Bacterial spore morphology remains highly recognizable after exposure to simulated enceladus and europa surface conditions
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT The subsurface oceans of Enceladus and Europa are thought to be some of the best candidate environments for finding life beyond Earth. Realistically, the first missions aimed at
searching for life on these worlds will likely be restricted to the shallow subsurface. Here, we investigated whether indicators of life, or _biosignatures_, deposited near the surface could
persist long enough to be detected, given that the extremely harsh conditions there would tend to degrade them. We exposed _Bacillus subtilis_ spores to Ocean World surface conditions and
used electron microscopy combined with spectroscopic approaches to assess the preservation potential of structural and morphological biosignatures derived from spores. Our results show that
spore structure is highly resilient in the face of extreme conditions long after they have been inactivated, suggesting that methods targeting cell morphology would be valuable components in
a suite of life detection strategies used in future missions to Ocean Worlds. SIMILAR CONTENT BEING VIEWED BY OTHERS AEROBIC MICROBIAL LIFE PERSISTS IN OXIC MARINE SEDIMENT AS OLD AS 101.5
MILLION YEARS Article Open access 28 July 2020 THE POLAR NIGHT SHIFT: SEASONAL DYNAMICS AND DRIVERS OF ARCTIC OCEAN MICROBIOMES REVEALED BY AUTONOMOUS SAMPLING Article Open access 11
December 2021 SEA SPRAY ALLOWS FOR THE GROWTH OF SUBAERIAL MICROBIALITES AT THE DRIEST DESERT ON EARTH Article Open access 28 August 2024 INTRODUCTION The frozen moons of the outer Solar
System, especially Europa and Enceladus, are some of the most enticing targets in the search for life beyond Earth. This stems primarily from the potential for rock-water interactions
occurring at their seafloors, which may produce environments that resemble hydrothermal vent ecosystems here on Earth1,2,3,4,5,6. Observations made by the Galileo and Cassini spacecraft
provided evidence for the presence of a salt-rich ocean hidden below the icy surfaces of these moons, suggesting that conditions favorable for the development of life may exist there7.
Upcoming missions including Europa Clipper and JUICE are likely to yield further insights into the subsurface composition of Europa and may provide important guidance for future lander
missions to the surfaces of icy moons8,9,10. However, the thick ice shells covering these bodies present considerable challenges for direct sampling of their subsurface oceans, where
conditions are most compatible with the chemistry of life11. While robotic solutions for subsurface exploration are in development, initial attempts at detecting life on these worlds may
have to rely on geological processes, such as plumes or cryovolcanism and ice sheet tectonics, to transport subsurface ocean material to accessible depths closer to the surface. Fortunately,
such processes appear to exist in the Southern Polar region of Enceladus, and possibly on Europa12,13,14,15. While the subsurface oceans of Europa and Enceladus may be hospitable, their
surface environments are hostile towards the chemistry of life as we know it. The surfaces of these moons are characterized by extremely low temperatures ranging from 60 to 150 Kelvin, and
are continually bombarded by ultraviolet (UV) photons from the Sun and high-energy charged particles accelerated by the magnetic fields of the giant planets16,17,18,19,20. These harsh
conditions would pose significant challenges to the survival of biological organisms if they exist on these worlds and were expressed on the surface. Ionizing and non-ionizing sources of
radiation rapidly degrade organic material, as demonstrated by the numerous studies on the degradation of bare organic molecules21,22,23,24,25,26,27,28,29,30,31. While laboratory studies
have demonstrated the effects of radiation on organics in ices, the specific effects of radiation at exposure levels relevant to Ocean World surface ages on biosignature preservation from
material that started as a cell remains relatively unexplored. Understanding the preservation of biosignatures originating from the most resilient life forms to these conditions is crucial
for informing future missions aimed at searching for life in such extreme environments. Bacterial spores are among the most resilient forms of life on Earth, with _Bacillus subtilis_ serving
as a model organism for studying the responses of biological systems to extreme conditions. In addition to being well studied, straightforward to culture, and amenable to genetic
manipulation, _B. subtilis_ spores exhibit remarkable resistance to many stressors, including exposure to space vacuum, temperature extremes, and radiation32. Because of this, they are
regularly used to test the efficacy of sterilization techniques and verify spacecraft cleanliness for planetary protection purposes33,34,35,36,37. As a result, the molecular and
physiological responses of spores under conditions replicating aspects of several planetary environments, including low-Earth orbit and the Martian surface, have been well characterized, as
have the specific mechanisms leading to spore inactivation38,39,40,41. Experiments exposing _B. subtilis_ spores to UV, microwave, gamma ray, and electron irradiation under different vacuum
and temperature conditions have collectively shown that while survival under these conditions is enhanced relative to less resilient microbial cells, prolonged exposure to both ionizing and
non-ionizing radiation results in rapid loss of viability42,43,44,45,46,47,48,49,50,51,52,53. In particular, when exposed to solar UV radiation at Ocean World surface temperatures, the
majority of spores are inactivated within minutes, with a slight enhancement in survival at lower temperatures54,55. This suggests that viable spores are unlikely to persist long enough to
be recovered at the surface of Ocean Worlds. However, whether spores leave behind biosignatures that can be detected long after they are inactivated remains an open question. The broad goal
of this work was to experimentally determine whether biosignatures derived from microbial spores persist under conditions representative of Ocean World surfaces over geologically relevant
timescales. Specifically, the objective was to use microscopic and spectroscopic methods to survey how spore morphology and cellular structure change in response to radiation and temperature
extremes, as well as compare the effects of ionizing and non-ionizing radiation. By simulating exposures relevant to the surface ages of Europa and Enceladus and their geological processes,
the results of this work may provide important insights into the likelihood of detecting biological material on these worlds and whether structural and morphological biosignatures are
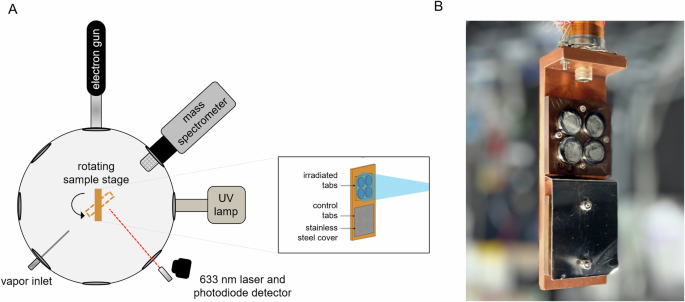
preserved over timescales that would enable detection by future astrobiology missions. The temperature, vacuum, and radiation conditions of Europa and Enceladus were simulated in a
high-vacuum chamber equipped with a cryostat, onto which stainless steel tabs containing a sub-monolayer of _B. subtilis_ spores were mounted and subjected to either electron radiation to
simulate the Europan surface, or a UV photon source to replicate conditions at Enceladus (Fig. 1). Current crater count-based estimates place the surface age of Europa at 40–90 million years
old, which suggests that any organic material that might be present at the surface could be heavily processed by multiple types of radiation bombarding the surface for extended periods of
time56. To reach less processed material, sampling would need to occur below this heavily processed layer where the effects of deeper penetrating electrons (e-) originating from Jupiter’s
magnetosphere would be dominant over other forms of radiation16. Therefore, our experiments simulating the shallow subsurface of Europa focused on electron radiation produced by an electron
flood gun aimed at spore tabs in our high-vacuum chamber set-up. Electron irradiation times were selected to reach a cumulative dose of 6 × 108 Grays (600 MGy), a community accepted standard
for experiments involving the irradiation of water ice at which all chemical bonds are presumed to have been broken at least once, equivalent to 100 eV per oxygen atom16,18,26,57,58. We
also performed an experiment simulating a cumulative dose of 9000 MGy, allowing us insight into even more heavily processed (i.e., shallower) depths near the surface of Europa. Models
constructed from surface bombardment patterns and particle tracing have provided dose-depth estimates for energetic electrons at Europa58. Based on these models, the time to reach a
cumulative dose of 600 MGy at the Europan surface is estimated to be about 10 years, while it would take 107 years to reach the same dose 10 cm below the surface, roughly equivalent to the
mean surface age of Europa56. Given these dose estimates at a sampling depth of 10 cm, our experiments therefore simulate timescales on the order of 107–108 years of electron radiation
exposure. Future lander missions to Enceladus are likely to target the South polar region, where plumes are actively depositing material originating from the deeper subsurface, and possibly
from the ocean itself, onto the surface7,11. This freshly deposited material is likely to be relatively unprocessed by ionizing radiation. However, a major concern in this case are the
effects of strong UV radiation from the sun, which is also highly damaging to biological molecules. Therefore, we simulated the Enceladus radiation environment by irradiating spores with
solar-like UV produced by a xenon arc lamp in the 200–300 nm range. We chose to simulate 1 year, 10 years, and 29 years at Enceladus, with the longest exposure time replicating one Saturnian
orbit around the Sun. We selected this value as it represents a highly conservative scenario in which material deposited from plumes remains at the surface without burial for an extended
period, resulting in more pronounced degradation. RESULTS All UV and electron irradiation experiments were carried out at room temperature with no temperature control, and at 100 K with and
without a 375 nm layer of amorphous water ice. This ice thickness was selected based on the stopping power of electrons in ice and represents a thickness that allows electrons to reach the
spore layer rather than being fully absorbed by the ice while shielding the underlying stainless-steel substrate to suppress the production of substrate-generated X-rays. In the UV case,
water ice below 1 micron in thickness is effectively transparent to UV photons but may act as a desorption barrier and therefore influence spore responses to irradiation. To visually inspect
spores for morphological changes in response to irradiation in our experimental set-up, we observed tab-mounted spores directly using a scanning electron microscope (SEM). Representative
micrographs for each radiation type can be seen in Fig. 2. Normal spores appear plump and cylindrical in shape. However, even in samples that were not exposed to radiation, a significant
number of spores (~20–30%) appear to have a deflated appearance. This fraction of spores is likely the result of spores becoming dehydrated under the high-vacuum conditions of the experiment
chamber and/or the SEM chamber. Examples of spore damage induced by electron and UV radiation in our experiments are highlighted in panels A–D of Fig. 2 and include distinct holes in the
outer-most visible layer of spores (likely the spore coat), signs of lysis, deflation, and scattered material surrounding spores. After counting the number of visibly damaged spores for each
treatment, we found that even the most extreme of exposures for both UV and electron experiments yielded marginal visual evidence of increased spore damage (Fig. 3). For reference, spores
specifically inactivated by lysis through exposure to wet heat in an autoclave can be seen in Supplementary Fig. 1, and in this case all spores visible are either heavily shriveled or fully
deflated. For UV irradiations at room temperature, we observed a slight increase in the fraction of damaged spores as a function of simulated exposure time, but no significant
time-dependence at low temperature both in the presence and absence of ice (ANOVA; _F_(14) = 1.22, _p_ = 0.3 and _F_(14) = 0.99, _p_ = 0.34, respectively). Temperature did not play a
significant role during electron irradiation (ANOVA; _F_(14) = 1.49, _p_ = 0.37), nor did the electron energy at 100 K with or without ice, although a slight but significant increase in
spore damage occurred at room temperature using 10 keV electrons (ANOVA; _F_(14) = 5.17, _p_ = 0.0098). At 100 K, increasing the cumulative dose from 600 MGy to 9000 MGy increased the
percentage of damaged spores by ~15% (Fig. 3) (_t_(14) = −2.52, _p_ = 0.012). Viability assays performed on spores recovered from experiments that simulated the shortest and least aggressive
UV and electron irradiations (1 year and 600 MGy with 3 keV electrons, respectively) revealed that all spores had been inactivated, despite appearing structurally intact by SEM (Fig. 4).
Elemental analysis was carried out alongside SEM imaging with energy-dispersive X-ray spectroscopy (EDS) to determine whether an elemental enrichment suggestive of material originating from
spores could still be detected after irradiation (Fig. 5). We found that intact spores were enriched in C and Ca2+ relative to the stainless-steel background in the majority of fields, and
that damaged spores retained the C enrichment while Ca2+ was not consistently detectable. We then analyzed tab-mounted spores by Fourier transform infrared spectroscopy (FTIR) to assess
whether chemical changes could be detected spectroscopically. Spore IR spectral features have previously been investigated to distinguish between _Bacillus_ species and between vegetative
cells and spores55,59,60,61. Thanks to this work, we were able to identify signals corresponding to amides, sugars, fatty acids, and dipicolinic acid, a compound specific to bacterial
endospores involved in their resistance to multiple stressors38 (Fig. 6). All of these features were consistently detected in control samples. In UV experiments, spores irradiated for the
equivalent of 1 year at Enceladus retained the majority of these features, with some decreases in intensity. A broad peak appeared around 3500 cm−1, which is likely to be an artifact
(perhaps due to irregularities on the tab surface) based on the peak shape. Extending the UV exposure time to the equivalent of 10 and 29 years decreased the intensity of all IR spectral
features, with peaks at around 2900 cm−1 and 1600 cm−1 remaining discernable. In the electron case, both doses resulted in the loss of most spectral features, with the most pronounced change
occurring at the highest dose of 9000 MGy. Irradiation with both sources produced color changes in the spore layer, with the electron-irradiated spores appearing more orange than the
UV-irradiated layer, which looked to be more yellow. To further assess the stability of the spore material observed by electron microscopy and simulate a generic liquid handling protocol for
chemical analysis as part of a hypothetical life detection mission, electron-irradiated and control spores were recovered from tabs by suspending them in water and re-depositing the
suspension on new tabs with the same membrane filtering method used to initially mount the spores for irradiation. SEM analysis of these re-deposited spores revealed that spore morphology
was still clearly recognizable and appeared similar in control and irradiated samples (Supplementary Fig. 2). Finally, to qualitatively determine if the spore form has behavior completely
different from standard cells, _B. subtilis_ vegetative cells were exposed to the shortest exposures of UV and electron irradiation (1 year and 600 MGy with 3 keV electrons, respectively) at
room temperature. These cells also did not show catastrophic signs of damage or morphological changes when observed by SEM, although the UV-irradiated cells specifically did display some
damage (Supplementary Fig. 3). DISCUSSION The effects of radiation on biological material and the resilience of _Bacillus subtilis_ spores under extreme conditions have been well documented,
but little work has been done to characterize how spores respond to Ocean World surface conditions over geologically relevant timescales to assess the preservation potential of the
resulting biosignatures. In this work, we exposed spores to radiation, vacuum, and temperature conditions representative of Europa and Enceladus surfaces and found that surprisingly, spore
structure and morphology remained recognizable even after the most extreme of exposures, despite all spores being rapidly inactivated. Neither the physical alteration of biomolecules or
indirect damage through the production of reactive oxygen species (ROS), which both cause irreparable damage to vital cellular components and prevent spores from germinating, lead to enough
destruction in the outermost spore layers to be readily discernable by microscopy. While we did observe a small increase in damage in spores exposed to UV at room temperature, this is more
likely due to the effects of heat generated by the lamp than to the UV photons alone. Without temperature control, the tabs reached ~60 °C during the irradiation experiments, which is
expected to sensitize the spores to chemical damage. The lack of observable damage to the outer spore layers in response to irradiation is consistent with prior work showing that exposing
spores to damaging electrical discharge in water, which produces some UV light, does not result in morphological damage as determined by scanning and transmission electron microscopy62.
While previous studies have demonstrated that the likelihood of finding viable spores on the surfaces of Europa and Enceladus is low, these results indicate that cellular morphology would
still be detectable long after spores have been inactivated even under extreme conditions. The qualitatively similar response of _B. subtilis_ vegetative cells irradiated as part of this
study, also hints at the fact that the high preservation potential of cellular morphology is not unique to spores, but further work is needed to generalize these observations. Elemental
analysis also revealed that the Ca2+ enrichment expected in visually intact spores in the control sample, due to the high intracellular concentrations of Ca2+ characteristic of spores in the
_Bacillus_ genus32, was also present in intact spores of irradiated samples. The loss of Ca2+ signal in damaged spores may be due both to the loss of intracellular Ca2+ as a result of
damage and to a reduction in spore thickness upon deflation and therefore a change in incident electron penetration depth through the spore. In contrast, most of the characteristic
vibrational spectral features have been significantly reduced in strength or eliminated completely, especially for the most intense radiation conditions. In both radiation environments, the
most long-lived of the observed spectral signatures correspond to stretching and bending of amide bonds. This persistence may be simply related to the fact that proteins are the largest
class of compounds by mass in any given cell, or it may also be that proteins degrade more slowly than other molecules under the conditions we tested. The spore coat is responsible for
conferring resistance to damage induced by heat, desiccation, chemical agents, and radiation, and is composed of more than 70 spore coat-specific proteins63, further supporting the idea that
the proteins themselves may generally outlast other biomolecules under these challenging conditions. Interestingly, both UV and electron irradiation led to similar changes in the spectra,
e.g., comparing the 600 MGy electron spectra to the 29-year UV spectra in Fig. 6. Given the differences in energy profiles for UV photons and electrons, it is somewhat surprising that a more
target- or radiation-specific change in spectra was not observed. This may be attributed to the fact that much of the cellular damage is caused by reactive photolysis products. Follow-on
work will recover the spores to extract and analyze specific molecular biosignatures such as fatty acids, amino acids, and chromosomal DNA to more quantitatively understand if some of the
differences in molecular-specific destruction are lost with the less quantitative IR analysis performed here. In this work, we were limited in the length of exposure times, radiation
energies, and doses we could feasibly execute in our experimental set-up. Despite these limitations, we were able to simulate timescales equivalent to or exceeding the mean surface age of
Europa (~107 years) assuming a 10-cm sampling depth. In our experiments, the electron source and safety considerations associated with our vacuum chamber set-up placed significant
constraints. To eliminate the risk of harmful X-ray production, experiments were carried out with a maximum electron energy of 10 keV. However, spore damage after irradiation with MeV
electrons has previously been characterized by SEM and while there are clear signs of damage, spores appear to largely retain their morphology as seen in our own investigations45. There are
always caveats with using discrete e- energies and then extrapolating to the entire spectrum of energies present at Europa. However, comparing 3 keV and 10 keV in our experiments, along with
the MeV electrons of the previous study does not reveal any discernable qualitative differences suggesting that maintaining morphological structure is a reliable feature under electron
irradiation across a wide range of energies. The specific energy of incident electrons is also unlikely to result in significantly different damage profiles, as it is secondary electrons
produced by substrate ionization that are responsible for inducing the majority of chemical change64. In the UV experiments, the conditions in our set-up may be more extreme than what we
would expect to encounter at the surface of Enceladus, where burial by plume material would provide additional shielding from UV radiation and presumably enhance biosignature preservation.
Recent estimates of Enceladus UV reflectance spectra from the Hubble telescope and resurfacing rates suggest that organic material would be buried to a UV-shielded depth of 100 microns
within several years65. Because our experiments involved depositing sub-monolayers of spores on stainless steel tabs, the effects of shielding were virtually eliminated, and spores were,
therefore, more vulnerable to damage than they would be in a plausible planetary scenario based on these estimates. Taken together, our results indicate that spore morphology is a highly
resilient biosignature that may withstand the harsh conditions found at the surfaces of Europa and Enceladus long enough to be reliably detected with the appropriate instrumentation. This
work also shows that the likely first step of any sample handling system, which would be to melt the sample and move it fluidically, does not disrupt this morphological biosignature, a
critical issue when thinking about how to design a mission and instruments for maximum life detection science return. Cellular morphology is readily discernable using various forms of
microscopy, and both the proposed Europa Lander and the Enceladus Orbilander mission concepts included microscopes as part of their life detection instrument suites8,10. While cellular
morphology by itself would not be a convincing biosignature given the high probability of false-positives, it would be a powerful complement to other measurements, and successful life
detection beyond Earth will almost certainly require different lines of evidence targeting distinct biosignature types66,67. The ability to identify cell morphology in addition to the
detection of complex organics and other definitive indicators of life would increase the certainty in a positive life detection scenario. Overall, the results of this work suggest that
including the capacity to identify cell-like structures would be especially important in the search for signs of life on the surfaces of Ocean Worlds. METHODS VACUUM CHAMBER SET-UP All
irradiation experiments were carried out in a high-vacuum chamber (_P_ < 5 × 10−7 Torr at room temperature) that allows for sample temperature control between 11 and 350 K using a
closed-cycle He cryostat, and a resistive element which are mounted on a rotary platform with 360° rotation and can be moved to face any of the chamber ports (Fig. 1). Eight tabs were
prepared for each irradiation experiment and attached to a copper mounting block, which was affixed to the end the cryostat. This cryostat was then placed in the chamber. With this setup,
the tabs were either irradiated at room temperature (without temperature control) or maintained at a constant temperature of 100 K, which was monitored with a Si diode sensor attached to the
copper mounting block. For experiments performed with water ice, a thin layer was deposited onto the spores by allowing HPLC-grade water vapor at 10−6 Torr to condense onto the chilled
tabs, with the ice thickness monitored using laser interferometry with a 633 nm laser and photodiode (Thorlabs CPS635F, PDA100A2). This technique allowed for the deposition of amorphous
compact water ice with a thickness of 375 nm in this study. For each irradiation experiment, four tabs were exposed to the radiation source, and the remaining four tabs were shielded with a
stainless-steel cover. For Europa experiments, spores were exposed to an electron gun (Kimball Physics) with beam washing to simultaneously irradiate the four exposed tabs with a uniform
electron flux. For Enceladus experiments, tabs were irradiated with a Xenon arc lamp (Oriel; model # 6266) with a solar-like UV spectrum. SPORE STOCK PREPARATION _Bacillus subtilis_ spores
(ATCC 27370) were prepared from the stock used in ref. 55. To begin, spores were plated onto tryptic soy agar (TSA) plates to stimulate germination at 37 °C for 2 days until vegetative cells
reached an exponential growth stage. The cells were then inoculated onto a sporulation medium composed of 1.6% nutrient broth, 1.7% agar, 0.2% KCl, 0.05% MgSO4, 1 mM Ca(NO3)2, 100 μM
MnCl2·4H2O, 1 μM FeSO4, and 1% glucose and incubated at 37 °C for a total of ~2.5 days, until sporulation reached 80%, which was verified by phase contrast microscopy. The bacterial spores
were separated from the remaining vegetative cells and debris by cycles of suspension, sonication, and centrifugation in the following solutions: sterile water (H2O, Molecular Biology Grade,
Fisher BioReagents), 1 M NaCl, sterile water, 0.5 M NaCl+, 1 M KCl, sterile water, 0.2 mg/mL lysozyme and 10 mM tris-HCl solution under constant shaking for 2 h at 37 °C and an additional
seven times with sterile water until no more cellular debris was visible. The concentration of spores was determined by plating on TSA and counting colonies. The resulting stock was used to
make solutions of fixed CFU ml−1 to use in irradiation experiments. SPORE MOUNTING ON STAINLESS STEEL TABS To prepare spores for irradiation, spores were deposited onto 14 mm diameter
stainless steel tabs by filtering an aqueous solution containing 1 × 108 CFU mL−1 onto a membrane filter (0.2 μm pore diameter, GE Healthcare Whatman Nucleopore Black Polycarbonate #110656)
and then wetting it by placing on a water-soaked filter paper (GE Healthcare Whatman Grade 93 Qualitative Filter Paper Wet-Strengthened #1093-110) to keep the membrane wet, which is
necessary for spore transfer, without disturbing the spore layer. The membrane was then placed onto the stainless-steel surface and dried under a slow flow of N2. This procedure ensures a
low-density distribution of the spores across the surface with sub-monolayer coverage to limit the risk of self-shielding68,69. UV FLUX AND ENCELADUS EXPOSURE TIME CALCULATIONS We used a
Xenon arc lamp (Oriel model # 6266) as a source of solar-like UV used to simulate the Enceladus surface environment. To determine the UV flux at the spore tabs, a photodiode (International
Radiation Detectors Inc. SXUV-100; model #09−718) was placed at the tab location and connected to a picoammeter outside the chamber to measure the current. The UV beam was passed through a
280 nm bandpass filter (Edmund Optics #67-880). We measured a current of 952 nA, which corresponds to a flux of 5.85 × 1018 photons s−1 m−2 based on the filter transmission and photodiode
quantum efficiency. This value and the published spectrum for the lamp were used to generate an interpolated UV spectrum and compared to the solar flux at Enceladus (9.6 a.u.), the data for
which were obtained from Woods et al.70 (Solar spectrum at 1 a.u.; Supplementary Fig. 4) with appropriate inverse square law scaling. The ratio between integrated areas was then used to
determine exposure time equivalencies used in our experiments. ELECTRON DOSE AND EUROPA EXPOSURE TIME CALCULATIONS The electron flux was calculated from current measurements obtained by
mounting a Faraday cup (Kimball Physics model # FC-72) fitted with a molybdenum mesh connected to a picoammeter and affixed the tab location in the vacuum chamber. The cup was positively
biased with 120 V supplied by batteries placed in series with the cup. Calibration curves were generated using both 3 keV and 10 keV electrons at room temperature and recording the average
current at the Faraday cup at different gun emission currents ranging from 1 μA to 80 μA (Supplementary Fig. 5). The resulting curves were used to determine the tab current obtained with the
33.2 μA gun emission current used in the spore experiments, which produced a current value of 130 nA cm−2 with 3 keV electrons. This value was then converted to Grays by multiplying the
recorded current by the estimated stopping power _S_ generated by the National Institutes of Standards and Technology ESTAR program with the following spore elemental composition: H 52.5%, O
17.5% C 25%, N 3.5%, Ca 1.5% (_S_ = 51 meV cm2 g−1). Based on these calculations, we found that using 3 keV electrons, a 33.2 μA emission current, and 30% spot size, a second of irradiation
in our chamber produces 8.6 kGy s−1. With 10 keV electrons, a second of irradiation in the chamber produces 21 kGy s−1. Based on these values, reaching the selected cumulative dose of 600
MGy requires 19 h in our chamber with 3 keV electrons, or 8 h with 10 keV electrons. We also carried out a more aggressive irradiation to reach 9000 MGy with 10 keV electrons, which required
120 h of irradiation. VIABILITY ASSAYS Spores were recovered from stainless steel tabs by dropping 100 μL of sterile nanopure water onto the tabs and scraping the spores with a sterile
silicone cell scraper. The spore suspension was transferred to a tube and the process was repeated 5 times yielding a total of 0.5 mL of spore suspension, which was added to 0.5 mL of
sterile water for a total volume of 1 mL. The solution was diluted 1/1000 and plated in triplicate on TSA plates, which were incubated at 37 °C overnight. Colonies were counted and used to
determine the recovered CFU mL−1, which corresponds to CFU recovered for each tab. SCANNING ELECTRON MICROSCOPY (SEM) AND ENERGY-DISPERSIVE X-RAY SPECTROSCOPY (EDS) Spores were coated with a
7 nm layer of platinum using a sputter coater and loaded into a high-resolution analytical SEM (Zeiss 1550 VP). Imaging was performed with the in-lens secondary electron detector. Three
tabs were analyzed at 15 kV for each treatment and five random fields at 1000x magnification were imaged per tab for a total of 15 fields per treatment. Spores were then counted and the
number of intact and damaged spores determined visually. For an example of how spores were scored and percent damage calculated, see Supplementary Fig. 6. The elemental composition of the
stainless-steel substrate, intact spores, and damaged spores was determined by EDS connected to the SEM (Oxford X-Max SDD EDS system). EDS spectra were obtained using a 10-kV accelerating
voltage and 30 s acquisition time. SEM fields used for EDS analysis were scored for the presence of a carbon enrichment relative to the stainless-steel background and for the presence of
absence of calcium. All raw SEM images can be accessed publicly via the JPL Open Repository71. FOURIER-TRANSFORM INFRARED SPECTROSCOPY (FTIR) Infrared spectra of tab-mounted spores were
collected directly with Fourier transform infrared spectrometer (Thermo Nicolet 6700) in reflectance using a PIKE Technologies DIFFUSIR reflection accessory. A blank stainless-steel tab was
used as a background. To ensure a good signal, a thicker layer of spores was deposited onto the tabs (5 × 108 CFU mL−1). Baseline correction was performed in the OMNIC software. A drop of
water was placed on a blank tab and the resulting spectrum was subtracted from the experimental spectra. DATA AVAILABILITY All raw data generated from this work, including electron
micrographs and infrared spectra, can be accessed publicly via the JPL Open Repository (https://doi.org/10.48577/jpl.98PC5B). REFERENCES * Parkinson, C. D., Liang, M.-C., Yung, Y. L. &
Kirschivnk, J. L. Habitability of Enceladus: planetary conditions for life. _Orig. Life Evol. Biosph._ 38, 355–369 (2008). Article CAS Google Scholar * Pappalardo, R. T. et al. Does
Europa have a subsurface ocean? Evaluation of the geological evidence. _J. Geophys. Res. Planets_ 104, 24015–24055 (1999). Article CAS Google Scholar * Hand, K. P., Carlson, R. W. &
Chyba, C. F. Energy, chemical disequilibrium, and geological constraints on Europa. _Astrobiology_ 7, 1006–1022 (2007). Article CAS Google Scholar * McCollom, T. M. Methanogenesis as a
potential source of chemical energy for primary biomass production by autotrophic organisms in hydrothermal systems on Europa. _J. Geophys. Res. Planets_ 104, 30729–30742 (1999). Article
CAS Google Scholar * Reynolds, R. T., Squyres, S. W., Colburn, D. S. & McKay, C. P. On the habitability of Europa. _Icarus_ 56, 246–254 (1983). Article Google Scholar * Russell, M.
J., Murray, A. E. & Hand K. P. The possible emergence of life and differentiation of a shallow biosphere on irradiated icy worlds: the example of Europa. _Astrobiology_ 17, 1265−1273
(2017). * Porco, C. C. A community grows around the geysering world of Enceladus. _Astrobiology_ 17, 815–819 (2017). Article Google Scholar * Hand, K. The Europa lander mission concept and
science goals of the 2016 Europa lander science definition team report in _42nd COSPAR Scientific Assembly_ (NASA, 2018). * Pappalardo, R. T. et al. Science potential from a Europa lander.
_Astrobiology_ 13, 740–773 (2013). Article CAS Google Scholar * MacKenzie, S. M. et al. The Enceladus orbilander mission concept: balancing return and resources in the search for life.
_Planet. Sci. J._ 2, 77 (2021). * Choukroun, M. et al. Sampling plume deposits on Enceladus’ surface to explore ocean materials and search for traces of life or biosignatures. _Planet. Sci.
J._ 2, 100 (2021). Article Google Scholar * Goodman, J. C., Collins, G. C., Marshall, J. & Pierrehumbert, R. T. Hydrothermal plume dynamics on Europa: implications for chaos formation.
_J. Geophys. Res. Planets_ 109, E3 (2004). * Howell, S. M. & Pappalardo, R. T. Band formation and ocean‐surface interaction on Europa and ganymede. _Geophys. Res. Lett._ 45, 4701–4709
(2018). Article Google Scholar * Jia, X., Kivelson, M. G., Khurana, K. K. & Kurth, W. S. Evidence of a plume on Europa from Galileo magnetic and plasma wave signatures. _Nat. Astron._
2, 459–464 (2018). Article Google Scholar * Roth, L. et al. Transient water vapor at Europa’s south pole. _Science_ 343, 171–174 (2014). Article CAS Google Scholar * Cooper, J.,
Johnson, R. E., Mauk, B. H., Garrett, H. B. & Gehrels, N. Energetic ion and electron irradiation of the icy Galilean satellites. _Icarus_ 149, 133–159 (2001). Article CAS Google
Scholar * Johnson, R. E. et al. Radiation effects on the surfaces of the Galilean satellites. _Jupit. Planet Satell. Magnetos._ 1, 485–512 (2004). Google Scholar * Paranicas, C., Cooper,
J. F., Garrett, H. B., Johnson, R. E. & Sturner, S. J. _Europa’s Radiation Environment and its Effects on the Surface_. In: _Europa_ (eds Pappalardo R. T., McKinnon W. B., Khurana K. K.)
(University of Arizona Press, 2009). * Paranicas, C. et al. Energetic charged particle weathering of Saturn’s inner satellites. _Planet. Space Sci._ 61, 60–65 (2012). Article CAS Google
Scholar * Spencer, J. R., Tamppari, L. K., Martin, T. Z. & Travis, L. D. Temperatures on Europa from Galileo photopolarimeter-radiometer: nighttime thermal anomalies. _Science_ 284,
1514–1516 (1999). Article CAS Google Scholar * Barnett, I. L., Lignell, A. & Gudipati M. S. Survival depth of organics in ices under low-energy electron radiation (≤2 keV).
_Astrophys. J._ 474, 13 (2012). * Bernstein, M. P. et al. UV irradiation of polycyclic aromatic hydrocarbons in ices: production of alcohols, quinones, and ethers. _Science_ 283, 1135–1138
(1999). Article CAS Google Scholar * Ehrenfreund, P. et al. The ORGANICS experiment on BIOPAN V: UV and space exposure of aromatic compounds. _Planet. Space Sci._ 55, 383–400 (2007).
Article CAS Google Scholar * Kate, I. L. et al. Amino acid photostability on the Martian surface. _Meteorit. Planet. Sci._ 40, 1185–1193 (2005). Article Google Scholar * Guan, Y. Y. et
al. UVolution: compared photochemistry of prebiotic organic compounds in low earth orbit and in the laboratory. _Planet. Space Sci._ 58, 1327–1346 (2010). Article CAS Google Scholar *
Hand, K. P. & Carlson, R. W. Laboratory spectroscopic analyses of electron irradiated alkanes and alkenes in solar system ices. _J. Geophys. Res. Planets_ 117, E3 (2012). * Maté, B.,
Tanarro, I., Escribano, R., Moreno, M. A. & Herrero V. J. Stability of extraterrestrial glycine under energentic particle radiation estimated from 2 kev electron bombardment experiments.
_Astrophys. J._ 806, 151 (2015). * Materese, C. K., Gerakines, P. A. & Hudson, R. L. The radiation stability of thymine in solid H2O. _Astrobiology_ 20, 956–963 (2020). Article CAS
Google Scholar * Orzechowska, G. E., Goguen, J. D., Johnson, P. V., Tsapin, A. & Kanik, I. Ultraviolet photolysis of amino acids in a 100K water ice matrix: application to the outer
solar system bodies. _Icarus_ 187, 584–591 (2007). Article CAS Google Scholar * Nuevo, M. et al. Irradiation of pyrimidine in pure H2O Ice with high-energy ultraviolet photons.
_Astrobiology_ 14, 119–131 (2014). Article CAS Google Scholar * Johnson, P. V., Hodyss, R., Chernow, V. F., Lipscomb, D. M. & Goguen, J. D. Ultraviolet photolysis of amino acids on
the surface of icy solar system bodies. _Icarus_ 221, 800–805 (2012). Article CAS Google Scholar * Setlow, P. Spores of Bacillus subtilis: their resistance to and killing by radiation,
heat and chemicals. _J. Appl. Microbiol._ 101, 514–525 (2006). Article CAS Google Scholar * Horneck, G., Klaus, D. M. & Mancinelli, R. L. Space microbiology. _Microbiol. Mol. Biol.
Rev._ 741, 121–156 (2010). Article Google Scholar * Horneck, G. et al. Resistance of bacterial endospores to outer space for planetary protection purposes- experiment PROTECT of the
EXPOSE-E mission. _Astrobiology_ 12, 445–456 (2012). Article Google Scholar * Horneck, G. et al. Protection of bacterial spores in space, a contribution to the discussion on panspermia.
_Orig. Life Evol. Biosph._ 31, 527–547 (2001). Article CAS Google Scholar * Nicholson, W. L., Schuerger, A. C. & Setlow, P. The solar UV environment and bacterial spore UV resistance:
considerations for earth-to-mars transport by natural processes and human spaceflight. _Mutat. Res._ 571, 249–264 (2005). Article CAS Google Scholar * Schuerger, A. C., Mancinelli, R.
L., Kern, R. G., Rothschild, L. J. & McKay, C. P. Survival of endospores of Bacillus subtilis on spacecraft surfaces under simulated Martian environments: implications for the forward
contamination of Mars. _Icarus_ 165, 253–276 (2003). Article CAS Google Scholar * Slieman, T. A. & Nicholson, W. L. Role of dipicolinic acid in survival of Bacillus subtilis spores
exposed to artificial and solar UV radiation. _Appl. Environ. Microbiol._ 67, 1274–1279 (2001). Article CAS Google Scholar * Slieman, T. A. & Nicholson, W. L. Artificial and solar UV
radiation induces strand breaks and cyclobutane pyrimidine dimers in Bacillus subtilis Spore DNA. _Appl. Environ. Microbiol._ 66, 199–205 (2000). Article CAS Google Scholar * Fairhead,
H., Setlow, B., Waites, W. M. & Setlow, P. Small, acid-soluble proteins bound to DNA protect Bacillus subtilis Spores from being killed by freeze-drying. _Appl. Environ. Microbiol._ 60,
2647–2649 (1994). Article CAS Google Scholar * Kozubek, S., Rýznar, L., Vitová, H., Mlejnek, P. & Šlotová, J. Cell inactivation, mutation and DNA strand-break induction by γ-rays at
very low temperatures. _Radiat. Environ. Biophys._ 33, 293–302 (1994). Article CAS Google Scholar * Celandroni, F. et al. Effect of microwave radiation on Bacillus subtilis spores. _J.
Appl. Microbiol._ 97, 1220–1227 (2004). Article CAS Google Scholar * Dose, K. & Gill, M. DNA stability and survival of Bacillus subtilis spores in extreme dryness. _Orig. Life Evol.
Biosph._ 25, 277–293 (1995). Article CAS Google Scholar * Dose, K. & Klein, A. Response of Bacillus subtilis spores to dehydration and UV irradiation at extremely low temperatures.
_Orig. Life Evol. Biosph._ 26, 47–59 (1996). Article CAS Google Scholar * Fiester, S. E., Helfinstine, S. L., Redfearn, J. C., Uribe, R. M. & Woolverton, C. J. Electron beam
irradiation dose dependently damages the Bacillus spore coat and spore membrane. _Int. J. Microbiol._ 2012, 1–9 (2012). Article Google Scholar * Moeller, R. et al. Resistance of Bacillus
subtilis spore DNA to lethal ionizing radiation damage relies primarily on spore core components and DNA repair, with minor effects of oxygen radical detoxification. _Appl. Environ.
Microbiol._ 80, 104–109 (2014). Article CAS Google Scholar * Moeller, R., Reitz, G., Li, Z., Klein, S. & Nicholson, W. L. Multifactorial resistance of Bacillus subtilis spores to
high-energy proton radiation: role of spore structural components and the homologous recombination and non-homologous end joining DNA repair pathways. _Astrobiology_ 12, 1069–1077 (2012).
Article CAS Google Scholar * Moeller, R., Schuerger, A. C., Reitz, G. & Nicholson, W. L. Protective role of spore structural components in determining Bacillus subtilis spore
resistance to simulated mars surface conditions. _Appl. Environ. Microbiol._ 78, 8849–8853 (2012). Article CAS Google Scholar * Panitz, C. et al. The SPORES experiment of the EXPOSE-R
mission: Bacillus subtilis spores in artificial meteorites. _Int. J. Astrobiol._ 14, 105–114 (2015). Article CAS Google Scholar * Wassmann, M. et al. Survival of spores of the
UV-resistant Bacillus subtilis strain MW01 after exposure to low-earth orbit and simulated martian conditions: data from the space experiment ADAPT on EXPOSE-E. _Astrobiology_ 12, 498–507
(2012). Article Google Scholar * Zhang, Y. et al. Geobacillus and Bacillus spore inactivation by low energy electron beam technology: resistance and influencing factors. _Front.
Microbiol._ 9, 2720 (2018). * Tauscher, C., Schuerger, A. C. & Nicholson, W. L. Survival and germinability of Bacillus subtilis exposed to simulated Mars solar radiation: implications
for life detection and planetary protection. _Astrobiology_ 6, 592–605 (2006). Article CAS Google Scholar * Weber, P. & Greenberg, J. M. Can spores survive in interstellar space?
_Nature_ 316, 403–407 (1985). Article CAS Google Scholar * Fayolle, E. C., Noell, A. C., Johnson, P. V., Hodyss, R. & Ponce, A. Viability of Bacillus subtilis spores exposed to
ultraviolet light at ocean world surface temperatures. _Astrobiology_ 20, 889–896 (2020). Article Google Scholar * Noell, A. C. et al. Spectroscopy and viability of Bacillus subtilis
spores after ultraviolet irradiation: implications for the detection of potential bacterial life on Europa. _Astrobiology_ 15, 20–31 (2015). Article CAS Google Scholar * Bierhaus, E. B.
et al. Europa’s crater distributions and surface ages. In: _Europa_ (eds Pappalardo, R. T., McKinnon, W. B. & Khurana, K. K.) (University of Arizona Press, 2009). * Hand, K. P. &
Carlson, R. W. H2O2 production by high-energy electrons on icy satellites as a function of surface temperature and electron flux. _Icarus_ 215, 226–233 (2011). Article CAS Google Scholar
* Nordheim, T. A., Hand, K. P. & Paranicas, C. Preservation of potential biosignatures in the shallow subsurface of Europa. _Nat. Astron._ 2, 673–679 (2018). Article Google Scholar *
Goodacre, R. et al. Detection of the dipicolinic acid biomarker in Bacillus spores using Curie-point pyrolysis mass spectrometry and Fourier transform infrared spectroscopy. _Anal. Chem._
72, 119–127 (2000). Article CAS Google Scholar * Johnson, T. J. et al. The infrared spectra of Bacillus bacteria part I: vegetative Bacillus versus sporulated cells and the contributions
of phospholipids to vegetative infrared spectra. _Appl. Spectrosc._ 63, 899–907 (2009). Article CAS Google Scholar * Johnson, T. J., Williams, S. D., Valentine, N. B. & Su, Y. F. The
infrared spectra of Bacillus bacteria part II: sporulated Bacillus—the effect of vegetative cells and contributions of calcium dipicolinate trihydrate, CaDP-3H2O. _Appl. Spectrosc._ 63,
908–915 (2009). Article CAS Google Scholar * Lamarche, C. et al. Electrical discharges in water induce spores’ DNA damage. _PLOS ONE_ 13, e0201448 (2018). Article Google Scholar *
McKenney, P. T., Driks, A. & Eichenberger, P. The Bacillus subtilis endospore: assembly and functions of the multilayered coat. _Nat. Rev. Microbiol._ 11, 33–44 (2013). Article CAS
Google Scholar * Bennett, C. J., Pirim, C. & Orlando, T. M. Space-weathering of solar system bodies: a laboratory perspective. _Chem. Rev._ 113, 9086–9150 (2013). Article CAS Google
Scholar * Hendrix, A. R. & House C. H. Low effective ultraviolet exposure ages for organics at the surface of Enceladus. _Commun. Earth Environ._ 4, 485 (2023). * Neveu, M., Hays, L.
E., Voytek, M. A., New, M. H. & Schulte, M. D. The ladder of life detection. _Astrobiology_ 18, 1375–1402 (2018). Article Google Scholar * Hays, L. E. et al. Biosignature preservation
and detection in Mars analog environments. _Astrobiology_ 17, 363–400 (2017). Article Google Scholar * Raguse, M. et al. Improvement of biological indicators by uniformly distributing
Bacillus subtilis spores in monolayers to evaluate enhanced spore decontamination technologies. _Appl. Environ. Microbiol._ 82, 2031–2038 (2016). Article CAS Google Scholar * Noell, A.
C., Greenwood, A. R., Lee, C. M. & Ponce, A. High-density, homogeneous endospore monolayer deposition on test surfaces. _J. Microbiol. Methods_ 94, 245–248 (2013). Article CAS Google
Scholar * Woods, T. N. et al. Solar EUV Experiment (SEE): Mission overview and first results. _J. Geophys. Res.: Space Physics_ 110, A1 (2005). * Vincent, L., Fayolle, E. C., Hodyss, R.,
Johnson, P. V., & Noell A. C. Publication Dataset for “_Bacterial Spore Morphology Remains Highly Recognizable after Exposure to Simulated Enceladus and Europa Surface Conditions_”). 1
edn https://doi.org/10.48577/jpl.98PC5B (2024). Download references ACKNOWLEDGEMENTS We would like to thank Chi Ma and the Caltech Geology and Planetary Science Analytical Facility for
providing access and support to the SEM and EDS. This work was funded by the NASA Exobiology Program and the NASA Postdoctoral Program. The research was carried out at the Jet Propulsion
Laboratory, California Institute of Technology, under a contract with the National Aeronautics and Space Administration (80NM0018D0004). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Jet
Propulsion Laboratory, California Institute of Technology, Pasadena, CA, USA Lena N. Vincent, Edith C. Fayolle, Robert Hodyss, Paul V. Johnson & Aaron C. Noell Authors * Lena N. Vincent
View author publications You can also search for this author inPubMed Google Scholar * Edith C. Fayolle View author publications You can also search for this author inPubMed Google Scholar *
Robert Hodyss View author publications You can also search for this author inPubMed Google Scholar * Paul V. Johnson View author publications You can also search for this author inPubMed
Google Scholar * Aaron C. Noell View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS L.V. performed the experiments, analyzed the data, and
wrote the manuscript, E.F., A.N., R.H., and P.J. contributed to the design of the experiments and the final version of the manuscript, E.F. and A.N. supervised the project, and E.F., A.N.,
R.H., and P.J. conceived of the original idea. All authors provided critical feedback to help guide the research, analyses, and manuscript. CORRESPONDING AUTHOR Correspondence to Aaron C.
Noell. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. PEER REVIEW PEER REVIEW INFORMATION _Communications Earth & Environment_ thanks Brent
Christner, Stewart Gault, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Ilka Peeken and Joe Aslin. A peer review
file is available. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
SUPPLEMENTARY INFORMATION TRANSPARENT PEER REVIEW FILE SUPPLEMENTARY INFORMATION RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons
Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give
appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission
under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons
licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by
statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit
http://creativecommons.org/licenses/by-nc-nd/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Vincent, L.N., Fayolle, E.C., Hodyss, R. _et al._ Bacterial spore morphology
remains highly recognizable after exposure to simulated Enceladus and Europa surface conditions. _Commun Earth Environ_ 5, 688 (2024). https://doi.org/10.1038/s43247-024-01872-z Download
citation * Received: 11 July 2024 * Accepted: 01 November 2024 * Published: 10 November 2024 * DOI: https://doi.org/10.1038/s43247-024-01872-z SHARE THIS ARTICLE Anyone you share the
following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer
Nature SharedIt content-sharing initiative