Scalable deoxygenative alkynylation of alcohols via flow photochemistry
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Internal alkynes are often contained in bioactive pharmaceuticals and crucial intermediates in material sciences, yet their production methods are often limited and challenging,
necessitating the development of more efficient and versatile synthetic routes. Here we report a method of deoxygenative alkynylation of alcohols via flow photochemistry. Formation of
_N_-heterocyclic carbene-alcohol adducts undergoes oxidation by a photocatalyst, generating alkyl radicals. These radicals are subsequently trapped by an alkynylation agent, yielding the
desired alkyne. Compared to batch reactions, the strategy using flow photochemistry is practical and efficient to complete the reaction in relatively short time with good yields. A wide
range of functional groups were tolerated. The broad application of this method for alkyne synthesis in industry settings is anticipated, supported by the potential in late-stage
functionalization of biomolecules and gram-scale synthesis. SIMILAR CONTENT BEING VIEWED BY OTHERS MODULAR ALKENE SYNTHESIS FROM CARBOXYLIC ACIDS, ALCOHOLS AND ALKANES VIA INTEGRATED
PHOTOCATALYSIS Article 27 September 2024 PHOTOCATALYTIC DEOXYGENATIVE _Z_-SELECTIVE OLEFINATION OF ALIPHATIC ALCOHOLS Article Open access 02 April 2025 HIGHLY SCALABLE PHOTOINDUCED SYNTHESIS
OF SILANOLS VIA UNTRAVERSED PATHWAY FOR CHLORINE RADICAL (CL•) GENERATION Article Open access 09 December 2023 INTRODUCTION Internal alkynes exist in various natural products1 and are also
widely used in drug discovery and material sciences for the synthesis of antibiotics2,3, antifungals4, polymers and liquid crystal materials5,6. The unique reactivity of alkynes also makes
them valuable as precious building blocks, including heterocycles, alkenes, and carbonyl compounds7,8. Transition metal-catalyzed Sonogashira coupling reaction is a common method for
synthesizing internal alkynes9,10,11. Under the catalysis of metal catalysts like palladium and copper salts, (pseudo)halogenated aromatics or alkanes react with terminal alkynes, which
showed good functional group compatibility12,13,14,15,16. However, this protocol often accompanies a few issues, such as the use of expensive metals and ligands, harsh reaction conditions
and competitive β-H elimination side reactions17. Despite great progress made by researchers by avoiding the utilization of transition metals18,19,20,21,22,23,24,25, there is still a
practical need to develop a mild, efficient and versatile method for synthesizing internal alkynes. Using the electron-deficient type of reagents, visible-light-mediated radical alkynylation
demonstrated the characteristics of mild reaction conditions and adaptation to various precursors, such as carboxylic acids or esters26,27,28,29,30,31, C(sp3)-H substrates32,33,34,35,36,37,
alkyl trifluoroborates38,39 and aldehydes40,41,42, etc.43,44, making it an ideal alternative to Sonogashira reaction (Scheme 1a). However, the deoxyalkynylation of alcohols, which are the
most diverse and commercially available substrates45,46,47, has seldom been reported. In 2016, Fu and co-workers described a visible-light photoredox synthesis of internal alkynes containing
quaternary carbons (Scheme 1b)48; Waser group and Xie group reported a similar visible-light-mediated deoxyalkynylation of activated tertiary alcohols in 2021 (Scheme 1c)49,50. The
limitation of substrates and the additional purification steps associated to the pre-activation step affected the practicality of the above two methods. The direct alkynylation of alcohols
(1°, 2° and 3°) has become an urgent problem that remained to be solved. Since 2021, MacMillan has reported a series of photoredox-enabled deoxygenative arylation51,
alkylation52,53,54,55,56, sulfination57, fluoromethylation58,59, phosphonylation60, and amination61 of alcohols, directly activated by _N,O_-heterocyclic carbenes (NHC) without purification.
This strategy offers a novel approach to sp3-sp2 and sp3-sp3 cross-coupling reactions using widely available alcohol-containing reagents. However, the sp3-sp coupling reaction has not yet
been explored. Additionally, the scalability of product synthesis via photochemistry remains a challenge to be addressed. According to the Bouguer-Lambert-Beer Law, the propagation of the
photons in the reaction mixture decays rapidly, especially in a large photoreactor62,63. This effect significantly prolonged the reaction time and increased energy consumption. Additionally,
it may lead to the formation of by-products, making purification difficult and costly. Nevertheless, flow photochemistry64,65,66,67,68, which combines the advantages of flow chemistry and
photochemistry, can effectively resolve these problems69,70,71,72,73. Herein, we report a practical, efficient, and scalable deoxygenative alkynylation of alcohols, which combines NHC
activation with flow photochemistry (Scheme 1d). RESULTS AND DISCUSSION Following the extensive optimization as described in the Supplementary Information “Reaction Optimization” (Tables
S1-S6), we provided the ideal reaction conditions as shown in Table 1. 2.0 equiv of _tert_-butyl 4-hydroxypiperidine-1-carboxylate (1) was condensed with NHC-1 affording the NHC-alcohol
adduct in 15 min (see Supplementary Information “General Procedure”), and the adduct then subjected to react with a reaction mixture, including 5 mol%
1,2,3,5-Tetrakis(carbazol-9-yl)-4,6-dicyanobenzene (4CzIPN), 1.0 equiv of ((methylsulfonyl)ethynyl)benzene (2) and 4 equiv of _n_Bu4NPO4H2 in DMF/_t_BuOH, in a polytetrafluoroethylene (PTFE)
capillary under the irradiation of 450 nm LEDs (for reaction setup see Supplementary Information “General information”). The alkynylation reaction completed to provide the product in 77%
yield via flow photochemistry (entry 1). The NHC variant with _p_-CF3 group resulted in a yield reduced to 46% (entry 2). The screening of the alkynylation reagents showed 2 with a simple
methyl group attached to alkyne demonstrating superior atom economy and reactivity to other analogs (entries 3–6). Moreover, other organic bases were also found to give inferior results
(entries 7–9). The attempts in the absence of light or 4CzIPN (entry 10) led to no reaction occurring. Although this sp3-sp coupling reaction can also be performed in a batch reactor,
significantly longer reaction time and yield drop were observed (entry 11). While we were concluding this study, a batch reaction on the deoxyalkynylation of alcohols was reported74.
Compared to the flow photochemistry method, the reported 36-hour reaction time and scalability present practical challenges. Furthermore, the use of their described reagent led to a slower
conversion rate and lower yield (entry 5 vs. entry 1). Under the optimized conditions, we explored the alcohol scope of the deoxygenative alkynylation with 2 (Scheme 2). Inactivated primary
alcohols attached to cyclic alkyl, chain alkyl, and fluorinated alkyl groups formed viable substrates in this reaction, affording 8, 9, and 10 in reasonable yields. Alcohols bearing cyclic
ethers, cyclic and acyclic carbamates provided the corresponding products (11–14) with 42%–55% yields. Primary alcohols with sterically hindered substitution at β position, lactam, and
pyrazole structures were well tolerated to give the desired products (15, 16, and 17) with 43%, 57%, and 56% yields, respectively. Secondary alcohols acted as better substrates for this
alkynylation reaction. Products (18, 19, and 20) were obtained from the corresponding cyclopentyl, cyclohexyl, and 4-phenyl-2-butyl alcohols with good yields (66–70%). Notably,
_exo_-norborneol and the lactone derivative reacted stereoselectively to give the corresponding products 21 (69% yield) and 22 (47% yield) with >20:1 diastereoselective ratio. Other
function groups in secondary alcohols, including ether (23, 24), acetal (25), thioether (26), and carbonyl (27) were compatible with the deoxygenative alkynylation conditions, giving
moderate to good yields (58–85% yield). A variety of medicinally relevant cyclic and acyclic carbamates (28–32) could be obtained by the direct alkynylation from the corresponding alcohols,
yielding the products in fair to good yields (48–82% yield). Additionally, the four-membered ring-containing spirocyclic system, attracting significant attention in drug discovery75, was
successfully alkynylated in 55% (33) and 49% (34) yields. Next, a series of tertiary alcohols were investigated as precursors under the activation of NHC-2, a more electrophilic reagent that
generated the NHC-_tert_-alcohol adduct effectively. To our gratification, except the product (37) derived from arylcyclopropanol, other tertiary alcohols afforded the desired products (35,
36, 38–42) with satisfactory yields (57–82%). Consequently, we turned our attention to the scope of the alkyne reagents. As expected, including thiophenyl alkyne, the substituent groups on
the aromatic rings, such as _p_-CH3, -F, -Cl, and _m_-Br, showed little effect on the yields of products 43–47 (62–88% yields). To further demonstrate the outstanding tolerance of functional
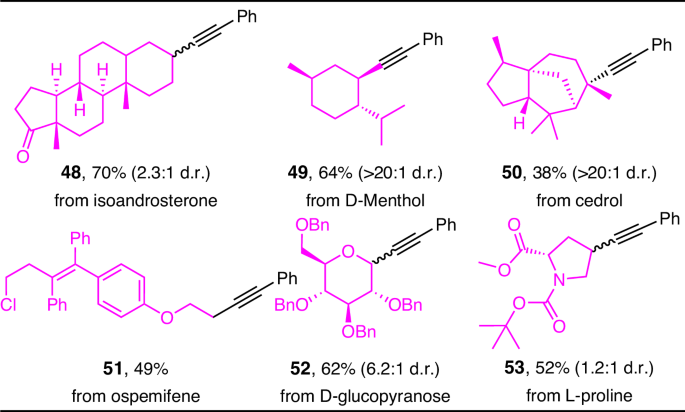
groups and the capability of this deoxygenative alkynylation protocol in late-stage derivatizations, a variety of natural products and their derivatives, as well as a marketed drug
containing the hydroxyl group were subjected to alkynylation under our conditions (Fig. 1). It was found that the secondary alcohols in isoandrosterone and D-Menthol provided the
corresponding products 48 and 49 in good yields, but cedrol containing a tertiary alcohol showed relatively moderate yield (50), probably due to steric hindrance, whereas the
diastereoselectivity was always excellent (>20:1 d.r.). Finally, ospemifene (51), as well as benzyl-protected D-glucopyranose (52) and protected L-proline (53) served as competent vectors
for the deoxygenative alkynylnation of alcohols. Reaction scale-up for photochemical reactions used to be hampered by the poor penetration of light through the reaction mixture in large
batch reactors, which can be overcome by the narrow channel of flow photochemistry. As a result, the synthesis of 3 could be conducted in 10 g scale within 2.5 h by employing flow
photochemistry (Scheme 3a). Moreover, with an additional step, product 3 can be converted to compounds 5448 and 5576, containing useful azido and olefin groups, respectively (Scheme 3b),
which can undergo diverse reactions thereafter. To further investigate reaction mechanism, we conducted the reaction in the presence of TEMPO to detect the generation of radicals, according
to the previously reported sp3-sp3 and sp3-sp2 coupling reactions51,52,53,54,55,56,57,58,59,60,61. As a result, several key intermediates in the reaction were captured: Under standard
conditions, the addition of TEMPO completely inhibited the formation of 3 (see Supplementary Information “Mechanistic experiments”). Meanwhile, compounds 56 and 57 were detected by HRMS
(Scheme 4a), indicating the presence of the alkyl radical (B) and the alkenyl radical (D). Additionally, compound 58 was also detected when the electrophilic reagent BnBr was introduced to
the reaction mixture (Scheme 4b), suggesting the presence of methyl sulfinate (F), which can be converted from the methylsulfonyl radical (E). In summary, we have developed a practical and
efficient method for the visible-light-promoted deoxygenative alkynylation of alcohols via flow photochemistry, utilizing NHC to activate alcohols without purification. This protocol
demonstrated broad compatibility with a wide range of alcohols and good late-stage derivatization possibilities of biomolecules. Gram-scale synthesis further showcased the potential of our
method. METHODS GENERAL PROCEDURE FOR DEOXYGENATIVE ALKYNYLATION OF ALCOHOLS To an oven-dried 25 mL Schlenk tube was added NHC (0.6 mmol, 2.0 equiv), alcohol (0.60 mmol, 2.5 equiv), and
anhydrous methyl _tert_-butyl ether (6 ml). Pyridine (0.6 mmol, 2.0 equiv) was added dropwise, and the suspension was stirred at room temperature under nitrogen atmosphere for 15 min.
Another oven-dried 25 mL Schlenk tube was charged with 4CzIPN (0.015 mmol, 5 mol%), _n_Bu4NPO4H2 (1.2 mmol, 4.00 equiv) and the alkynylation reagent (0.30 mmol, 1.0 equiv). DMF (6 mL) and
_t_BuOH (6 mL) were added to the mixture. The methyl _tert_-butyl ether suspension was transferred to a 10 mL syringe under air. Then a syringe filter and new needle were installed on the
syringe. The methyl _tert_-butyl ether solution was injected through the syringe filter into the DMF/_t_BuOH solution. Then the reaction mixture was transferred to a 20 mL syringe. The LEDs
were turned on and the reaction solution was slowly injected using an injection pump. For primary alcohols, the flow rate was 0.75 mL min−1, 5.8 min residence time; For secondary and
tertiary alcohols, the flow rate was 1.2 mL min−1, 3.6 min residence time. The inner diameter of PTFE capillary is 0.75 mm. For tertiary alcohols, methyl _tert_-butyl ether was replaced by
PhCF3. The mixture in the receiving bottle was diluted with ethyl acetate, washed with H2O and brine. The organic phase was dried with Na2SO4, then filtered, and concentrated _in vacuo_. The
crude product was purified by flash column chromatography. DATA AVAILABILITY All data generated during this study are included in this article and Supplementary Information. Experimental
procedure, condition optimization, product characterization, and NMR spectra are provided in the Supplementary Information. REFERENCES * Sugimoto, K. et al. Protecting-group-free total
synthesis of (−)-rhazinilam and (−)-rhazinicine using a gold-catalyzed cascade cyclization. _Angew. Chem. Int. Ed._ 52, 7168–7171 (2013). Article CAS Google Scholar * Qiu, Y. et al.
Synthesis and biological evaluation of nusbiarylin derivatives as bacterial rRNA synthesis inhibitor with potent antimicrobial activity against MRSA and VRSA. _Bioorg. Chem._ 124, 105863
(2022). Article CAS PubMed Google Scholar * Zheng, S. et al. Synthesis, chemical reactivity as Michael acceptors, and biological potency of monocyclic cyanoenones, novel and highly
potent anti-inflammatory and cytoprotective agents. _J. Med. Chem._ 55, 4837–4846 (2012). Article CAS PubMed Google Scholar * Beutler, U., Mazacek, J., Penn, G., Schenkel, B. &
Wasmuth, D. Die Entwicklung eines neuen, umweltgerechten Produktionsprozesses für Terbinafin. _CHIMIA_ 50, 154 (1996). Article CAS Google Scholar * Anastasia, L. & Negishi, E. i.
Highly satisfactory procedures for the Pd-catalyzed cross coupling of aryl electrophiles with in situ generated alkynylzinc derivatives. _Org. Lett._ 3, 3111–3113 (2001). Article CAS
PubMed Google Scholar * Plenio, H., Hermann, J. & Sehring, A. Optically and redox-active ferroceneacetylene polymers and oligomers. _Chem. Eur. J._ 6, 1820–1829 (2000). Article CAS
PubMed Google Scholar * Bortolami, M., Petrucci, R., Rocco, D., Scarano, V. & Chiarotto, I. Alkynes as building blocks, intermediates and products in the electrochemical procedures
since 2000. _ChemElectroChem_ 8, 3604–3613 (2021). Article CAS Google Scholar * Wu, W. & Jiang, H. Haloalkynes: a powerful and versatile building block in organic synthesis. _Acc.
Chem. Res._ 47, 2483–2504 (2014). Article CAS PubMed Google Scholar * Cassar, L. Synthesis of aryl- and vinyl-substituted acetylene derivatives by the use of nickel and palladium
complexes. _J. Organomet. Chem._ 93, 253–257 (1975). Article CAS Google Scholar * Dieck, H. A. & Heck, F. R. Palladium catalyzed synthesis of aryl, heterocyclic and vinylic acetylene
derivatives. _J. Organomet. Chem._ 93, 259–263 (1975). Article CAS Google Scholar * Sonogashira, K., Tohda, Y. & Hagihara, N. A convenient synthesis of acetylenes: catalytic
substitutions of acetylenic hydrogen with bromoalkenes, iodoarenes and bromopyridines. _Tetrahedron Lett._ 16, 4467–4470 (1975). Article Google Scholar * Bakherad, M. Recent progress and
current applications of Sonogashira coupling reaction in water. _Appl. Organomet. Chem._ 27, 125–140 (2013). Article CAS Google Scholar * Chinchilla, R. & Nájera, C. The Sonogashira
reaction: a booming methodology in synthetic organic chemistry. _Chem. Rev._ 107, 874–922 (2007). Article CAS PubMed Google Scholar * Chinchilla, R. & Nájera, C. Recent advances in
Sonogashira reactions. _Chem. Soc. Rev._ 40, 5084–5121 (2011). Article CAS PubMed Google Scholar * Thomas, A. M., Sujatha, A. & Anilkumar, G. Recent advances and perspectives in
copper-catalyzed Sonogashira coupling reactions. _Rsc Adv._ 4, 21688–21698 (2014). Article CAS Google Scholar * Wang, D. & Gao, S. Sonogashira coupling in natural product synthesis.
_Org. Chem. Front._ 1, 556–566 (2014). Article CAS Google Scholar * Choi, J. & Fu, G. C. Transition metal–catalyzed alkyl-alkyl bond formation: another dimension in cross-coupling
chemistry. _Science_ 356, 7230 (2017). Article Google Scholar * Appukkuttan, P., Dehaen, W. & Van der Eycken, E. Transition-metal-free Sonogashira-type coupling reactions in water.
_Eur. J. Org. Chem._ 2003, 4713–4716 (2003). Article Google Scholar * Dale, H. J. A., Nottingham, C., Poree, C. & Lloyd-Jones, G. C. Systematic evaluation of 1,2-migratory aptitude in
alkylidene carbenes. _J., Am. Chem. Soc._ 143, 2097–2107 (2021). Article CAS PubMed Google Scholar * Deol, H., Singh, G., Kumar, M. & Bhalla, V. Phenazine-based donor acceptor
systems as organic photocatalysts for “Metal-free” C–N/C–C cross-coupling. _J. Org. Chem._ 85, 11080–11093 (2020). Article CAS PubMed Google Scholar * Maji, M. S., Murarka, S. &
Studer, A. Transition-metal-free Sonogashira-type coupling of ortho-substituted aryl and alkynyl Grignard reagents by using 2,2,6,6-tetramethylpiperidine-n-oxyl radical as an oxidant. _Org.
Lett._ 12, 3878–3881 (2010). Article CAS PubMed Google Scholar * Prüger, B. et al. Transition-metal-free formal Sonogashira coupling and α-carbonyl arylation reactions. _Chem. Eur. J._
16, 3783–3790 (2010). Article PubMed Google Scholar * Truong, T. & Daugulis, O. Transition-metal-free alkynylation of aryl chlorides. _Org. Lett._ 13, 4172–4175 (2011). Article CAS
PubMed PubMed Central Google Scholar * Yang, L., Li, H., Du, Y., Cheng, K. & Qi, C. Visible light-catalyzed decarboxylative alkynylation of arenediazonium salts with alkynyl
carboxylic acids: direct access to aryl alkynes by organic photoredox catalysis. _Adv. Synth. Catal._ 361, 5030–5041 (2019). Article CAS Google Scholar * Ye, M. et al. Arylation of
terminal alkynes: transition-metal-free Sonogashira-type coupling for the construction of C(sp)–C(sp2) bonds. _Org. Lett._ 25, 1787–1792 (2023). Article CAS PubMed Google Scholar *
Jiang, M., Jin, Y., Yang, H. & Fu, H. Visible-light photoredox synthesis of unnatural chiral α-amino acids. _Sci. Rep._ 6, 26161 (2016). Article CAS PubMed PubMed Central Google
Scholar * LeVaillant, F., Courant, T. & Waser, J. Room-temperature decarboxylative alkynylation of carboxylic acids using photoredox catalysis and EBX reagents. _Angew. Chem. Int. Ed._
54, 11200–11204 (2015). Article CAS Google Scholar * Vaillant, F. L. & Waser, J. Decarboxylative alkynylation and cyanation of carboxylic acids using photoredox catalysis and
hypervalent iodine reagents. _Chimia_ 71, 226–230 (2017). Article PubMed Google Scholar * Yang, C., Yang, J.-D., Li, Y.-H., Li, X. & Cheng, J.-P. 9,10-dicyanoanthracene catalyzed
decarboxylative alkynylation of carboxylic acids under visible-light irradiation. _J. Org. Chem._ 81, 12357–12363 (2016). Article CAS PubMed Google Scholar * Yang, J., Zhang, J., Qi, L.,
Hu, C. & Chen, Y. Visible-light-induced chemoselective reductive decarboxylative alkynylation under biomolecule-compatible conditions. _Chem. Commun._ 51, 5275–5278 (2015). Article CAS
Google Scholar * Zhou, Q.-Q. et al. Decarboxylative alkynylation and carbonylative alkynylation of carboxylic acids enabled by visible-light photoredox catalysis. _Angew. Chem. Int. Ed._
54, 11196–11199 (2015). Article CAS Google Scholar * Capaldo, L. & Ravelli, D. Decatungstate as direct hydrogen atom transfer photocatalyst for SOMOphilic alkynylation. _Org. Lett._
23, 2243–2247 (2021). Article CAS PubMed PubMed Central Google Scholar * Hoshikawa, T., Kamijo, S. & Inoue, M. Photochemically induced radical alkynylation of C(sp3)–H bonds. _Org.
Biomol. Chem._ 11, 164–169 (2013). Article CAS PubMed Google Scholar * Liu, Z. et al. Hypervalent iodine reagents enable C–H alkynylation with iminophenylacetic acids via alkoxyl
radicals. _Org. Lett._ 24, 5951–5956 (2022). Article CAS PubMed Google Scholar * Matsumoto, K., Nakajima, M. & Nemoto, T. Visible light-induced direct S(0) –> T(n) transition of
benzophenone promotes C(sp(3))-H alkynylation of ethers and amides. _J. Org. Chem._ 85, 11802–11811 (2020). Article CAS PubMed Google Scholar * Voutyritsa, E. et al. Photochemical
functionalization of heterocycles with EBX reagents: C−H alkynylation versus deconstructive ring cleavage. _Chem. Eur. J._ 26, 14453–14460 (2020). Article CAS PubMed Google Scholar *
Xie, X., Liu, J., Wang, L. & Wang, M. Visible-light-induced alkynylation of α-C–H bonds of ethers with alkynyl bromides without external photocatalyst. _Eur. J. Org. Chem._ 2020,
1534–1538 (2020). Article CAS Google Scholar * Huang, H., Zhang, G., Gong, L., Zhang, S. & Chen, Y. Visible-light-induced chemoselective deboronative alkynylation under
biomolecule-compatible conditions. _J., Am. Chem. Soc._ 136, 2280–2283 (2014). Article CAS PubMed Google Scholar * Pan, Y., Jia, K., Chen, Y. & Chen, Y. Investigations of
alkynylbenziodoxole derivatives for radical alkynylations in photoredox catalysis. _Beilstein J. Org. Chem._ 14, 1215–1221 (2018). Article CAS PubMed PubMed Central Google Scholar *
Mukherjee, S., Garza-Sanchez, R. A., Tlahuext-Aca, A. & Glorius, F. Alkynylation of C (O)–H bonds enabled by photoredox-mediated hydrogen-atom transfer. _Angew. Chem. Int. Ed._ 56,
14723–14726 (2017). Article CAS Google Scholar * Tanaka, I., Sawamura, M. & Shimizu, Y. Visible light-induced reductive alkynylation of aldehydes by umpolung approach. _Org. Lett._
24, 520–524 (2022). Article CAS PubMed Google Scholar * Yan, J. et al. Divergent functionalization of aldehydes photocatalyzed by neutral eosin Y with sulfone reagents. _Nat. Commun._
12, 7214 (2021). Article CAS PubMed PubMed Central Google Scholar * Liang, S., Angnes, R. A., Potnis, C. S. & Hammond, G. B. Photoredox catalyzed C(sp3) C(sp) coupling of
dihydropyridines and alkynylbenziodoxolones. _Tetrahedron. Lett_. 60, 151230 (2019). * Zhao, Y. et al. Gold catalysed site-selective cross-coupling of tertiary α-silylamines with
1-iodoalkynes under UVA LED light. _Org. Chem. Front._ 10, 759–766 (2023). Article CAS Google Scholar * Ertl, P. An algorithm to identify functional groups in organic molecules. _J.
Cheminform._ 9, 36 (2017). Article PubMed PubMed Central Google Scholar * Ertl, P. & Schuhmann, T. A systematic cheminformatics analysis of functional groups occurring in natural
products. _J. Nat. Prod._ 82, 1258–1263 (2019). Article CAS PubMed Google Scholar * Henkel, T., Brunne, R. M., Müller, H. & Reichel, F. Statistical investigation into the structural
complementarity of natural products and synthetic compounds. _Angew. Chem. Int. Ed._ 38, 643–647 (1999). Article CAS Google Scholar * Gao, C., Li, J., Yu, J., Yang, H. & Fu, H.
Visible-light photoredox synthesis of internal alkynes containing quaternary carbons. _Chem. Commun._ 52, 7292–7294 (2016). Article CAS Google Scholar * Amos, S. G. E., Cavalli, D., Le
Vaillant, F. & Waser, J. Direct photoexcitation of ethynylbenziodoxolones: an alternative to photocatalysis for alkynylation reactions*. _Angew. Chem. Int. Ed._ 60, 23827–23834 (2021).
Article CAS Google Scholar * Li, M. et al. Visible-light-mediated deoxyalkynylation of activated tertiary alcohols. _J. Org. Chem._ 86, 12386–12393 (2021). Article CAS PubMed Google
Scholar * Dong, Z. & Macmillan, D. W. C. Metallaphotoredox-enabled deoxygenative arylation of alcohols. _Nature_ 598, 451–456 (2021). Article CAS PubMed PubMed Central Google
Scholar * Chen, R. et al. Alcohol-alcohol cross-coupling enabled by SH2 radical sorting. _Science_ 383, 1350–1357 (2024). Article CAS PubMed Google Scholar * Gould, C. A., Pace, A. L.
& Macmillan, D. W. C. Rapid and modular access to quaternary carbons from tertiary alcohols via bimolecular homolytic substitution. _J., Am. Chem. Soc._ 145, 16330–16336 (2023). Article
CAS PubMed Google Scholar * Lyon, W. L. & Macmillan, D. W. C. Expedient access to underexplored chemical space: deoxygenative C(sp3)–C(sp3) cross-coupling. _J., Am. Chem. Soc._ 145,
7736–7742 (2023). Article CAS PubMed Google Scholar * Sakai, H. A. & Macmillan, D. W. C. Nontraditional fragment couplings of alcohols and carboxylic acids: C(sp3)–C(3)
cross-coupling via radical sorting. _J., Am. Chem. Soc._ 144, 6185–6192 (2022). Article CAS PubMed Google Scholar * Wang, J. Z., Sakai, H. A. & Macmillan, D. W. C. Alcohols as
alkylating agents: photoredox‐catalyzed conjugate alkylation via in situ deoxygenation. _Angew. Chem. Int. Ed._ 61, e202207150 (2022). Article CAS Google Scholar * Carson Ii, W. P.,
Sarver, P. J., Goudy, N. S. & MacMillan, D. W. C. Photoredox catalysis-enabled sulfination of alcohols and bromides. _J. Am. Chem. Soc._ 145, 20767–20774 (2023). Article CAS PubMed
Google Scholar * Intermaggio, N. E., Millet, A., Davis, D. L. & Macmillan, D. W. C. Deoxytrifluoromethylation of alcohols. _J. Am. Chem. Soc._ 144, 11961–11968 (2022). Article CAS
PubMed PubMed Central Google Scholar * Mao, E., Prieto Kullmer, C. N., Sakai, H. A. & MacMillan, D. W. C. Direct bioisostere replacement enabled by metallaphotoredox
deoxydifluoromethylation. _J. Am. Chem. Soc._ 146, 5067–5073 (2024). Article CAS PubMed Google Scholar * Bissonnette, N. B., Bisballe, N., Tran, A. V., Rossi-Ashton, J. A. &
MacMillan, D. W. C. Development of a general organophosphorus radical trap: deoxyphosphonylation of alcohols. _J. Am. Chem. Soc._ 146, 7942–7949 (2024). Article CAS PubMed Google Scholar
* Carson, W. P. et al. Free-radical deoxygenative amination of alcohols via copper metallaphotoredox catalysis. _J. Am. Chem. Soc._ 146, 15681–15687 (2024). Article CAS PubMed Google
Scholar * Mayerhöfer, T. G., Pahlow, S. & Popp, J. The Bouguer-Beer-Lambert law: shining light on the obscure. _Chemphyschem_ 21, 2029–2046 (2020). Article PubMed PubMed Central
Google Scholar * Thomas, O. & Causse, J. in _UV-Visible Spectrophotometry of Waters and Soils_ _(__Third Edition_) (eds Olivier T & C. Burgess) 59–94 (Elsevier, 2022). * Cambié, D.,
Bottecchia, C., Straathof, N. J. W., Hessel, V. & Noël, T. Applications of continuous-flow photochemistry in organic synthesis, material science, and water treatment. _Chem. Rev._ 116,
10276–10341 (2016). Article PubMed Google Scholar * Rehm, T. H. Flow photochemistry as a tool in organic synthesis. _Chem. Eur. J._ 26, 16952–16974 (2020). Article CAS PubMed Google
Scholar * Buglioni, L., Raymenants, F., Slattery, A., Zondag, S. D. A. & Noël, T. Technological innovations in photochemistry for organic synthesis: flow chemistry, high-throughput
experimentation, scale-up, and photoelectrochemistry. _Chem. Rev._ 122, 2752–2906 (2022). Article CAS PubMed Google Scholar * Oelgemöller, M., Zhang, L., Zhao, F. & Su, Y. Editorial:
novel technologies for sustainable and energy-efficient flow photochemistry. _Front. Chem._ 11, 1322556 (2023). Article PubMed PubMed Central Google Scholar * Williams, J. D. &
Kappe, C. O. Recent advances toward sustainable flow photochemistry. _Curr. Opin. Green Sust._ 25, 100351 (2020). * Knowles, J. P., Elliott, L. D. & Booker-Milburn, K. I. Flow
photochemistry: old light through new windows. _Beilstein J. Org. Chem._ 8, 2025–2052 (2012). Article CAS PubMed PubMed Central Google Scholar * Garlets, Z. J., Nguyen, J. D. &
Stephenson, C. R. J. The development of visible-light photoredox catalysis in flow. _Isr. J. Chem._ 54, 351–360 (2014). Article CAS PubMed PubMed Central Google Scholar * Schuster, E.
M. & Wipf, P. Photochemical flow reactions. _Isr. J. Chem._ 54, 361–370 (2014). Article CAS Google Scholar * Plutschack, M. B., Correia, C. A., Seeberger, P. H. & Gilmore, K. in
_Organometallic Flow Chemistry_ (ed T. Noël) 43–76 (Springer International Publishing, 2016). * Su, Y., Straathof, N. J. W., Hessel, V. & Noël, T. Photochemical transformations
accelerated in continuous-flow reactors: basic concepts and applications. _Chem. Eur. J._ 20, 10562–10589 (2014). Article CAS PubMed Google Scholar * Wang, Q. et al. Photoredox catalytic
deoxygenative divergent functionalizations of alcohols assisted by N,O-heterocyclic carbenes. _Org. Chem. Front._ 11, 3471–3477 (2024). Article CAS Google Scholar * Carreira, E. M. &
Fessard, T. C. Four-membered ring-containing spirocycles: synthetic strategies and opportunities. _Chem. Rev._ 114, 8257–8322 (2014). Article CAS PubMed Google Scholar * Wu, Y. et al.
Modulation of metal species as control point for Ni-catalyzed stereodivergent semihydrogenation of alkynes with water. _Nat. Commun._ 14, 1655 (2023). Article CAS PubMed PubMed Central
Google Scholar Download references ACKNOWLEDGEMENTS We gratefully acknowledge the financial support from the Research Grants Council of the Hong Kong Special Administrative Region, China
(PolyU 15100021), Hong Kong Polytechnic University (State Key Laboratory of Chemical Biology and Drug Discovery). We thank the University Research Facility in Life Sciences (ULS) of the Hong
Kong Polytechnic University for the technical assistance. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * State Key Laboratory of Chemical Biology and Drug Discovery, Department of Applied
Biology and Chemical Technology, The Hong Kong Polytechnic University, Hung Hom, Hong Kong SAR, China Pin Xu & Cong Ma Authors * Pin Xu View author publications You can also search for
this author inPubMed Google Scholar * Cong Ma View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS P.X. and C.M. conceived the idea. C.M.
supervised the project and acquired the funding. P.X. conducted the laboratory work. P.X. and C.M. analyzed the data. P.X. and C.M. wrote the manuscript. CORRESPONDING AUTHOR Correspondence
to Cong Ma. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. PEER REVIEW INFORMATION _Communications Chemistry_ thanks the anonymous reviewers for their
contribution to the peer review of this work. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional
affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons
Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give
appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission
under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons
licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by
statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit
http://creativecommons.org/licenses/by-nc-nd/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Xu, P., Ma, C. Scalable deoxygenative alkynylation of alcohols via flow
photochemistry. _Commun Chem_ 7, 276 (2024). https://doi.org/10.1038/s42004-024-01363-4 Download citation * Received: 11 September 2024 * Accepted: 11 November 2024 * Published: 26 November
2024 * DOI: https://doi.org/10.1038/s42004-024-01363-4 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable
link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative