Auditory pallial regulation of the social behavior network
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
Sensory cues such as vocalizations contain important social information. Processing social features of vocalizations (e.g., vocalizer identity, emotional state) necessitates unpacking the
complex sound streams in song or speech; this depends on circuits in pallial cortex. But whether and how this information is then transferred to limbic and hypothalamic regions remains a
mystery. Here, using gregarious, vocal songbirds (female Zebra finches), we identify a prominent influence of the auditory pallium on one specific node of the Social Behavior Network, the
lateral ventromedial nucleus of the hypothalamus (VMHl). Electrophysiological recordings revealed that social and non-social auditory stimuli elicited stimulus-specific spike trains that
permitted stimulus differentiation in a large majority of VMHl single units, while transient disruption of auditory pallium elevated immediate early gene activity in VMHl. Descending
functional connections such as these may be critical for the range of vertebrate species that rely on nuanced communication signals to guide social decision-making.
The sensory environment is a key part of our social lives. For example, in many social animals like humans and birds, dynamic vocal exchanges shape interactions and impact subsequent
encounters. Social cues such as vocalizations are processed hierarchically in the brain along the ascending auditory pathway from hindbrain to pallium. In parallel, several limbic and
hypothalamic regions are critically involved in the control of social behaviors; these ‘social behavior’ nuclei are densely interconnected, enriched in steroid signaling, and are often
referred to as the Social Behavior Network (SBN)1,2. The SBN is thought to control social behavior in part by integrating external social signals, like vocalizations, with internal state. In
order to understand social behavioral control, it is essential to understand how socially-relevant cues, once fully unpacked by sensory systems, reach the SBN to modulate its output.
It has long been known that SBN nuclei respond to all major sensory modalities tested (e.g., olfaction3,4,5; auditory6,7,8,9,10,11; somatosensory12,13,14; visual15,16,17), suggesting rich
connections with sensory systems that are critical for how these nuclei participate in the control of social behavior18,19. Social stimulus selectivity in sensory processing
areas20,21,22,23,24,25,26, taken together with the rich sensory representations in the SBN, appear to blur the lines between regions delineated as strictly sensory processing and those that
control social behavior18. This is most evident in studies that directly link the social behavioral impacts of olfactory processing in the pallium of mammals to specific SBN circuit
nodes1,27,28,29,30,31,32,33,34. For example, in mice, juvenile pheromones processed in olfactory pallium (i.e., the vomeronasal organ) decreased sexual receptivity in females; this
sensory-guided social decision-making relied on SBN nuclei31. For mammals, network models and schematics of social circuits now regularly include specific olfactory pallial areas18,33,35.
When it comes to other sensory modalities prominent across vertebrate species, such as vision and audition, there is less known about circuits that transform sensory representations into
social behavior. For the auditory system, brain areas relatively ‘early’ in the ascending auditory pathway clearly provide input to the SBN. Comparative work in frogs, birds, and rodents,
has revealed that the auditory midbrain and thalamus influence the SBN11,36,37,38,39,40,41. However, auditory signals in many species are complex and are not fully processed until reaching
higher-order circuits such as the auditory pallium. In many vertebrate taxa, the auditory thalamus sends a large projection to a primary recipient area of the auditory pallium (including the
cortex in mammals); this primary area then projects to a number of secondary auditory pallial areas42,43,44,45,46. In mice, inactivation or lesion of the primary auditory cortex impact pup
retrieval in mothers but not virgin females47,48. In birds, lesion studies of auditory pallium alter female preferences for male vocalizations, disrupt pair-bond formations, and alter entire
social networks of individuals49,50,51. The auditory pallium is clearly instrumental for the complete processing of sociosensory stimuli, including dissection of auditory scenes, extracting
meaning from complex auditory signals (i.e., language, song), learning new sounds, and recognizing conspecifics25,52,53,54,55,56,57. These functions are vital to social interactions, and so
these higher-order sensory percepts must eventually reach circuits that control social behavior.
To uncover the influence of auditory pallium on circuits controlling social behavior, birds are an excellent study system. Zebra finches (Taeniopygia guttata) live in large social groups and
can remember upwards of forty individuals by their vocalizations alone58, and the auditory pallium of this species has been well-characterized (e.g.21,43,49,52,59,60,61,62,63,64,65,66,). In
birds, current evidence supports the possibility that auditory pallium is functionally connected to SBN nuclei, though the available data are correlational. Auditory-evoked immediate early
gene expression in secondary auditory pallium and SBN nuclei are highly correlated in female Zebra finches7, and secondary auditory pallium projects to potential intermediate nuclei, which
may then project to the SBN67,68.
We asked whether auditory pallium is necessary for auditory responses observed in SBN nuclei, consistent with an auditory pallial influence on circuits that control social behavior.
Alternatively, auditory pallium could process sensory cues in parallel to the SBN, and in this way, both systems could guide behaviors via efferents to effector regions. In this latter case,
sensory representations in SBN nuclei would be unaltered by disruption of the auditory pallium. We focused on female Zebra finches in this study, given the well-studied social role of
females in this species in evaluating conspecific vocalizations (e.g.69,). We transiently inactivated the auditory pallium of female Zebra finches during male song playback and examined
song-induced immediate early gene activation in SBN nuclei. We observed an effect of pallial inactivation solely in a specific nucleus of the SBN: the lateral ventromedial nucleus of the
hypothalamus (VMHl). We then characterized the auditory responses of VMHl cells using extracellular electrophysiology. Our findings identify the first known functional connection between
auditory pallium and a specific node of the SBN, the VMHl.
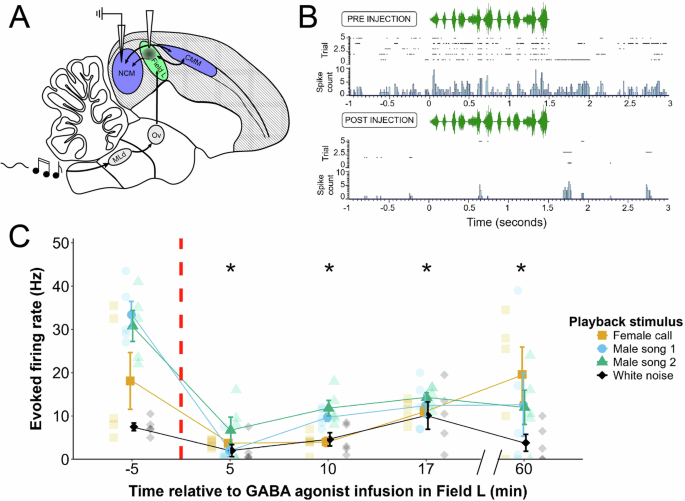
To determine whether inactivation of primary auditory pallium disrupted coding in secondary auditory regions, we injected GABA receptor agonists (baclofen & muscimol) centered on primary
auditory pallium (i.e., Field L) and recorded extracellularly from the caudomedial nidopallium (NCM), a secondary auditory pallial region that receives dense synaptic input from Field L.
Prior to injection in Field L, multiunit traces in NCM displayed characteristic baseline and stimulus-response properties as in previous work (e.g. ref. 70, Fig. 1B). In multiunit traces,
NCM is characterized by irregular spontaneous activity and heightened activity throughout the duration of natural acoustic sounds such as bird songs and calls. Upon baclofen & muscimol
injection into Field L, after 5 minutes spontaneous NCM activity was entirely altered, qualitatively shifting to intermittent bursts of activity in between periods of inactivity. With
respect to stimulus-driven activity, song playback now evoked only brief onset responses to some syllables, and overall evoked firing was suppressed. We moved the probe 100 μm ventral to a
second recording site to confirm this suppression was not due to attrition of the signal, and recorded at this site at three further time points, 10, 17, and 60 minutes, to assess the
duration of the effect GABA receptor agonists in Field L had on NCM. We conducted this experiment on one bird, using a linear mixed model with auditory stimulus as a random effect to
determine the effect of time of recording relative to GABA receptor agonist infusion in Field L on NCM firing rates (2 recording sites total, 5 trials of each of 4 auditory stimuli per
5-time points (i.e., 20 multiunit firing rates at each of 5 total time points); see methods and Fig. 1 caption for further detail). This model revealed a main effect of time of recording on
song-evoked multiunit firing rates (F(4,92) = 14.89, p