudotyped virus construct or backbone (Supplementary Fig. 3b,c), cell line (Supplementary Fig. 3d,e) or assay conditions,
which could vary across studies (Supplementary Fig. 3f,g). The variability was thus not attributable to these potential confounding factors and appeared to be intrinsic to the NAbs,
indicating the spectrum of NAbs elicited. Furthermore, akin to HIV-1 antibodies45, the variations in IC50 and _m_ appeared independent. For example, the NAbs BD-361 and REGN10954 had similar
IC50 values (both ~0.04 µg ml−1) but vastly different _m_ (~0.7 and ~1.5, respectively), whereas the NAbs CC12.3 and 515-5 had different IC50 values (~0.02 µg ml−1 and 1.6 µg ml−1,
respectively) but similar _m_ (both ~1). IC50 and _m_ were thus not only sufficient but also necessary for quantifying the neutralization efficiencies of NAbs. We therefore employed IC50 and
_m_ as the shape parameters. Plotting the NAbs on an IC50–_m_ plot, we identified the NAb shape space (Fig. 2), which, because of its two-dimensional nature, we termed the ‘landscape of
SARS-CoV-2 NAbs’. The landscape contains potent NAbs, with low IC50 and high _m_, as well as weak NAbs, with the opposite traits. To compare the NAbs, we employed the instantaneous
inhibitory potential (IIP), a composite metric of IC50 and _m_ (refs. 45,46,47). IIP_D_ represents the log10 decline in viral load in a single round infection assay due to the NAb present at
concentration _D_. Thus, the higher the IIP_D_, the more potent is the NAb at that concentration. NAbs displayed a wide distribution of IIP100 values: six NAbs had the highest IIP100
values, >5, and seven had the least, ≤1 (_D_ = 100 µg ml−1) (Fig. 2b and Supplementary Data 1). This distribution demonstrated further the wide spectrum of neutralization efficiencies of
NAbs. Using another NAb concentration did not affect this distribution substantially (Supplementary Fig. 4). PREDICTION OF THE NAB RESPONSES OF CONVALESCENT PATIENTS The landscape
established bounds on the neutralization efficiencies of the NAbs elicited. We reasoned next that the diversity of the NAb responses across individuals would arise from the way NAbs are
sampled from the landscape. Although a large number of NAbs can be isolated from individuals, studies of convalescent patient plasma26,27,48,49,50 as well as on NAb epitope profiling51 have
argued that the NAb response of an individual can be attributed to a small subset of five to ten distinct NAbs. Furthermore, although some epitopes on the SARS-CoV-2 spike protein, S, are
targeted more than others by NAbs, the collection of NAbs produced differs substantially across individuals52. We therefore assumed that the response elicited by an infected individual would
be a small, random subset of the landscape. We analyzed DRCs of NAbs isolated from individual patients and found that they indeed constituted such subsets (Supplementary Fig. 5).
Accordingly, we sampled random combinations of ten NAbs each, each combination representing the response of an individual. Our results were robust to an increase in the number of NAbs
sampled beyond ten (Supplementary Fig. 6). We let NAb concentrations vary across individuals, to mimic the observed variation of the NAb titers48,49,50. We quantified the neutralization
efficiency of the NAb response by simulating standard plasma dilution assays (Methods and Fig. 3a). Experimental plasma dilution assay results follow an inverse sigmoidal pattern and are
characterized by NT50, the dilution at which the neutralization efficiency of the plasma decreases by 50% (Fig. 3b). In our simulations, we let the NAbs exhibit Bliss independence or Loewe
additivity, the former representing NAbs targeting distinct, non-occluding epitopes and the latter the same or occluding epitopes53. Our simulations recapitulated the dilution curves
associated with patient plasma (Fig. 3b). Furthermore, the values of NT50 we predicted were in close agreement with experimental observations49 (Fig. 3c). (Note that immunoglobulin-G (IgG)
targeting the receptor binding domain of the SARS-CoV-2 spike protein, reported in the experiments in Fig. 3c, do not represent all NAbs. They are, however, a major component of the NAbs.
The dilution assays in Fig. 3b do include all NAbs, indicating that our predictions capture the measured NT50 values reliably.) The latter data were described better by Bliss independence at
low NAb titers and Loewe additivity at high titers. This is expected, because, at low titers, the NAbs are unlikely to interact with each other and would thus follow Bliss independence,
whereas at high titers, they may compete for binding sites on S or occlude each other and exhibit Loewe additivity53. At any NAb titer, there existed substantial variation in NT50,
attributed to the random combinations of NAbs sampled. The variation, however, was outweighed by the overall rise of NT50 with the NAb titer, consistent with patient data (Fig. 3c). For
example, the geometric mean NT50 computed using Loewe additivity was 7.2 at an IgG titer of 0.1 µg ml−1 and 455.1 at 10 µg ml−1. Sampling from the NAb landscape thus successfully
recapitulated patient responses. Note that no adjustable parameters were involved in this comparison. We were thus able to describe the diversity of the NAb responses elicited across
patients. Armed with this description, we examined next the protection accorded by vaccines in clinical trials. PREDICTION OF COVID-19 VACCINE EFFICACIES Following vaccination, NAb titers
rise and are expected to remain stable (or decay slowly) over weeks to months54, protecting individuals exposed to the virus during this period. Individuals were assumed to be protected if
they did not report symptomatic infection. Loss of protection involved symptoms and a positive result on a nucleic acid amplification test10,11. Protection with NAbs is not expected to be
sterilizing, as suggested by animal studies21,55; NAbs help suppress virus load and facilitate more rapid clearance of the infection. We assumed that the severity of the symptoms would be
proportional to the virus load. If the peak is sufficiently suppressed, no symptoms may result, as is the case with naturally asymptomatic infections56. Here we assumed that an individual
would be detected as symptomatically infected if the viral load rose above a threshold. To estimate the peak viral load, we developed a mathematical model (Fig. 4a) of the early time course
of the infection, where the viral load typically rises, attains a peak and declines57. To ascertain its ability to describe these dynamics in vivo, we fit the model to longitudinal viral
load data from individual patients from three cohorts57,58,59. The model fit the data well (Fig. 4b and Supplementary Figs. 7 and 8) and yielded parameter estimates quantifying inter-patient
variability in the within-host dynamics (Supplementary Tables 2 and 3). We applied the model to describe the effect of vaccination (Methods). We assumed that NAbs generated following
vaccination would exist at the start of infection. Although NAbs may perform many functions, including stimulating the cellular adaptive response56,60, direct evidence of these functions in
SARS-CoV-2-infected humans is yet to be gathered56. (The vaccines may stimulate other immune arms independently of the NAbs, which, as we argue above, is expected to make limited
contributions to vaccine efficacy against the wild-type SARS-CoV-2 strain10,11,17,18.) We therefore focused on their ability to neutralize free viruses, effectively reducing viral
infectivity. We recall that protection due to NAbs is not expected to be sterilizing21,55. The reduced infectivity is also consistent with in vitro pseudovirus neutralization assays, which
measure the drop in infectivity with increasing NAb concentrations. Our model captures these in vitro assays quantitatively (Fig. 3). The greater the reduction in infectivity, the lower the
peak viral load in vivo (Fig. 4c, curves with efficacy _ε_ > 0). This assumption of the activity of NAbs is thus also consistent with the inverse correlation between the peak viral load
and NAb titers elicited by vaccination in mouse models21 and in macaques61. Substantial de novo NAb production post-infection typically occurs after the peak in virus load56 and has been
argued to have limited impact on viral clearance62. We therefore considered pre-existing NAbs as responsible for protection and assumed their titers not to vary substantially during the
course of the infection, given the typically short course of the infection and the much longer durability of the NAb response to vaccination54. We let the pre-existing NAbs be drawn as
random subsets from the landscape, as we did above. We assumed that the NAbs neutralized free viruses with an efficiency that we estimated using Loewe additivity. NAb titers in the lung
airways are expected to be similar to those in the blood given the close coupling between the lungs and the circulatory system56. The efficiency of NAbs in vivo can differ from that in
vitro63, which we took into account. We simulated a virtual patient population of 3,500 individuals, similar to the number of individuals infected in the placebo arms of clinical trials. The
individuals all had distinct viral dynamics parameters drawn from previously known and/or estimated ranges (Supplementary Tables 1 and 2), to mimic inter-patient variability in addition to
the variability arising from NAb sampling from the landscape. Our model predicted wide variability in the peak viral load (Fig. 4d). At low pre-existing NAb concentrations (0.01 µg ml−1),
indicative of the scenario without vaccination, the predicted peak viral load ranged from ~103 to 109 copies per milliliter, consistent with the range in symptomatic individuals64. The peaks
declined as NAb titers increased. Following clinical trials, we set the limit of detection to ~102 copies per milliliter (ref. 65). (Note that, even if symptoms were to arise with lower
viral loads in some individuals, such individuals would not be diagnosed as infected because of assay limitations.) The fraction of individuals with peaks below detection would indicate the
level of protection due to the vaccine. To quantify the mean level of protection and test it against data from clinical trials, we used viral dynamics parameters representative of detectable
infections66,67 (Supplementary Tables 1 and 2) and simulated the dynamics in a cohort of 10,000 infected individuals. Vaccination studies report the NT50 values of the NAb responses
elicited and the associated mean protection level, or efficacy7,10,12,68,69,70 (Supplementary Table 4). We binned individuals into narrow NT50 bands and calculated the mean protection and
95% confidence interval (CI) in each band. We found that the mean protection was low for an NT50 of ~1. It increased in a sigmoidal manner to 50% at an NT50 of ~15 and asymptotically
reached 100% at an NT50 of ~200 (Supplementary Fig. 9). To compare these predictions with clinical data across trials, we normalized the data using the NT50 values from convalescent patients
reported by the respective trials (Supplementary Table 4). This ensured that assay variations across studies did not confound our comparisons. We accordingly also normalized our predictions
of NT50 with those corresponding to convalescent patients captured by our model (Methods, Fig. 3 and Supplementary Fig. 10). For the set of parameters employed, the data for all the eight
approved vaccines we considered fell on this ‘protection curve’ (Fig. 5). Thus, for example, a single dose of the vaccine Ad26.COV2.S elicited NAbs with a scaled NT50 of 0.43 and accorded
66.1% protection. Following two doses of the vaccine BNT162b2, the corresponding values were 3.84% and 94%, respectively. These values were captured accurately by our model predictions. This
agreement extended to data from phase III trials of the other vaccines we considered as well (Fig. 5). We tested the robustness of these predictions to model parameter variations. Using
local (Supplementary Fig. 11a) and global sensitivity analysis (Supplementary Fig. 11b), we found that our predictions of vaccine efficacy were robust to parameter variations so long as the
parameter values were consistent with patient data (Supplementary Fig. 11c–e). One parameter not involved in the patient data fitting was _ω_, the assumed ratio of the IC50 values of the
NAbs in vivo and in vitro, because NAb responses, given their limited role, were not part of our model of unvaccinated individuals62. The protection curve was sensitive to _ω_ (Supplementary
Fig. 11f). Recent studies71,72 have estimated _ω_ for a few NAbs of SARS-CoV-2 and found it to be in the range ~5–40 (Supplementary Table 1), similar to estimates for other viruses63,73. In
the above predictions we set _ω_ to ~10, which lies in the above range and yielded the protection curve that captured clinical data. Future studies may provide independent estimates of the
model parameters, limiting these uncertainties and sharpening the mechanistic links between COVID-19 vaccine efficacies and NAb responses assumed in our formalism. DISCUSSION Our formalism
for predicting COVID-19 vaccine efficacies as a function of the NAb responses they elicit required the description and integration of several key phenomena, varying over many length and time
scales. These include (1) the neutralization potential of individual NAbs, (2) the diversity of the NAb response within and across individuals, (3) the relationship between NAb titers in
individuals and their neutralization potential, (4) the within-host dynamics of disease progression, (5) the influence of vaccination on the within-host dynamics and (6) the variability of
the latter influence across a patient population in clinical trials. At each step, we established quantitative connects with experimental data, rendering our approach rigorous. Identifying
correlates of the protection offered by COVID-19 vaccines has been challenging74. Several recent studies have pointed to NAb responses as strong correlates of vaccine-mediated
protection17,18,19,20. Our study independently arrived at the correlation between vaccine efficacy and NT50 and generated testable hypotheses of its mechanistic origins. Briefly, our study
assumed that, in response to vaccination, different individuals in a population produce NAbs that are well represented by random samples from the NAb landscape. The neutralization potential
of the NAbs elicited, quantified by NT50, accordingly varies across individuals in a dose-dependent manner. The presence of the NAbs, in our model, caused a reduction in the peak viral load
following infection, also in a dose-dependent manner. A sufficient reduction in the peak viral load was assumed to prevent symptoms and manifest as protection conferred by vaccination,
potentially giving rise to the correlation of vaccine efficacy with NT50. Thus, our study suggests a plausible mechanism that can be tested experimentally for how multiple NAbs generated
within an individual following vaccination provide protection by being members of a shape space. Our model proposes a mechanism to reduce the net neutralization effect of these antibodies
into a single metric. Further, our formalism deduced a relationship between NT50 and the concentration of NAbs in plasma, so that the latter may also be used to estimate vaccine efficacies.
Our formalism employs several assumptions and hypotheses underlying the protection conferred by NAbs elicited by vaccination. First, NAbs are assumed to be generated randomly from the NAb
landscape. Although this appears plausible (Supplementary Fig. 5), it remains to be established. Furthermore, that the NAb landscape in convalescent patients and following vaccination is
similar needs to be ascertained. Second, assay variations in the estimates of NAb characteristics (IC50 and _m_) are assumed not significant. To verify this, estimates of IC50 and _m_ could
be standardized across studies, for example, by normalizing them using the IC50 and _m_ values generated in each study for the same ‘reference’ NAb and by using the same viral backbone and
cell line. Third, our model assumed that protection following vaccination is predominantly due to NAbs and that other immune arms play a secondary role. Although the extent to which COVID-19
vaccines trigger other immune arms is being investigated20, the high NAb titers generated after the boost support this assumption. Fourth, NAbs are assumed to act primarily by neutralizing
virus and thus reducing viral infectivity and the peak viral load in a dose-dependent manner. It is possible that NAbs trigger other immune arms, via Fc-mediated mechanisms20. Innate immune
responses and CD8 T cells may also reduce the peak viral load56,75. The contributions of these mechanisms to vaccine efficacy remain to be ascertained. Fifth, the severity of symptoms is
assumed to depend on the viral load. In clinical trials of vaccine efficacy, individuals are diagnosed after symptom onset. If the minimum viral load for symptom onset, which is unknown, is
greater than the assay limit of 100 copies per milliliter, fewer people would display symptoms than would test positive on a nucleic acid test if all individuals were frequently monitored.
Vaccine efficacy trials thus report protection against symptoms, whereas our model predicts protection against positivity in a nucleic acid test. The latter might be slightly lower than the
former. Sixth, our within-host model assumes that innate immune responses would render target cells refractory to infection. Although this has been observed with other viruses76, and has
been used in models of SARS-CoV-2 dynamics77, it remains to be demonstrated explicitly in vivo. Seventh, although we assessed the robustness of our model to parameter variations, several of
the parameter values employed were based on previous studies or from other viral infection settings. For example, we assumed that the ratio of the neutralization efficiency of NAbs in vitro
and in vivo, _ω_, was a constant. We set its value, based on estimates for a few NAbs, to one that helped recapitulate clinical data. However, _ω_ might vary across NAbs. Knowledge of these
parameter values would make our model more robust and improve its predictive ability. Challenge studies on macaques post vaccination could help test these assumptions. Finally, we note that
our study did not consider viral mutations. With five to ten NAbs active, viral escape from NAb responses is expected to be unlikely51,78. With the new circulating mutant strains7, however,
the NAb landscape may have to be reconstructed. Future studies may report DRCs of NAbs against the new strains, facilitating such reconstruction. Our formalism could then be applied to
predict the efficacies of vaccines against the new strains. METHODS DATA OF DRCS We considered data from studies that reported in vitro DRCs of NAbs using SARS-CoV-2 pseudotyped
virions26,27,30,31,32,34,35,36,37,38,39,40,41,42,43,44. We included early studies in our analysis so that the NAbs considered were unlikely to be against mutant virus strains. The assays
estimated the fraction of infection events unaffected by the NAbs as a function of the NAb concentration (Fig. 1 and Supplementary Fig. 2). We extracted the data using Engauge Digitizer 12.1
and ensured consistency with reported details, such as dilution levels used. ANALYSIS OF DRCS We used the median-effect equation (equation (1)) to analyze the data: $${\log _{10}\left(
{\frac{{f_{\rm{a}}}}{{f_{\rm{u}}}}} \right)} = {{m}{\log _{10}}\left( {\frac{D}{{{\rm{IC}}_{50}}}} \right)}$$ (1) where \({f_{\rm{u}}}\) and \({f_{\rm{a}}}\) are the fraction of infection
events unaffected and affected, respectively, by the NAbs in a single round of infection, _D_ is the NAb concentration, IC50 is the half-maximal inhibitory concentration and _m_ is the
slope. Data were fitted using the tool REGRESS in MATLAB R2017b. Data points with 1% < _f_u < 99% were considered for parameter estimation. We fit the data using equation (1), with _m_
and \({- {m}{\log _{10}}({\rm{IC}}_{50})}\) as adjustable parameters, and obtained estimates of IC50 and _m_ for each NAb. We then computed \({\rm{IIP}}_{100} = {{\log _{10}}\left( {{1} +
{\left( {\frac{{100}}{{{\rm{IC}}_{50}}}} \right)^m}} \right)}\). We did not include NAbs for which the fits were not satisfactory (_R_2 ≤ 0.8; Supplementary Data 1), possibly arising from
large uncertainties in the data. The details of the NAbs and parameter estimates are presented in Supplementary Data 1. IN SILICO SIMULATION OF PLASMA DILUTION ASSAYS We simulated plasma
dilution experiments as follows. We assumed that the plasma contained _N_ NAbs in equimolar concentrations sampled from the landscape (Fig. 2a). The reciprocal plasma dilution curve was
predicted assuming Loewe additivity (equation (2)) or Bliss independence (equation (3)) between the different NAbs79,80,81 using $${\mathop {\sum}\limits_{i = 1}^{N} {\frac{{{D_i}/\gamma
}}{{{{\rm{IC}}_{50_i}}\left( {\frac{1}{{\varepsilon _{\rm{L}}}} - {1}} \right)^{ - 1/{m_i}}}}}} = {1}$$ (2) $${\varepsilon _{\rm{B}}} = {{1} - {\mathop {\prod}\limits_{i = 1}^{N}}
{\frac{{\left( {{\rm{IC}}_{50_i}} \right)^{m_i}}}{{\left( {{\rm{IC}}_{50_i}} \right)^{m_i} + \left( {{D_i}/\gamma } \right)^{m_i}}}}}$$ (3) where _γ_ is the plasma dilution factor, _ε_L and
_ε_B are the fractions of infection events affected by the plasma in a single round of infection estimated using Loewe additivity and Bliss independence, respectively, _D__i_ is the
concentration of the _i_th NAb in the plasma before dilution, and IC50 is its half-maximal inhibitory concentration and _m__i_ its slope, with \({{i} \in \left\{ {1,\,2,\,...,\,N}
\right\}}\). We let _N_ = 10 in our simulations26. We estimated the value of _γ_ at which _ε_ = 0.5 as the corresponding NT50. We chose _D__i_ as _D_0/_N_, and varied _D_0 between 0.1 and
100 µg ml−1 (_D_0 is the total NAb concentration). We repeated these simulations 100 times at different NAb titers, with each simulation representative of an individual patient. We compared
the resulting predictions at _D_0 = 30 µg ml−1 with observations from three patients (Fig. 3b)82, which we also digitized. The equation \({f_{\rm{u}}} = {\frac{{(\gamma )^{n}}}{{{(\gamma
)^{n}} + {({\rm{NT}}_{50})^n}}}}\) was fit to the observations from three patients, merely to ascertain the shapes of the curves and their similarity to those predicted by our calculations.
Here, _n_ is the Hill coefficient, γ is the plasma dilution and NT50 is the half-maximal inhibitory plasma neutralizing titer. We also compared predictions of the dependence of NT50 on NAb
titers with experimental observations (Fig. 3c)49. MODEL OF SARS-COV-2 DYNAMICS To predict the protection conferred by vaccines, we developed a mathematical model of within-host SARS-CoV-2
infection post vaccination. We adapted previous models58,67,77,83 by focusing on early dynamics, required to accurately predict the reduction in the peak viral load due to pre-existing NAbs.
The following equations described the resulting infection dynamics in vaccinated individuals exposed to the virus: $${\frac{{{\rm{d}}T}}{{{\rm{d}}t}}} = {- \beta ({1} - \varepsilon ){VT} -
{\rho _X}{XT}}$$ (4) $${\frac{{{\rm{d}}R}}{{{\rm{d}}t}}} = {\rho _XXT}$$ (5) $${\frac{{{\rm{d}}I_1}}{{{\rm{d}}t}}} = {\beta ({1} - \varepsilon ){VT} - {k}{I_1}}$$ (6)
$${\frac{{{\rm{d}}{I_2}}}{{{\rm{d}}t}}} = {{k}{I_1} - \delta {I_2}}$$ (7) $${\frac{{{\rm{d}}V}}{{{\rm{d}}t}}} = {{p}{I_2} - {cV}}$$ (8) $${\frac{{{\rm{d}}X}}{{{\rm{d}}t}}} = {\frac{{{\sigma
_X}{I_2}{(1 - X)}}}{{{\phi _X} + {I_2}}} - {d_X}{X}}$$ (9) Here, uninfected target cells, _T_, are infected by SARS-CoV-2 virions, _V_, with second-order rate constant _β_, producing
infected cells in eclipse phase, _I_1. Cells _I_1 convert to productively infected cells, _I_2, with a rate constant _k_. Cells _I_2 produce virions at rate _p_ per cell and are lost with
rate constant _δ_. This transition from target cells to infected cells in the eclipse phase and then the productive phase has been employed in previous models58,77. The virions are cleared
with rate constant _c_. The activation of the innate immune response, quantified phenomenologically using _X_, is assumed to be a saturable function of _I_2, with maximal rate _σ__X_ and
half-maximal activation parameter _ϕ__X_. If _I_2 is not limiting, _X_ would rise at rate _σ__X_ at low _X_ and cease to rise as _X_ approaches 1. This form for the innate immune response
has been proposed previously84. _X_ converts uninfected cells to an infection-refractory state, _R_, at a per capita rate _ρ__X_, and decays with rate constant _d__X_. The presence of the
refractory population has also been proposed in previous models77,85,86. The pre-existing NAbs are drawn as random subsets from the landscape (Fig. 2a) to block new infections with an
efficacy _ε_, which is a function of NAb titers and computed using $${\mathop {\sum}\limits_{i = 1}^{N}} {\frac{{{D_i}/\gamma }}{{\omega {\rm{IC}}_{50_i}\left( {\frac{1}{\varepsilon } - {1}}
\right)^{ - 1/m_i}}}} = {1}$$ where _ω_ accounts for the difference between in vitro and in vivo IC50 values63,71. The neutralizing activity of NAbs follows from our observations above
(Fig. 3), where this activity recapitulated plasma dilution experiments. FITS TO LONGITUDINAL PATIENT DATA We considered viral load data from non-vaccinated individuals in three cohorts. One
dataset57 has patients with mild symptoms. The second dataset59 has measurements in the viral expansion phase. We chose nine individuals from the latter dataset who had frequent viral load
measurements. The third dataset58 has data from hospitalized patients classified by their age (above and below 65 years). We simultaneously fit our viral dynamics model (equations (4)–(9))
to viral loads from all three datasets using a population-based fitting approach via nonlinear mixed effects models (Supplementary Section 1). PREDICTION OF VACCINE EFFICACY VACCINE EFFICACY
To examine the variation in peak viral loads with NAb titers (Fig. 4d), we predicted the viral dynamics of 3,500 infected individuals, each individual with different pre-existing NAbs
sampled from the landscape as well as different values of viral dynamics parameters (Supplementary Tables 1 and 2). To obtain the protection curve (Fig. 5), we sampled antibody
concentrations from a uniform distribution ranging between 0.05 and 100 μg ml−1, mimicking the range seen experimentally, and simulated viral dynamics in 10,000 virtual individuals, as we
describe above, and obtained peak viral loads. Furthermore, for each individual, we also simulated plasma dilution assays and estimated NT50. We then binned individuals into narrow ranges of
NT50 values. In each bin, we estimated the fraction of individuals with peak viral load below 100 copies per milliliter. This fraction yielded the protection curve. The error bars (95% CI)
were obtained using the Clopper–Pearson method and following the protocols used in clinical trials10,87,88. CONVALESCENT NT50 Our model captured the experimentally observed dependence of
NT50 on NAb titers in 15 convalescent patients (Fig. 3c)49. NT50 as a function of time post symptom onset has also been measured in the study described in ref. 49. Following vaccine
immunogenicity studies28,89, we randomly sampled one timepoint per patient at least 20 days after initial symptoms and obtained a geometric mean NT50 of 565 (Supplementary Fig. 10). We used
this value to normalize the NT50 predicted by our model. PARAMETER SENSITIVITY The available longitudinal patient data did not allow estimation of all the parameters in our model. We thus
fixed several parameters (_ρ__X_, _σ__X_, _Φ__X_, _k_, _c_ and _d__X_) and initial conditions (_T_(0) and _I_2(0)) to values from previous studies and estimated the rest (_β_, _p_ and _δ_)
from fits to patient data (Methods). Overall, the good fits (Fig. 4b and Supplementary Fig. 7) indicate that the parameters recapitulate the patient viral load data. Yet, the choice of
values of the ‘fixed’ parameters could introduce uncertainties in the overall parameter estimates. We therefore tested the sensitivity of model predictions of vaccine efficacy to fixed model
parameter values and initial conditions by increasing or decreasing the parameter values by twofold, one at a time (Supplementary Fig. 11a). We also performed a global sensitivity analysis
and estimated the partial rank correlation coefficients of each parameter (Supplementary Fig. 11b). We found that the vaccine efficacy predictions were most sensitive to the viral clearance
rate, _c_. We therefore varied _c_, refitted the model to patient data using the same adjustable parameters, and repeated our vaccine efficacy predictions. The protection curves were close
to the one described above (Supplementary Fig. 11c). We also obtained protection curves using two different iterations after fitting using Monolix (Supplementary Fig. 11d) and when fixed
parameter values were varied over a defined range or held constant (Supplementary Fig. 11e). Finally, we tested the sensitivity of the protection curve to the IC50 values of the NAbs in vivo
by varying _ω_ (Supplementary Fig. 11f). DATA AVAILABILITY All data supporting the findings of this study are available within the paper and its Supplementary Information files. Source data
are provided with this paper. CODE AVAILABILITY The codes are written in MATLAB and are available on GitHub (https://github.com/PraneshPadmanabhan/COVID-19-vaccine-efficacies) and on
Zenodo90. REFERENCES * Wouters, O. J. et al. Challenges in ensuring global access to COVID-19 vaccines: production, affordability, allocation and deployment. _Lancet_ 397, 1023–1034 (2021).
Article Google Scholar * Forni, G. & Mantovani, A. COVID-19 vaccines: where we stand and challenges ahead. _Cell Death Differ._ 28, 626–639 (2021). Article Google Scholar * Shrotri,
M., Swinnen, T., Kampmann, B. & Parker, E. P. K. An interactive website tracking COVID-19 vaccine development. _Lancet Glob. Health_ 9, e590–e592 (2021). Article Google Scholar * Koup,
R. A. et al. A government-led effort to identify correlates of protection for COVID-19 vaccines. _Nat. Med._ 27, 1493–1494 (2021). Article Google Scholar * Saad-Roy, C. M. et al.
Epidemiological and evolutionary considerations of SARS-CoV-2 vaccine dosing regimes. _Science_ 372, 363–370 (2021). Article Google Scholar * Bubar, K. M. et al. Model-informed COVID-19
vaccine prioritization strategies by age and serostatus. _Science_ 371, 916–921 (2021). Article Google Scholar * Voysey, M. et al. Single-dose administration and the influence of the
timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. _Lancet_ 397, 881–891 (2021). Article Google
Scholar * Garg, A. K., Mittal, S., Padmanabhan, P., Desikan, R. & Dixit, N. M. Increased B cell selection stringency in germinal centers can explain improved COVID-19 vaccine efficacies
with low dose prime or delayed boost. _Front. Immunol._ 12, 776933 (2021). Article Google Scholar * Tauzin, A. et al. Strong humoral immune responses against SARS-CoV-2 spike after
BNT162b2 mRNA vaccination with a 16-week interval between doses. _Cell Host Microbe_ 30, 97–109 (2022). Article Google Scholar * Baden, L. R. et al. Efficacy and safety of the mRNA-1273
SARS-CoV-2 vaccine. _N. Engl. J. Med._ 384, 403–416 (2021). Article Google Scholar * Polack, F. P. et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 Vaccine. _N. Engl. J. Med._ 383,
2603–2615 (2020). Article Google Scholar * Logunov, D. Y. et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a
randomised controlled phase 3 trial in Russia. _Lancet_ 397, 671–681 (2021). Article Google Scholar * Ella, R. et al. Efficacy, safety, and lot-to-lot immunogenicity of an inactivated
SARS-CoV-2 vaccine (BBV152): interim results of a randomised, double-blind, controlled, phase 3 trial. _Lancet_ 398, 2173–2184 (2021). Article Google Scholar * Jara, A. et al.
Effectiveness of an inactivated SARS-CoV-2 vaccine in Chile. _N. Engl. J. Med._ 385, 875–884 (2021). Article Google Scholar * Sadoff, J. et al. Safety and efficacy of single-dose
Ad26.COV2.S vaccine against COVID-19. _N. Engl. J. Med._ 384, 2187–2201 (2021). Article Google Scholar * Heath, P. T. et al. Safety and efficacy of NVX-CoV2373 COVID-19 vaccine. _N. Engl.
J. Med._ 385, 1172–1183 (2021). Article Google Scholar * Khoury, D. S. et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection.
_Nat. Med._ 27, 1205–1211 (2021). Article Google Scholar * Earle, K. A. et al. Evidence for antibody as a protective correlate for COVID-19 vaccines. _Vaccine_ 39, 4423–4428 (2021).
Article Google Scholar * Feng, S. et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. _Nat. Med._ 27, 2032–2040 (2021). Article Google Scholar *
Barrett, J. R. et al. Phase 1/2 trial of SARS-CoV-2 vaccine ChAdOx1 nCoV-19 with a booster dose induces multifunctional antibody responses. _Nat. Med._ 27, 279–288 (2021). Article Google
Scholar * Israelow, B. et al. Adaptive immune determinants of viral clearance and protection in mouse models of SARS-CoV-2. _Sci. Immunol._ 6, eabl4509 (2021). Article Google Scholar *
Gilbert, P. B. et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. _Science_ 375, 43–50 (2022). Article Google Scholar * Addetia, A. et al.
Neutralizing antibodies correlate with protection from SARS-CoV-2 in humans during a fishery vessel outbreak with a high attack rate. _J. Clin. Microbiol._ 58, e02107–e02120 (2020). Article
Google Scholar * Lumley, S. F. et al. Antibody status and incidence of SARS-CoV-2 infection in health care workers. _N. Engl. J. Med._ 384, 533–540 (2020). Article Google Scholar *
Callow, K. A. Effect of specific humoral immunity and some non-specific factors on resistance of volunteers to respiratory coronavirus infection. _J. Hyg._ 95, 173–189 (1985). Article
Google Scholar * Liu, L. et al. Potent neutralizing antibodies against multiple epitopes on SARS-CoV-2 spike. _Nature_ 584, 450–456 (2020). Article Google Scholar * Robbiani, D. F. et al.
Convergent antibody responses to SARS-CoV-2 in convalescent individuals. _Nature_ 584, 437–442 (2020). Article Google Scholar * Folegatti, P. M. et al. Safety and immunogenicity of the
ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. _Lancet_ 396, 467–478 (2020). Article Google Scholar * Perelson,
A. S. & Oster, G. F. Theoretical studies of clonal selection: minimal antibody repertoire size and reliability of self-non-self discrimination. _J. Theor. Biol._ 81, 645–670 (1979).
Article MathSciNet Google Scholar * Chi, X. et al. A neutralizing human antibody binds to the N-terminal domain of the Spike protein of SARS-CoV-2. _Science_ 369, 650–655 (2020). Article
Google Scholar * Wang, C. et al. A human monoclonal antibody blocking SARS-CoV-2 infection. _Nat. Commun._ 11, 2251 (2020). Article Google Scholar * Seydoux, E. et al. Analysis of a
SARS-CoV-2-infected individual reveals development of potent neutralizing antibodies with limited somatic mutation. _Immunity_ 53, 98–105 e105 (2020). Article Google Scholar * Shi, R. et
al. A human neutralizing antibody targets the receptor-binding site of SARS-CoV-2. _Nature_ 584, 120–124 (2020). Article Google Scholar * Wec, A. Z. et al. Broad neutralization of
SARS-related viruses by human monoclonal antibodies. _Science_ 369, 731–736 (2020). Article Google Scholar * Lei, C. et al. Neutralization of SARS-CoV-2 spike pseudotyped virus by
recombinant ACE2-Ig. _Nat. Commun._ 11, 2070 (2020). Article Google Scholar * Lv, Z. et al. Structural basis for neutralization of SARS-CoV-2 and SARS-CoV by a potent therapeutic antibody.
_Science_ 369, 1505–1509 (2020). Article Google Scholar * Zost, S. J. et al. Potently neutralizing and protective human antibodies against SARS-CoV-2. _Nature_ 584, 443–449 (2020).
Article Google Scholar * Ju, B. et al. Human neutralizing antibodies elicited by SARS-CoV-2 infection. _Nature_ 584, 115–119 (2020). Article Google Scholar * Cao, Y. et al. Potent
neutralizing antibodies against SARS-CoV-2 identified by high-throughput single-cell sequencing of convalescent patients' B cells. _Cell_ 182, 73–84 (2020). Article Google Scholar *
Hansen, J. et al. Studies in humanized mice and convalescent humans yield a SARS-CoV-2 antibody cocktail. _Science_ 369, 1010–1014 (2020). Article Google Scholar * Rogers, T. F. et al.
Isolation of potent SARS-CoV-2 neutralizing antibodies and protection from disease in a small animal model. _Science_ 369, 956–963 (2020). Article Google Scholar * Barnes, C. O. et al.
Structures of human antibodies bound to SARS-CoV-2 spike reveal common epitopes and recurrent features of antibodies. _Cell_ 182, 828–842 (2020). Article Google Scholar * Pinto, D. et al.
Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody. _Nature_ 583, 290–295 (2020). Article Google Scholar * Hanke, L. et al. An alpaca nanobody neutralizes
SARS-CoV-2 by blocking receptor interaction. _Nat. Commun._ 11, 4420 (2020). Article Google Scholar * Webb, N. E., Montefiori, D. C. & Lee, B. Dose–response curve slope helps predict
therapeutic potency and breadth of HIV broadly neutralizing antibodies. _Nat. Commun._ 6, 8443 (2015). Article Google Scholar * Padmanabhan, P. & Dixit, N. M. Inhibitors of hepatitis C
virus entry may be potent ingredients of optimal drug combinations. _Proc. Natl Acad. Sci. USA_ 114, E4524–E4526 (2017). Article Google Scholar * Jilek, B. L. et al. A quantitative basis
for antiretroviral therapy for HIV-1 infection. _Nat. Med._ 18, 446–451 (2012). Article Google Scholar * Isho, B. et al. Persistence of serum and saliva antibody responses to SARS-CoV-2
spike antigens in COVID-19 patients. _Sci. Immunol._ 5, eabe5511 (2020). Article Google Scholar * Iyer, A. S. et al. Persistence and decay of human antibody responses to the receptor
binding domain of SARS-CoV-2 spike protein in COVID-19 patients. _Sci. Immunol._ 5, eabe0367 (2020). Article Google Scholar * Röltgen, K. et al. Defining the features and duration of
antibody responses to SARS-CoV-2 infection associated with disease severity and outcome. _Sci. Immunol._ 5, eabe0240 (2020). Article Google Scholar * Shrock, E. et al. Viral epitope
profiling of COVID-19 patients reveals cross-reactivity and correlates of severity. _Science_ 370, eabd4250 (2020). Article Google Scholar * Yuan, M. et al. Structural basis of a shared
antibody response to SARS-CoV-2. _Science_ 369, 1119–1123 (2020). Article Google Scholar * Meyer, C. T. et al. Quantifying drug combination synergy along potency and efficacy axes. _Cell
Syst._ 8, 97–108 (2019). Article Google Scholar * Widge, A. T. et al. Durability of responses after SARS-CoV-2 mRNA-1273 vaccination. _N. Engl. J. Med._ 384, 80–82 (2021). Article Google
Scholar * McMahan, K. et al. Correlates of protection against SARS-CoV-2 in rhesus macaques. _Nature_ 590, 630–634 (2021). Article Google Scholar * Sette, A. & Crotty, S. Adaptive
immunity to SARS-CoV-2 and COVID-19. _Cell_ 184, 861–880 (2021). Article Google Scholar * Wölfel, R. et al. Virological assessment of hospitalized patients with COVID-2019. _Nature_ 581,
465–469 (2020). Article Google Scholar * Neant, N. et al. Modeling SARS-CoV-2 viral kinetics and association with mortality in hospitalized patients from the French COVID cohort. _Proc.
Natl Acad. Sci. USA_ 118, e2017962118 (2021). Article Google Scholar * Kissler, S. M. et al. Viral dynamics of acute SARS-CoV-2 infection and applications to diagnostic and public health
strategies. _PLoS Biol._ 19, e3001333 (2021). Article Google Scholar * Desikan, R., Raja, R. & Dixit, N. M. Early exposure to broadly neutralizing antibodies may trigger a dynamical
switch from progressive disease to lasting control of SHIV infection. _PLoS Comput. Biol._ 16, e1008064 (2020). Article Google Scholar * Yu, J. et al. DNA vaccine protection against
SARS-CoV-2 in rhesus macaques. _Science_ 369, 806–811 (2020). Article Google Scholar * Yang, S., Jerome, K. R., Greninger, A. L., Schiffer, J. T. & Goyal, A. Endogenously produced
SARS-CoV-2 specific IgG antibodies may have a limited impact on clearing nasal shedding of virus during primary infection in humans. _Viruses_ 13, 516 (2021). Article Google Scholar * van
Gils, M. J. & Sanders, R. W. In vivo protection by broadly neutralizing HIV antibodies. _Trends Microbiol._ 22, 550–551 (2014). Article Google Scholar * Fajnzylber, J. et al.
SARS-CoV-2 viral load is associated with increased disease severity and mortality. _Nat. Commun._ 11, 5493 (2020). Article Google Scholar * Arnaout, R. et al. SARS-CoV2 testing: the limit
of detection matters. Preprint at _bioRxiv_ https://doi.org/10.1101/2020.06.02.131144 (2020). * Gonçalves, A. et al. Timing of antiviral treatment initiation is critical to reduce SARS-CoV-2
viral load. _CPT Pharmacometrics Syst. Pharm._ 9, 509–514 (2020). Article Google Scholar * Goyal, A., Cardozo-Ojeda, E. F. & Schiffer, J. T. Potency and timing of antiviral therapy as
determinants of duration of SARS-CoV-2 shedding and intensity of inflammatory response. _Sci. Adv._ 6, eabc7112 (2020). Article Google Scholar * Dagan, N. et al. BNT162b2 mRNA COVID-19
vaccine in a nationwide mass vaccination setting. _N. Engl. J. Med._ 384, 1412–1423 (2021). Article Google Scholar * Walsh, E. E. et al. Safety and immunogenicity of two RNA-based COVID-19
vaccine candidates. _N. Engl. J. Med._ 383, 2439–2450 (2020). Article Google Scholar * Anderson, E. J. et al. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adult. _N.
Engl. J. Med._ 383, 2427–2438 (2020). Article Google Scholar * Maisonnasse, P. et al. COVA1-18 neutralizing antibody protects against SARS-CoV-2 in three preclinical models. _Nat.
Commun._ 12, 6097 (2021). Article Google Scholar * Chigutsa, E., O'Brien, L., Ferguson-Sells, L., Long, A. & Chien, J. Population pharmacokinetics and pharmacodynamics of the
neutralizing antibodies bamlanivimab and etesevimab in patients with mild to moderate COVID-19 infection. _Clin. Pharmacol. Ther._ 110, 1302–1310 (2021). Article Google Scholar * Saha, A.
& Dixit, N. M. Pre-existing resistance in the latent reservoir can compromise VRC01 therapy during chronic HIV-1 infection. _PLoS Comput. Biol._ 16, e1008434 (2020). Article Google
Scholar * Krammer, F. A correlate of protection for SARS-CoV-2 vaccines is urgently needed. _Nat. Med._ 27, 1147–1148 (2021). Article Google Scholar * Chatterjee, B., Sandhu, H. S. &
Dixit, N. M. The relative strength and timing of innate immune and CD8 T-cell responses underlie the heterogeneous outcomes of SARS-CoV-2 infection. Preprint at _medRxiv_
https://doi.org/10.1101/2021.06.15.21258935 (2021). * Padmanabhan, P., Garaigorta, U. & Dixit, N. M. Emergent properties of the interferon-signalling network may underlie the success of
hepatitis C treatment. _Nat. Commun._ 5, 3872 (2014). Article Google Scholar * Perelson, A. S. & Ke, R. Mechanistic modeling of SARS-CoV-2 and other infectious diseases and the effects
of therapeutics. _Clin. Pharmacol. Ther._ 109, 829–840 (2020). Article Google Scholar * Baum, A. et al. Antibody cocktail to SARS-CoV-2 spike protein prevents rapid mutational escape seen
with individual antibodies. _Science_ 369, 1014–1018 (2020). Article Google Scholar * Padmanabhan, P. & Dixit, N. M. Modeling suggests a mechanism of synergy between hepatitis C virus
entry inhibitors and drugs of other classes. _CPT Pharmacometrics Syst. Pharm._ 4, 445–453 (2015). Article Google Scholar * Chou, T. C. Theoretical basis, experimental design, and
computerized simulation of synergism and antagonism in drug combination studies. _Pharm. Rev._ 58, 621–681 (2006). Article Google Scholar * Padmanabhan, P., Desikan, R. & Dixit, N. M.
Targeting TMPRSS2 and Cathepsin B/L together may be synergistic against SARS-CoV-2 infection. _PLoS Comput. Biol._ 16, e1008461 (2020). Article Google Scholar * Brouwer, P. J. M. et al.
Potent neutralizing antibodies from COVID-19 patients define multiple targets of vulnerability. _Science_ 369, 643–650 (2020). Article Google Scholar * Kim, K. S. et al. A quantitative
model used to compare within-host SARS-CoV-2, MERS-CoV and SARS-CoV dynamics provides insights into the pathogenesis and treatment of SARS-CoV-2. _PLoS Biol._ 19, e3001128 (2021). Article
Google Scholar * Zarnitsyna, V. I. et al. Mathematical model reveals the role of memory CD8 T cell populations in recall responses to influenza. _Front. Immunol._ 7, 165 (2016). Article
Google Scholar * Benotmane, I. et al. Biomarkers of cytokine release syndrome predict disease severity and mortality from COVID-19 in kidney transplant recipients. _Transplantation_ 105,
158–169 (2021). Article Google Scholar * Ke, R. et al. Daily sampling of early SARS-CoV-2 infection reveals substantial heterogeneity in infectiousness. Preprint at _medRxiv_
https://doi.org/10.1101/2021.07.12.21260208 (2021). * Golob, J. L., Lugogo, N., Lauring, A. S. & Lok, A. S. SARS-CoV-2 vaccines: a triumph of science and collaboration. _JCI Insight_ 6,
e149187 (2021). Article Google Scholar * Zhang, Y. et al. Safety, tolerability and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: a randomised,
double-blind, placebo-controlled, phase 1/2 clinical trial. _Lancet Infect. Dis._ 21, 181–192 (2021). Article Google Scholar * Jackson, L. A. et al. An mRNA vaccine against
SARS-CoV-2—preliminary report. _N. Engl. J. Med._ 383, 1920–1931 (2020). Article Google Scholar * Padmanabhan, P., Desikan, R. & Dixit, N. M. COVID-19 vaccine efficacies. _Zenodo_
https://doi.org/10.5281/zenodo.5879304 (2022). Download references ACKNOWLEDGEMENTS This work was supported by the DBT/Wellcome Trust India Alliance Senior Fellowship IA/S/14/1/501307 to
N.M.D. AUTHOR INFORMATION Author notes * Rajat Desikan Present address: Certara QSP, Certara UK Limited, Sheffield, UK AUTHORS AND AFFILIATIONS * Clem Jones Centre for Ageing Dementia
Research, Queensland Brain Institute, The University of Queensland, Brisbane, Queensland, Australia Pranesh Padmanabhan * Department of Chemical Engineering, Indian Institute of Science,
Bangalore, India Rajat Desikan & Narendra M. Dixit * Centre for Biosystems Science and Engineering, Indian Institute of Science, Bangalore, India Narendra M. Dixit Authors * Pranesh
Padmanabhan View author publications You can also search for this author inPubMed Google Scholar * Rajat Desikan View author publications You can also search for this author inPubMed Google
Scholar * Narendra M. Dixit View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS P.P. provided conceptualization, carried out investigations and
formal analysis, contributed to writing the original draft and reviewed and edited the manuscript. R.D. performed formal analysis and reviewed and edited the manuscript. N.M.D. provided
conceptualization, contributed to writing the original draft and reviewed and edited the manuscript. CORRESPONDING AUTHORS Correspondence to Pranesh Padmanabhan or Narendra M. Dixit. ETHICS
DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. PEER REVIEW PEER REVIEW INFORMATION _Nature Computational Science_ thanks Joshua T. Schiffer and the other,
anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Ananya Rastogi, in collaboration with the _Nature Computational Science_ team.
ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION
SUPPLEMENTARY INFORMATION Supplementary Section 1, Figs. 1–11 and Tables 1–4. SUPPLEMENTARY DATA 1 Best-fit estimates of dose–response curve parameters of NAbs. SOURCE DATA SOURCE DATA FIG.
1 Statistical source data. SOURCE DATA FIG. 2 Statistical source data. SOURCE DATA FIG. 3 Statistical source data. SOURCE DATA FIG. 4 Statistical source data. SOURCE DATA FIG. 5 Statistical
source data. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Padmanabhan, P., Desikan, R. & Dixit, N.M. Modeling how antibody responses may determine
the efficacy of COVID-19 vaccines. _Nat Comput Sci_ 2, 123–131 (2022). https://doi.org/10.1038/s43588-022-00198-0 Download citation * Received: 27 April 2021 * Accepted: 20 January 2022 *
Published: 28 February 2022 * Issue Date: February 2022 * DOI: https://doi.org/10.1038/s43588-022-00198-0 SHARE THIS ARTICLE Anyone you share the following link with will be able to read
this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative