Kindlin-2 mediates mechanotransduction in bone by regulating expression of sclerostin in osteocytes
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Osteocytes act as mechanosensors in bone; however, the underlying mechanism remains poorly understood. Here we report that deleting Kindlin-2 in osteocytes causes severe osteopenia
and mechanical property defects in weight-bearing long bones, but not in non-weight-bearing calvariae. Kindlin-2 loss in osteocytes impairs skeletal responses to mechanical stimulation in
long bones. Control and cKO mice display similar bone loss induced by unloading. However, unlike control mice, cKO mice fail to restore lost bone after reloading. Osteocyte Kindlin-2
deletion impairs focal adhesion (FA) formation, cytoskeleton organization and cell orientation in vitro and in bone. Fluid shear stress dose-dependently increases Kindlin-2 expression and
decreases that of Sclerostin by downregulating Smad2/3 in osteocytes; this latter response is abolished by Kindlin-2 ablation. Kindlin-2-deficient osteocytes express abundant Sclerostin,
contributing to bone loss in cKO mice. Collectively, we demonstrate an indispensable novel role of Kindlin-2 in maintaining skeletal responses to mechanical stimulation by inhibiting
Sclerostin expression during osteocyte mechanotransduction. SIMILAR CONTENT BEING VIEWED BY OTHERS A FAK/HDAC5 SIGNALING AXIS CONTROLS OSTEOCYTE MECHANOTRANSDUCTION Article Open access 01
July 2020 GENETIC INTERACTIONS BETWEEN POLYCYSTIN-1 AND WWTR1 IN OSTEOBLASTS DEFINE A NOVEL MECHANOSENSING MECHANISM REGULATING BONE FORMATION IN MICE Article Open access 26 October 2023
OSTEOCYTES REGULATE BONE ANABOLIC RESPONSE TO MECHANICAL LOADING IN MALE MICE VIA ACTIVATION OF INTEGRIN Α5 Article Open access 18 July 2022 INTRODUCTION Bone constantly remodels in response
to mechanical forces during physical exercise and daily life. This concept is the well-known “Wolff’s law” introduced by German anatomist and surgeon Julius Wolff in the 19th century1. This
golden rule for bone remodeling is now widely accepted to explain the force-induced bone formation process and disuse-induced bone loss in humans2,3,4 and animals5. The mechanical forces
applied on bone tissue modify both bone structure and bone strength6. Even though the Wolff’s law for mechanical bone remodeling is well accepted and utilized in modern clinical
practices7,8, mechanisms behind it are still poorly understood. Osteocytes, as the major and long-lived cell type in bone environment, have been considered as the multifunctional regulators
in skeletal tissue9, such as the origin of local calcium abundancy10,11 and the endocrine center for phosphate metabolism12,13. For the past two to three decades, osteocytes are gradually
gaining more attentions from a “passive placeholder” to be the major orchestrator for bone mechanobiology14,15,16,17. Embedded in the mineralized extracellular matrix (ECM), osteocytes sense
various environmental physical stimuli, such as direct bending, compression, osmotic pressure, and shear stress derived from fluid flow in the lacuno-canalicular system (LCS)18. During
mechanical experiences, osteocytes take use of several molecular mechanosensors to transmit external forces into internal biochemical reactions. These mechanosensors include osteocyte
cytoskeleton, dendritic processes, integrin-based focal adhesions (FA), connexin-based gap junctions, primary cilium, ion channels, et al.17. Among all the osteocyte mechanosensors reported
so far, FA is the key player in bone mechanobiology19. Consisting of more than 200 proteins20 and extensive protein–protein interactions in adhesome21, FA links the external microenvironment
and internal cellular cytoskeleton22,23. Structurally and functionally, FA mediates bidirectional controls for cell mechanobiology17,19,24. On one hand, the “outside-in” signaling indicates
that the external ECM and physical environment control integrin activity, FA-associated protein dynamics, cell cytoskeleton, and overall cellular responses to mechanical signals. On the
other hand, the “inside-out” signaling states that the internal cellular status can also influence FA-associated protein abundancy and distribution, FA composition, and integrin sensitivity
in ECM binding and degradation. As one important FA protein, Kindlin-2 serves as a protein scaffold for multiple and dynamic protein–protein interactions in FA25. First, Kindlin-2 regulates
the bidirectional integrin signals26 through direct protein–protein interaction with integrin β1 and integrin β3 subunits27,28,29,30. Second, Kindlin-2 also has direct interactions with
other FA complex proteins, such as Migfilin25, Integrin-like kinases31, Talin, and Paxillin32. Third, Kindlin-2 is reported with direct binding to actin cytoskeleton33,34 and connects with
multiple actin-associated proteins, such as Arp2/335 and RhoGDIα36. As a consequence, Kindlin-2 is largely involved in mechano-related processes, such as FA formation, cell-ECM adhesion,
cell spreading, and cell migration27,29,32,37. However, little is known so far for the function of Kindlin-2 in mechanobiology in vivo. In mammals, Kindlin-2 is reported with ubiquitous
expression in major tissues38,39. Global deletion of _Kindlin-2_ gene caused embryonic lethality at peri-implantation stage in mice at E7.526. Kindlin-2 is reported with indispensable roles
at different tissue backgrounds, such as mesenchymal stem cell (MSC) fate decision40,41, muscle development42,43,44,45, pancreatic development46, adipogenesis and lipid metabolism47,
podocyte structure and function in kidney36. In bone tissue, Kindlin-2 regulates chondrogenesis41, osteogenesis and osteocyte survival48. Our group previously reported that
osteocyte-specific _Kindlin-2_ deletion through 10-kb mouse dentin matrix protein 1 (_Dmp1-Cre)_ caused obvious osteopenia in mice, which deteriorates bone accrual and homeostasis and
parathyroid hormone bone anabolism48,49. Deletion of _Kindlin-2_ in osteocytes is tightly associated with increased osteocyte apoptosis and abnormal expression of secretary Sclerostin
protein in these cells48. Sclerostin is a well-known negative regulator for bone formation in mechanical stimulation50. The expression of Sclerostin in osteocytes and its secretion in blood
circulation have been widely used as indicators for osteoporosis and fracture risks in both animals51,52 and humans53,54,55,56,57. However, detailed mechanisms behind osteocyte Kindlin-2 and
Sclerostin in the regulation of bone homeostasis are still unclear. The aim of this study was to determine whether Kindlin-2 in osteocytes plays an important role in mediation of bone
mechanotransduction. Using in vitro and in vivo model systems, we demonstrate that osteocyte Kindlin-2 largely modulates bone responses to mechanical loading through downregulation of
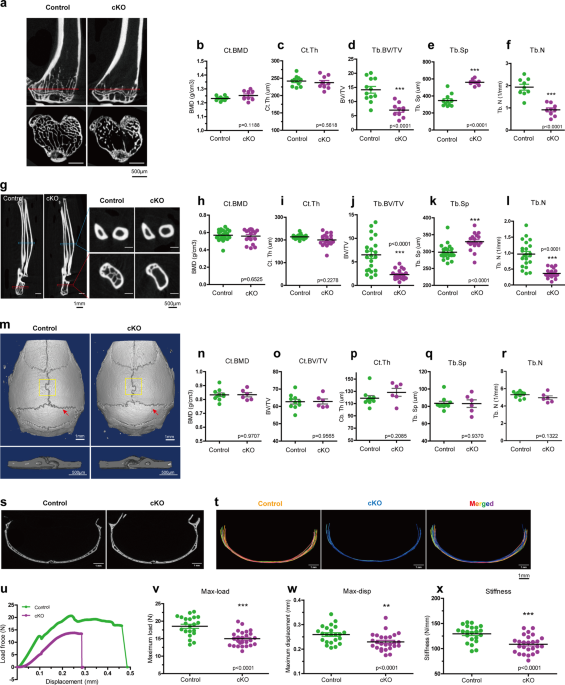
Sclerostin expression in osteocytes. RESULTS KINDLIN-2 DELETION IN OSTEOCYTES RESULTS IN NOTABLE BONE LOSS IN WEIGHT-BEARING LONG BONES, BUT NOT IN NON-WEIGHT-BEARING CALVARIAE We recently
generated osteocyte conditional _Kindlin-2_ knockout mice (hereafter referred to as _Kindlin-2__Dmp1_ or cKO) by breading the _Kindlin-2__fl/fl_ mice with the _Dmp1_-Cre transgenic mice48.
Our previous study demonstrated a striking osteopenia of _Kindlin-2__Dmp1_ mice in distal femurs and lumbar spine (L4)48. We wondered whether this bone loss is universal for other parts of
skeleton in cKO mice. We therefore performed micro-computerized tomography (μCT) analyses of other bones, including hindlimb tibia, forelimb ulna, and calvaria for cKO mice and their control
littermates at 4 month age (Fig. 1), when their skeletal system is fully matured and the expression of _Dmp1-Cre_ reaches to a relative high level58. We first compared the bone mass from
the cortical and distal trabecular bones of hindlimb tibia and forelimb ulna in control and cKO mice (Fig. 1a–l). Similar to our previous observations48, μCT analysis exhibited a remarkable
bone loss in the trabecular bone of both tibia and ulna in cKO mice (Fig. 1a, g). Compared with their age-matched controls, cKO mice showed a 50.7% reduction in bone volume fraction (BV/TV),
62.2% increase in trabecular separation (Tb. Sp), and 52.9% reduction in trabecular number (Tb. N) in the trabecular bone of tibia (Fig. 1d–f). Moreover, ulna bone showed a 64.4% reduction
in BV/TV, 10.4% increase in Tb.Sp, and a 62.5% reduction in Tb. N in the trabecular bone of cKO mice (Fig. 1j–l). Consistent with our previous observations in femurs48, there were no marked
alterations in cortical bone mineral density (BMD) and cortical thickness (Ct.Th) in either tibia or ulna of the two genotypes (Fig. 1b, c, h, i). Detailed parameter detection from μCT
analysis was listed in Supplementary Fig. 1b, c. Next, we examined the calvaria difference between control and cKO mice. As presented in Fig. 1m–r, calvaria from 4-month-old male mice showed
a comparable bone mass for all parameters between cKO and control mice, including BMD, BV/TV, Ct.Th, Tb.N, Tb.Sp, et al. (Supplementary Fig. 1a). To examine any possible structure
differences in calvaria, we compared the μCT cross sections at the interphase between pariental and interpariental bones in control and cKO mice (Fig. 1s). Super-imposed images from control
(in red) and cKO (in blue) revealed that the cross-section structure of calvariae was highly similar between the two genotypic mice (Fig. 1t). Results from immunofluorescence staining showed
that osteocytes from both femurs and calvariae of control mice expressed high level of Kindlin-2 protein, which was dramatically reduced in both bone types in age- and sex-matched cKO mice
(Supplementary Fig. 2a, b). Likewise, western blotting using extracts from femurs and calvariae revealed high expression level of Kindlin-2 protein in control mice, which was significantly
decreased in femurs and calvariae in cKO mice (Supplementary Fig. 2c). These results demonstrate that osteocytes of long bones and calvariae express high level of Kindlin-2 and that the
_Dmp1-Cre_ displays a similar Kindlin-2 deletion efficiency in osteocytes of both types of bones. Thus, it is unlikely that the lack of osteopenia in the calvariae of cKO mice is due to
differences of Kindlin-2 expression and/or Dmp1-Cre activity in osteocytes between the two types of bones. Furthermore, we analyzed the bone mass of alveolar bone, which is derived from
intramembrane ossification, like calvaria, but experiences frequent mechanical loading59. An obvious alveolar loss was observed in cKO mice, compared to control mice (Supplementary Fig.
3a–f). Together, these results suggest that the remarkable bone loss due to osteocyte Kindlin-2 deletion is restricted in weight-bearing bones, i.e., femur, tibia, ulna, and alveolar bone,
but not in non-weight-bearing calvaria. KINDLIN-2 LOSS IN OSTEOCYTES IMPAIRS MECHANICAL PROPERTIES IN MOUSE LONG BONES Wondering whether Kindlin-2 loss affects the long bone quality, we next
tested the mechanical properties of femurs from 4-month-old control and cKO male mice through three-point-bending test. As presented in the load-displacement curve (Fig. 1u), femurs from
control mice had higher maximum load (max-load, Fig. 1v) and higher maximum displacement (max-disp, Fig. 1w) than cKO mice. In this test, the average stiffness of femurs from control mice is
129.5 ± 3.569 N/mm (Fig. 1x). However, this number was decreased to 108.7 ± 3.373 N/mm in cKO femurs (Fig. 1x), which was a 16.62% reduction from control mice. Collectively, these results
clearly demonstrate that Kindlin-2 loss impairs the mechanical properties in long bones. Femur, tibia, ulna, and lumber spine are the important load-bearing bones in body, which experience
higher strain during daily physical activities than the flat calvaria60,61. Therefore, we hypothesized that the specific bone loss in cKO is associated with the mechanical environment in
long bones and that osteocyte Kindlin-2 is crucial for the bone responses for mechanical stimulation. KINDLIN-2 LOSS IN OSTEOCYTES IMPAIRS RESPONSES OF LONG BONES TO MECHANICAL LOADING
STIMULATION IN MICE To test our hypothesis that specific loss in cKO long bones is associated with mechanical loading, we challenged cKO mice and their control littermates with two in vivo
loading models. The first model we used was the ulna loading mouse model (Fig. 2a). In general, we loaded the right ulna of 4-month-old male mice with 6 times of 2.5 N cyclic mechanical
compression within 2 weeks. Meanwhile, we traced the bone mass changes through in vivo μCT detection before (Day 1) and after (Day 14) loading, and monitored the bone formation with the
double calcein labeling during loading experiments. In this model, the left ulnas were served as internal unload controls. As expected, we found that the BMD was increased in the load ulna
of control mice after 2 week experiments (Fig. 2b). Surprisingly, mechanical loading failed to stimulate bone mass increment in cKO ulna. In the comparison of the percentage changes of bone
mass after 14 days’ experiment, the cortical ulna of control mice displayed a 5% increase in BMD (Figs. 2c) and 2.1% increase in BV/TV (Fig. 2e), which are consistent with previously
published results in wild-type animals51,52. However, the cortical ulna of cKO mice showed 2.4% decrease in BMD (Fig. 2c) and 3.2% decrease in BV/TV (Fig. 2e). In this model, in vivo ulna
loading involves two parallel bones in mouse forelimb (Fig. 2f), i.e., ulna (red arrow in Fig. 2f) and radius (green arrow in Fig. 2f). Since radius also experiences mechanical loading and
shares ~23–35% of force during external mechanical stimulation62, we also examined the bone density changes of load radius in both control and cKO mice (Fig. 2o–r). From μCT analysis, radius
from control mice showed slight increases in BMD and BV/TV in the cortical bone (Fig. 2o, q). Similar to ulna results, cKO mice showed significant bone loss in radius by −4.53% and −4.93%
of BMD and BV/TV changes, respectively (Fig. 2p, r). Full set of μCT scanning results for loading samples were summarized in Supplementary Fig. 5 (Supplementary Fig. 5a, b). Together, these
results demonstrate that mechanical ulna loading enhances ulna and radius bone formation in control mice, but induces bone loss in cKO mice. To further confirm the force-induced bone loss in
ulna and radius, we performed the double calcein labeling experiments to measure the in vivo bone-formation activities in load ulnas and unload ulnas after mechanical stimulation. As shown
in Fig. 2g, h, mechanical loading largely increased the calcein labeling intensity, mineral apposition rate (MAR), and bone formation rate (BFR) in both ulna and radius of control mice ulna.
Whereas, cKO mice had reduced MAR, BFR, and mineralizing surface per bone surface (MS/BS) for the load ulna (Fig. 2i–k) and load radius (Fig. 2l–n). Notably, even though the unload ulna did
not show statistically significant differences between control and cKO mice, external mechanical loading lowered the bone formation rate in cKO load ulna and radius compared to that in the
control load ulna. We next wanted to know whether the mechanical properties of forelimbs were affected after loading in the presence and absence of osteocyte Kindlin-2. To this end, we
conducted nano-indentation experiments over non-decalcified unload and load bone samples from control and cKO mice and tested the Young’s modulus and hardness of cortical bone of ulna (Fig.
2t–v) and radius (Fig. 2s, w, x). Results from nano-indentation showed that both ulna and radius from cKO mice had significantly lower hardness compared to that in their control littermates,
but no obvious Young’s modulus difference was observed between these two genotypes. After mechanical loading, the Young’s modulus of ulna and radius from control mice was increased 16.9%
(Fig. 2u) and 20.8% (Fig. 2w), respectively. Comparably, the Young’s modulus of cKO mice had only 7.5% increase (Fig. 2u) for ulna and no significant change for radius (Fig. 2w). Moreover,
the Young’s modulus and Hardness of load ulna and load radius between control and cKO mice showed significant difference, which indicates that mechanical loading enlarges the difference of
bone mechanical properties in control and cKO mice. Therefore, these data reported so far suggest that deletion of Kindlin-2 in osteocytes affects not only bone mass, but also bone
mechanical property enhancement during force-adaptation process in mouse forelimbs. To further determine the involvement of osteocyte Kindlin-2 in the mediation of bone mechanotransduction,
we performed the second in vivo tibia loading model with control and cKO mice (Supplementary Fig. 4). We loaded cyclic compression (4 Hz, triangular waveform) with 9.0 N peak force on the
right tibia of 4-month-old male mice for 2 weeks. Consistent with ulna loading results, control mice showed obvious bone mass increase, whereas cKO mice exhibited a clear reduction in BV/TV
after mechanical loading (Supplementary Fig. 4a–d). Furthermore, the double calcein labeling experiments demonstrated significantly reductions of MAR and BFR in the load tibia of cKO mice,
compared to that of control mice (Supplementary Fig. 4e–g). Detailed parameter detection from μCT analysis in loading experiments were summarized in Supplementary Fig. 5. Collectively, both
ulna and tibia loading results demonstrate that mice with osteocytes-specific Kindlin-2 deletion fail to properly respond to mechanical stimulation. These results suggest a critical role for
Kindlin-2 in osteocyte in bone formation triggered by mechanical stimulation. CONTROL AND CKO MICE DISPLAY SIMILAR BONE LOSS IN RESPONSE TO MECHANICAL UNLOADING; BUT CKO MICE FAIL TO
RESTORE LOST BONE AFTER RELOADING After confirming that the skeleton of cKO mice fails to properly respond to mechanical loading, we wondered whether Kindlin-2 loss affects osteocyte
sensation to unloading conditions. To do so, we conducted 21 days hindlimb-unloading (HLU) experiments with 14-week-old male control and cKO mice and detected bone mass changes in the
hindlimb femurs (Fig. 3a). As shown in Fig. 3a, both control and cKO mice exhibited significant bone mass alternations in their femurs with substantial reductions of BV/TV and Tb.N, and
marked increase of Tb. Sp after HLU experiments. Quantitatively, the percentage changes for bone mass loss after HLU were ranged from 33.8 to 36.1% (−35.6% for BV/TV, −36.1% for Tb.N, and
+33.8% for Tb.Sp) in control mice, and 41.8–47.8% (−43.6% for BV/TV, −41.8% for Tb.N, and +47.8% for Tb.Sp) in cKO mice (Fig. 3b–d). The bone loss appeared to be slightly larger in cKO mice
than that in control mice, but loss-of-Kindlin-2 seems to have no effects on unloading sensation and disuse-induced bone loss. To further investigate whether Kindlin-2 is involved in
mechanical reloading sensation, we extended the HLU experiments with extra 21 days recovery, which allows the experimental animals to freely explore and experience mechanical loading in
daily activities (Fig. 3e). As expected, the control mice restored their bone mass with comparable BV/TV, Tb.Sp, and Tb.N in control and HLU-recovery group (Fig. 3f–h). However, the cKO mice
failed to regain their bone mass at the recovery stage, but still presented significant decreases in BV/TV (−57.9%) and Tb.N (−57.5%), and increase in Tb. Sp (37.1%) in HLU-recovery group
(Fig. 3f–h). These results further demonstrate that compared to unloading sensation, Kindlin-2 is more responsible for mechanical loading sensation in osteocytes. KINDLIN-2 DELETION REDUCES
OSTEOCYTE DENDRITE FORMATION AND CELL ATTACHMENT IN VITRO AND IN BONE To understand the mechanism behind Kindlin-2 in the regulation of osteocyte mechanical responses, we next utilized a
widely used osteocyte-like cell line, MLO-Y4 cells63, as an in vitro model for further studies. We previously deleted _Kindlin-2_ gene in MLO-Y4 cells through the Crispr-Cas9 technology48.
Considering the importance of Kindlin-2 in FA complex26,28 and the participation of FA in osteocyte mechanobiology17, we first tested the changes of FA components under different Kindlin-2
genetic backgrounds. Through western blotting, we found that, compared to the wild-type (WT) cells, _Kindlin-2_ knockout (K2KO) led to remarkable reductions in the expression of integrin and
FA-associated proteins in osteocytes (Fig. 4a). The expression of two integrin isoforms that were highly detected in osteocytes, i.e. integrin β1 and integrin β323 and several Kindlin-2
binding proteins, i.e. Talin129,32, focal adhesion kinase (FAK)64 and their phosphorylated isoforms were decreased by the Kindlin-2 ablation in MLO-Y4 cells. Interestingly, the expression of
Connexin-43, an important mechanosensor in osteocyte biology17, was not markedly altered in K2KO MLO-Y4 cells. With this broad influence in FA and FA-associated proteins, Kindlin-2 deletion
in osteocytes affected the focal adhesion formation and cellular morphology in MLO-Y4 cells (Fig. 4b, c). As shown in Fig. 4b, Kindlin-2 loss caused dramatic alternation in FA, whose loosen
and less-concentrated attachment to substrate (white arrows) indicated a reduced tension in K2KO FA sites. Moreover, Kindlin-2 deletion also brought notably morphological changes in MLO-Y4
cells with dramatic reductions in spreading area (Fig. 4e) and dendritic length (Fig. 4g). Specifically, WT MLO-Y4 cells had a broader frequency distribution for single dendritic length than
K2KO cells (Fig. 4h). The majority of dendritic length in WT cells ranged from 15 to 20 μm, whereas K2KO cells only extended dendrites in 5–10 μm length (Fig. 4h). Furthermore, we found
that Kindlin-2 controls plasma membrane continuances and cytoskeleton arrangement in MLO-Y4 cells. As shown in the scanning electron microscopy (SEM) images in Fig. 4c, WT MLO-Y4 cells had
large spreading area with normal and continuous plasma membrane, while K2KO cells displayed smaller spreading area with clear membrane curvature under SEM. Because the presence of nonionic
detergent Triton X-100 (TX-100) can induce cell membrane destabilization, permeabilization, and lysis65, it has been used for plasma membrane removal and in situ cytoskeleton detection66. To
observe the cellular cytoskeleton, we pre-incubated WT and K2KO cells with TX-100 before fixation and SEM imaging. We found that WT cells presented less and sparse cytosol components around
nucleus, but high and dense cytoskeleton component at the dendrites (yellow arrows). However, in K2KO cells, this difference of component distribution disappeared and the whole cells were
filled with dense cytoskeleton (red arrows). Based on these cytoskeleton and FA defects in K2KO cells, we further tested the spreading and attachment ability for MLO-Y4 cells at different
genetic backgrounds. We first traced the cell spreading dynamics within the first 30 min after cell seeding (Fig. 4i and Supplementary movie 1). Through live cell imaging, we found that,
right after cell seeding, WT cells rapidly protruded outside and formed multiple dendrites within the first 30 min. However, K2KO cells failed to generate any protrusions or form any
dendrites within the first 30 min (Fig. 4j, k). We next monitored the spreading and attachment of MLO-Y4 cells at different time points. As presented in Fig. 4j, on uncoated glass surface,
WT cells achieved 93.3% of attachment 3 h after seeding and reached to 98.3% 24 h after seeding. However, K2KO cells only reached to 18.1% of attachment at 3 h, with increased percentage at
6 h and reached to 73.7% 24 h after seeding (Fig. 4l). Since Collagen-I (Col-I) is one abundant ECM protein67 that activates integrin pathways68, we also tested the attachment ability of
MLO-Y4 cells on Col-I coated surface. Interestingly, this spreading difference was enlarged on Col-1 coated surface. As shown in Fig. 4k, m, WT cells needed some time to adapt to Col-1
coated surface and finally reached up to 88.5% 24 h after seeding. However, K2KO cells rarely spread out on Col-1 surface for the first 6 h, and only reached to 9.8% and 27.6% at 24 h in two
K2KO Crispr-Cas9 cell lines (#3 and #10). Together, these results demonstrate that Kindlin-2 is essential for cell cytoskeleton integrity, FA formation, and cell spreading in MLO-Y4 cells.
These observations also indicate that loss of Kindlin-2 in osteocytes may affect its cellular mechanical responses through the defects in cytoskeleton and FA of these cells. To further
confirm the relationship between Kindlin-2 and in vivo osteocyte morphology, we stained the actin cytoskeleton with Phalloidin-Rhodamine over tibia sections from both control and cKO mice
(Fig. 4n and Supplementary movie 2). As presented in Fig. 4N, the osteocytes from control mice displayed a typical ellipsoid cell shape, extended massive connections with neighboring cells,
and all were aligned in parallel to bone marrow. These extensive intracellular connections and parallel orientation allow osteocytes a fast and efficient transition and communications during
mechanical experiences69,70. However, osteocytes with Kindlin-2 deletion altered their morphology in several ways (Fig. 4n). First, the osteocyte cell body in cKO mice presented less
elongated cell body with dense F-actin filaments accumulation in the perinuclear region (white arrows). Second, compared to the osteocytes from control mice, osteocytes in cKO mice exhibited
less and shorter dendrites extended from cell body (yellow arrows in Fig. 4n zoom-in images), and their dendrites had dramatic discontinuity between osteocytes (yellow asterisks). These
observations are similar to the cytoskeleton defects in K2KO MLO-Y4 cells (Fig. 4b, c). Third, cKO mice had more round-up and miss-oriented osteocytes in tibia sections (red arrows).
Collectively, these results suggest that Kindlin-2 deletion in osteocytes influences its cytoskeleton organization and FA formation both in vitro and in vivo. FLUID SHEAR STRESS UP-REGULATES
KINDLIN-2 AND DOWN-REGULATES SCLEROSTIN AND SMAD2/3 EXPRESSION IN A DOSE-DEPENDENT MANNER IN MLO-Y4 CELLS To explore the mechanism behind Kindlin-2 in osteocyte mechanobiology, we first
conducted steady fluid shear stress (FSS) treatment in WT MLO-Y4 cells to mimic the mechanical stimulations that osteocytes experience in vivo71. As shown in Fig. 5a, we observed an obvious
increase of Kindlin-2 expression through immune-fluorescence (IF) staining in MLO-Y4 cells upon FSS treatment. Moreover, western blotting revealed that FSS induced expression of Kindlin-2
protein in a dose-dependent manner in MLO-Y4 cells (Fig. 5b, c). These results further confirm that Kindlin-2 is tightly associated with external mechanical stimulation in osteocytes. It is
known that the expression and secretion of Sclerostin are closely regulated by mechanical stimulation both in vivo51 and in vitro72. Published study focused on osteoblast cells suggests that
traditional TGF-β/Smad signaling is involved in transcription of _Sost_ in UMR106.01 mature osteoblast cell line73. Therefore, we next tested any possible involvements of Sclerostin and
Smad2/3 in FSS through western blotting. Consistent with previous results, we found that FSS stimulation decreased Sclerostin expression in MLO-Y4 cells (Fig. 5b, c). Interestingly, we also
detected an obvious reduction of Smad2/3 expression upon FSS treatment (Fig. 5b, c). KINDLIN-2 SUPPRESSES THE EXPRESSION OF SCLEROSTIN THROUGH SMAD2/3 IN MLO-Y4 CELLS We next analyzed the
protein expression of Smad2/3 and Sclerostin in two K2KO Crispr-Cas9 cell lines (#3 and #10) through western blotting (Fig. 5d). In K2KO cells, we observed dramatic increases in expression
of both Sclerostin and Smad2/3 proteins relative to that in WT cells (Fig. 5e). Similar increases in the expression of Smad2/3 and Sclerostin were observed in the K2KO cells through IF
staining (Fig. 5f, g). These data suggest that Kindlin-2 may function as a suppressor of Smad2/3 and Sclerostin expression in MLO-Y4 cells. A previous study by Loots and coworkers has shown
that Smad2/3 transcriptionally activates the expression of Sclerostin in osteoblasts73. We found that siRNA knock down of Smad2, Smad3, or both (Smad2/3) dramatically reduced the level of
Sclerostin protein in MLO-Y4 cells (Fig. 5h–m). Together, these results suggest that Kindlin-2 suppresses Sclerostin expression in MLO-Y4 cells probably through its suppression of Smad2/3.
To further analyze the influence of Kindlin-2 on Smad2/3 and Sclerostin upon FSS stimulation, we performed FSS at 10 dyns/cm2 for 2 h with WT and K2KO cells. As shown in Fig. 5n, under FSS
administration, WT MLO-Y4 cells experienced a clear Kindlin-2 upregulation, which was associated with reduced expression of both Smad2/3 and Sclerostin in these cells. However, in the
absence of Kindlin-2, the FSS stimulation failed to downregulate neither Smad2/3 nor Sclerostin expression in MLO-Y4 cells (Fig. 5n). Moreover, quantitative analysis of western blot results
(Fig. 5o) and qPCR results (Fig. 5p) further confirmed that Kindlin-2 could downregulate Smad2/3 and Sclerostin expression transcriptionally and translationally upon FSS treatment. Published
results suggest that Smad2/3 indirectly activates _Sost_ transcription through Mef2C, which binds to a distal gene enhancer of the _Sost_ promoter, ECR573,74. In MLO-Y4 cells, we found that
Mef2C mRNA and protein levels were decreased upon FSS treatment in WT cells (Fig. 5o, p). However, this FSS-induced suppression was abolished in K2KO cells (Fig. 5o, p). Together, these
data indicate that FSS inhibits Sclerostin expression at least partially through Kindlin-2-Smad2/3-Mef2C axis in MLO-Y4 cells. IN VIVO LOADING INCREASES SERUM SCLEROSTIN, AND ENHANCES
SCLEROSTIN AND SMAD2/3 EXPRESSION IN OSTEOCYTES OF CKO MICE We further examined expression of Kindlin-2 in osteocyte upon mechanical loading in mice. We conducted fluorescence staining of
the unloaded and loaded tibial sections from control mice using anti-Kindlin-2 antibody. Results displayed a significant increase in Kindlin-2 protein expression in osteocytes embedded in
mineralizing matrix in the loaded tibial bone (Fig. 6a). Furthermore, western blotting using protein extracts from osteocyte-enriched mid-diaphyseal tibial shafts (with their bone marrow
flushed) from age- and sex-matched control and cKO mice revealed that mechanical loading dramatically increased the level of Kindlin-2, which was largely reduced in cKO mice (Fig. 6b). We
next measured the Sclerostin level in the serum of in vivo loading mice. To monitor the serum Sclerostin changes at different loading time points, we collected the serum at Day 3 and Day 14
after in vivo tibia loading and compared it with serum collected at Day 1 (before loading experiments; Fig. 6c). As expected, we observed a clear serum Sclerostin drop in control mice after
loading with only 86.3% serum Sclerostin remain at Day 3 and 82.2% at Day 14. However, the serum Sclerostin level in cKO mice was increased after mechanical loading, which showed 32.8%
increase at Day 3 and 28.9% increase at Day 14. With these observations, we next determined the Sclerostin and Smad2/3 expression levels in osteocytes by IF staining over unload and load
tibia samples. As presented in Fig. 6d, while mechanical loading did not alter Sclerostin-positive osteocytes in control mice, Sclerostin-positive osteocytes, and the overall Sclerostin
expression in each osteocyte were largely increased in cKO mice than those in control mice, especially after mechanical loading (Fig. 6f). Consistent with in vitro data, Smad2/3 expression
shared similar patterns, i.e., osteocytes of load tibia had reduced Smad2/3 expression compared with those of unload tibia in control mice, but cKO tibia presented dramatic increase of
Smad2/3-positive osteocytes after loading (Fig. 6e, g). Together, these data confirmed that mice with conditional Kindlin-2 deletion in osteocytes experienced Sclerostin and Sma2/3
upregulation upon mechanical stimulation. Based on these results, our working model proposes an important function of Kindlin-2 in osteocyte mechanobiology (Fig. 6h). Mechanical forces
activate the transmembrane integrins through its influence over ECM. The cytoplasmic tails of integrins recruit and activate Kindlin-2 and other FA-associated proteins, such as Talin and
FAK. Mechanical forces upregulate Kindlin-2 expression in osteocytes. Kindlin-2 inhibits the expression of _Sost_ by, at least in part, downregulation of Smad2/3 in osteocytes under
mechanical force-stimulated conditions. Kindlin-2 also influences the organization of actin cytoskeleton. DISCUSSION In the present study, we demonstrate the importance of the focal adhesion
protein, Kindlin-2, in the regulation of force adaptation during osteocyte mechanobiology. Through μCT scanning, we find that mice with Kindlin-2 loss in osteocytes exhibit remarkable
osteopenia phenotype, which is only restricted to load-bearing bones, such as ulna, tibia, femur, and lumbar spine48, but not in calvariae. Considering that the _Dmp1_ promoter expression is
detected in both calvariae and long bones75 and a comparable deletion efficiency of Kindlin-2 in both long bones and calvariae is observed in this study, it is unlikely that the osteopenia
in load-bearing bones of cKO mice is due to a result of differential Kindlin-2 deletion between long bones and calvariae. One explanation for this osteopenia phenotype discrepancy between
long bones and calvariae is the gene expressing profiling difference originated from intramembrane ossification and endochondral ossification processes. Our group previously showed that
conditional deletion of Kindlin-2 in Prx1-expressing mesenchymal progenitors resulted in neonatal lethality, long bone shortening, and loss of skull vault in mice41. This result demonstrates
that Kindlin-2 is essential for both intramembrane and endochondral ossification during bone development. Considering the different origins and gene profiling of intramembrane and
endochondral osteocytes, it is possible that Kindlin-2 functions differentially in two types of osteocytes, for instance, differential functions in mechanotransduction. However, our μCT
scanning results with alveolar bone, in which osteocytes are derived from intramembrane process but under frequent mechanical loading, show a remarkable bone loss in alveolar process from
cKO mice, but not in control mice. At the tissue level, strain and load estimation from daily activities showed that fibula and tibia exhibited higher load than calvaria61,76. At the
cellular level, osteocytes derived from long bones have various gene expression profiles77, different cell morphology60, and diverse protein secretion upon strain application76, compared to
osteocytes derived from calvaria. These results suggest that osteocytes in load-bearing bones experience larger and more dynamic mechanical stimulations than osteocytes in calvariae. In
short, the osteopenia phenotype and reduced bone mechanical properties in long bones are due to a gradual accumulation of defects associated with Kindlin-2 loss during daily mechanical
stimulation in these bones. Published results showed that, besides osteocyte, _Dmp1-Cre_ expression is observed in osteoblasts and stromal cells78,79. Therefore, in the current study, we
cannot rule out the possible contributions of Kindlin-2 loss in these two cell types to the impairment of the mechanotransduction in cKO bone. However, considering the large number of
osteocytes in bone tissue (osteocyte occupying 90–95% of total bone cells, almost 10 times of cell number of osteoblasts80) and the contribution of mechanosensation of osteocyte in
mechanical stimulation14, we believe that this load-bearing bone-related phenotype in cKO mice is primarily due to Kindlin-2 loss in osteocytes. In this study, we conducted two in vivo
mechanical loading models and one in vivo unloading-reloading model for cKO mice and their control littermates. Consistent with previous reports51,52, we find that mechanical loading
preserves bone mass and stimulates bone formation in control mice. However, Kindlin-2 loss in osteocytes reverses the mechanical loading induced bone formation process, rather decreases the
bone mass quantity as well as bone quality in loading limbs. Moreover, mechanical unloading leads to similar bone loss in control and cKO mice. Surprisingly, extending HLU experiment with
recovery stage shows that cKO mice fail to restore lost bone mass after recovery. This result indicates that Kindlin-2 deletion in osteocytes cannot reverse, but exacerbate mechanical
unloading-induced bone loss. These data also suggest that the molecular mechanism for unloading sensation is different from that in loading sensation. Together, current data demonstrate that
Kindlin-2 in osteocytes is mainly involved in mechanical loading sensation process, but with limited contribution in unloading sensation. Mechanistically, we propose Kindlin-2 as a
Sclerostin suppressor that contributes to force-induced bone formation during osteocyte mechanobiology. Here, we demonstrate that Kindlin-2 is an essential negative regulator for Sclerostin
expression in mechanical loading process. In cultured MLO-Y4 cells, we show that mechanical stimulation enhances Kindlin-2 expression, which further suppresses expression of both Sclerostin
and Smad2/3 under shear stress conditions. Moreover, the expression of Sclerostin is tightly controlled by Smad2/3 proteins in MLO-Y4 cells. Consistent with these in vitro data, enhanced
Sclerostin secretion in the serum samples in cKO mice and upregulation of Sclerostin and Smad2/3 in loaded tibia samples from cKO mice were detected. These data together demonstrate that
osteocytes with Kindlin-2 deletion fail to suppress Sclerostin expression when stimulated with mechanical loading, resulting in reduced bone formation. The importance for Kindlin-2 in
osteocyte mechanotransduction could be related to its functions in FA. Previous studies showed that FA proteins are essential for bone mass maintenance in mechanical stimulation, such as
integrin-β181,82,83, FAK84, and Pinch1/285,86. Our results demonstrate that Kindlin-2 controls osteocyte FA formation, cell spreading, cell attachment, and cell morphology both in vitro and
in vivo. Consistent with that Kindlin-2 is an essential scaffold protein in FA26,33,87, deletion of its expression reduces integrin β1, integrin β3, Talin1 and FAK expression, and affects
the plasma membrane continuousness and cytoskeleton integrity in MLO-Y4 cells. Previous studies demonstrate that FA and cell cytoskeleton are active mechanosensors in osteocytes17,83. Our
results suggest that the abnormal osteocyte responses to mechanical loading in cKO mice could be resulted from the defects of FA formation and cytoskeleton integrity associated with
Kindlin-2 loss in osteocytes. It should be noted that, in addition to FAs, osteocytes contain several other mechanosensors, including primary cilium, gap junctions, ion channels, cell
cytoskeleton, and ECMs17. It would be interesting to study the possible involvement of Kindlin-2 in other mechanosensors in future studies. In short, we identify Kindlin-2 as a novel and
important regulator in osteocyte mechanotransduction during the process of force-induced bone formation. The results presented in current study shed new light on the molecular mechanism
between mechanical stimulation and Sclerostin suppression in osteocytes. METHODS ANIMAL STUDY Floxed _Kindlin-2_ mice (_Kindlin-2__fl/fl_) were generated as we previously described in Wu et
al.41. Mice with 10-kb mouse _Dmp1_ gene promoter-driving Cre recombinase expression (_Dmp1-Cre_) were generated as described in Lu et al.58. We obtained heterozygous mice (_Dmp1-Cre_ mice;
_Kindlin-2__fl/+_) by crossing _Kindlin-2__fl/lf_ mice with _Dmp1-Cre_ mice. By crossing heterozygous mice with _Kindlin-2__fl/fl_ mice, we obtained homozygous mice (_Dmp1-Cre_ mice;
_Kindlin-2__fl/fl_). Next, we crossed male _Dmp1-Cre_ ; _Kindlin-2__fl/fl_ mice with female _Kindlin-2__fl/fl_ mice. As a result, we obtained conditional knockout _Dmp1-Cre_ mice;
_Kindlin-2__fl/fl_ mice (cKO); and their control littermates (e.g., _Kindlin-2__fl/fl_). All research protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of
Southern University of Science and Technology. MICRO-COMPUTERIZED TOMOGRAPHY (ΜCT) For in vivo μCT, mice were anesthetized with 2.5% Avertin (100 μl per 10 g body weight) and subjected for
μCT scan. For ex vivo micro μCT, calvaria and long bones (femur, tibia, and ulna) were dissected and immediately fixed with 4% PFA for 24 h and post fixed with 70% ethanol at 4 °C. Either
live or fixed non-demineralized bones were subjected to μCT analyses in the Department of Biology of Southern University of Science and Technology using a Bruker μCT (SkyScan 1172 Micro-CT,
Bruker Micro-CT, Kontich, Belgium). The scan resolution was 13 μm for tibiae and calvariae, 16 μm for ulna and femur with setting of 60 kV, 100 μA and AI 0.5 mm filter. A lower/upper
threshold of 80/255 and 60/255 were used to segment bone from other tissues in ex vivo and in vivo μCT scan, respectively. Calvaria parameters were analyzed in ROI at the center of skull
with 2 mm×2 mm size. Ulna parameters were assessed at the proximal spongiosa from the end of growth plate to 1200 μm extending distally for trabecular bone, and 500 μm thick of cortical bone
at the midshaft for both ulna and radius cortical bones. Tibia parameters were assessed as described in Sugiyama et al.88, i.e., at the proximal spongiosa from the end of growth plate to
750 μm extending distally for trabecular bone, and from the 37% position of tibia length extending to 500 μm distally for cortical bone. Femur parameters were assessed from the distal growth
plate; the trabecular bone rages from 500 μm to 2000 μm; the cortical bone rages from 4000 μm to 5000 μm. The parameters for cortical bone included the bone mineral density (BMD, g/cm3),
bone volume fraction (BV/TV), and cortical thickness (Ct.Th, mm). The parameters for trabecular bone included the bone mineral density (BMD, g/cm3), bone volume fraction (BV/TV), trabecular
thickness (Tb.Th, μm), trabecular separation (Tb.Sp, μm), and trabecular number (Tb.N, 1/mm). IN VIVO ULNA LOADING AND TIBIA LOADING Force loading experiments were conducted as previously
described in refs. 89,90 using an electro actuator (Bose ElectroFore 3200; EndureTEC Minnetonka, MN, USA). Briefly, mice were under anesthesia with 2.5% Avertin injection (100 μl per 10 g
body weight). For ulna loading, the right ulna was loaded with cyclic compression force (2 Hz, sine waveform, 2.5 N peak fore, 450 cycles per loading). For tibia loading, the right tibia was
loaded with cyclic compression force (4 Hz, triangular waveform, 9.0 N peak force, 1200 cycles per loading). The left limbs were intact and served as unloading internal controls. Mice were
loaded on alternative day for 2 weeks. Injection of calcein (30 mg/kg body weight; Sigma, Saint Louis, MO, USA) was administered at Day 4 and Day 12, respectively. Animals were killed on Day
14 for bone histomorphometry. IN VIVO HINDLIMB UNLOADING Hindlimb unloading experiments were conducted as described before in Robling et al.51. Specifically, 14-week-old male mice were
outfitted with tail harnesses and their hindlimbs suspended in air and lose the ground reaction forces. HLU experiments were designed for 21 days. The HLU-recovery experiments included 21
days HLU treatment followed by another 21 days free exploration stage. The experimental control mice were husbandry with normal activities. FEMUR THREE-POINT BENDING Femurs were dissected
free of soft tissue and immediately kept in 1x PBS at 4 °C. Samples were kept in wet and tested right after dissection or 1 day after dissection. The strength test was performed at the
midshaft of femur. The machine used for this test is ElectroForce (Bose ElectroFore 3200; EndureTEC Minnetonka, MN, USA) with continuous displacement of 0.05 mm/sec in a single stop setting
(Ramp waveform, span length, 7 mm). Whole femur mechanical properties, including maximum load, maximum displacement, and stiffness, were determined using load-displacement diagrams. BONE
HISTOLOGY AND IMMUNOHISTOCHEMISTRY For double calcein labeling samples, the bones were embedded in methyl methacrylate following the manufactory plastic embedding protocol (EM0200, Osteo-Bed
Bone Embedding Kit, Sigma, MO, USA). Transverse section in 6 μm thickness of tibiae and ulnas were obtained by cutting with an annular diamond saw. Images of double calcein labeling bone
sections were visualized using the argon 488 nm laser of fluorescent microscopy (Olympus, BX53). For bone immunohistochemistry (IHC) and immunofluorescence (IF), bone samples were
decalcified with 10% ethylenedinitrilotetraacetic acid (EDTA, Sigma) for 2 weeks and 3 weeks for ulna and tibia samples, respectively. Samples were embedded in paraffin and cut in 5 μm
transversal sections. IHC and IF experiments were performed using our standard protocols as we previously descripted41,48. Antibodies used in this study are listed in Supplementary Table 3.
SERUM ELISA Serum samples were collected from the supernatant of centrifuged (4°C, 12,000×_g_, 20 min) mouse whole blood after coagulation for 1 h at room temperature. Samples were
immediately kept at -80°C fridge before usage. Serum levels of Sclerostin were measured by a Mouse/Rat Sost Immunoassay (R&D systems, Inc., Minneapolis, MN, USA, cat#: MSST00).
IMMUNOFLUORESCENCE AND CONFOCAL ANALYSIS Cells were cultured on glass coverslips or rat tail Collagen-I coated coverslips (Corning, cat# 354236, 40 ng/cm2) for 24–48 h. Cultured cells were
fixed with 4% PFA, penetrated with 0.25% Triton X-100, blocked with 1% BSA and then incubated with antibodies. Antibodies used in this study are listed in Supplementary Table 3. Live imaging
and Z-stack imaging were conducted by Nikon A1R laser-scanning confocal microscopy with 40x objective set at 5 s time interval and 0.5 μm z-stack interval. CELL CULTURE AND TRANSFECTION
Kindlin-2 deletion in MLO-Y4 cells was generated as previously described in48. Cells were maintained in α-MEM with 10% FBS and 1% P/S, in 37 °C, 5% CO2 cell culture incubator. For transient
transfection, 60–80% confluent cells were transfected with indicated siRNA using the Lipofectamine ® RNAiMAX Reagent according to the manufacturer’s instructions. SiRNA target sequences were
listed in Supplementary Table 2. QUANTITATIVE REAL-TIME PCR AND WESTERN BLOT ANALYSES RNA and protein isolation, quantitative real-time PCR, and western blot analyses were performed as
previously described91. In brief, total RNA was extracted from cultured cells using Trizol reagents. Synthesis of cDNA was performed using 2 μg of RNA by a Transcriptor First Strand cDNA
Synthesis Kit according to the manufacturer’s instructions. Relative mRNA expression levels were determined using a SYBR Green qPCR kit with CFX96 Real-Time System. β-Actin mRNA was used for
normalization. The specific primers for gene expression analysis were listed in Supplementary Table 1. For the protein samples extracted from bones, calvariae were dissected from control
and cKO mice free of muscle or other tissues; long bone samples were dissected free of other tissues, flashed out the bone marrow and only kept the osteocyte-enriched mid-diaphyseal shafts.
For western blotting, cell lysates were harvested in RIPA lysis buffer. Protein concentration was measured with a BCA kit. Aliquots of 30 μg total protein were separated and transferred onto
a PVDF membranes. Membranes were blocked at room temperature in 5% non-fat powdered milk for 1 h, followed by an overnight incubation at 4 °C with primary antibodies. The specific primary
antibodies for western blotting were listed in Supplemental Table 3. After incubation with appropriate HRP-conjugated secondary antibodies, blots were developed using an enhanced
chemiluminescence and exposed in ChemiDoc XRS chemiluminescence imaging system. FLUID-INDUCED FLOW SHEAR STRESS TREATMENT Fluid-induced flow shear stress was performed with Streamer ® System
STR-4000 (Flexcell International Corporation, Burlington, NC, USA) as discussed previously in Chen et al.92. MLO-Y4 cells with a total cell number of 3.0 × 105 cells were seed on Collagen-I
pre-coated culture slips (75 mm × 25 mm × 1 mm, Flexcell) for 48 h before FSS treatment. Upon FSS treatment, the culture slips were transferred into a parallel plat flow chamber and cells
were exposed to 1, 5, or 10 dyne/cm2 fluid flow for 2 h. For static controls, MLO-Y4 cells were kept in incubator without any further treatment. Protein and mRNA samples were collected right
after FSS treatment. SCANNING ELECTRON IMAGING Wild-type and Kindlin-2 knockout MLO-Y4 cells were seed on 12-mm glass coverslips at a density of 1.0 × 104 cells/slip and cultured for 24 h.
Cells were fixed with cold 100% methanol at −20 °C for 15 min. SEM sample preparation was conducted as previously descripted93. Cells were dehydrated by incubation in a series of methanol
solutions for 10 min per solution: 35%, 50%, 75%, 90%, and 100% methanol. Cells were completely dehydrated with hexamethyldisilazane (HMDS) treatment for 10 min and left to air dry
overnight. The samples were mounted using double-sided conductive tapes, and coated with Au/Pt (Gold/Platinum) particles in pumper to increase the sample conductivity. The cellular
morphology of MLO-Y4 cells was observed on scanning electron microscope (SEM, ZEISS Merlin) at 5.0 kV. For permeabilization, cells were incubated with 0.15% Triton X-100 in 1x PBS for 60 s
before fixation66. NANO-INDENTATION The indentation experiments were performed with a Nano Indenter G200 (Keysight Technologies, Inc., Santa Rosa, CA, USA), equipped with a three-sided
pyramid Berkovich diamond tip. The identical loading scheme applied consists of a loading stage at a constant rate of 20 mN/min to a depth of 1000 nm, holding at this load for a period of 10
s and then unloading to 15% of the peak load at a rate of 10 mN/min. Each sample was performed on twenty indentations at the midshaft of bone samples. For the indenter tip, Young’s modulus
(Ei) = 1140 GPa and Poisson’s ratio (vi) = 0.07. In calculating the modulus values from the nano-indentation data, the Poisson ratio for bone is taken to be 0.3, as suggested in previous
literature94. STATISTICS AND REPRODUCIBILITY All data were analyzed in this study by using the GraphPad Prism software (Version 5.0). The differences between two groups were analyzed by
two-tailed Student’s _t_ test. The differences among different time points were analyzed by two-way ANOVA. Results are expressed as mean ± standard deviation (s.d.). Difference with _P_ <
0.05 was considered as statistically significant. All experiments were repeated at least three times. Highly reproducible results were obtained. REPORTING SUMMARY Further information on
research design is available in the Nature Research Reporting Summary linked to this article. DATA AVAILABILITY All data generated or analyzed during this study are included in the
Supplementary Data 1. Any other data are available from the corresponding author upon reasonable request. REFERENCES * Wolff, J. _Das Gesetz der Transformation der Knochen Kirschwald_
(Hirschwald, 1892). * Sterck, J. G., Klein-Nulend, J., Lips, P. & Burger, E. H. Response of normal and osteoporotic human bone cells to mechanical stress in vitro. _Am. J. Physiol._ 274,
E1113–E1120 (1998). CAS PubMed Google Scholar * Klein-Nulend, J. et al. Donor age and mechanosensitivity of human bone cells. _Osteoporos. Int._ 13, 137–146 (2002). Article CAS PubMed
Google Scholar * LeBlanc, A. D. et al. Skeletal responses to space flight and the bed rest analog: a review. _J. Muscuoskelet. Neuronal Interact._ 7, 33–47 (2007). CAS Google Scholar *
Turner, C. H., Forwood, M. R., Rho, J. Y. & Yoshikawa, T. Mechanical loading thresholds for lamellar and woven bone formation. _J. Bone Miner. Res._ 9, 87–97 (1994). Article CAS PubMed
Google Scholar * Petit, M. A. et al. A randomized school-based jumping intervention confers site and maturity-specific benefits on bone structural properties in girls: a hip structural
analysis study. _J. Bone Miner. Res._ 17, 363–372 (2002). Article CAS PubMed Google Scholar * Frost, H. M. Wolff’s Law and bone’s structural adaptations to mechanical usage: an overview
for clinicians. _Angle Orthod._ 64, 175–188 (1994). CAS PubMed Google Scholar * Frost, H. M. From Wolff’s law to the Utah paradigm: insights about bone physiology and its clinical
applications. _Anat. Rec._ 262, 398–419 (2001). Article CAS PubMed Google Scholar * Bonewald, L. F. The amazing osteocyte. _J. Bone Miner. Res._ 26, 229–238 (2011). Article CAS PubMed
Google Scholar * Schaffler, M. B. & Kennedy, O. D. Osteocyte signaling in bone. _Curr. Osteoporos. Rep._ 10, 118–125 (2012). Article PubMed PubMed Central Google Scholar *
Schaffler, M. B., Cheung, W. Y., Majeska, R. & Kennedy, O. Osteocytes: master orchestrators of bone. _Calcif. Tissue Int._ 94, 5–24 (2014). Article CAS PubMed Google Scholar * Han,
Y., You, X., Xing, W., Zhang, Z. & Zou, W. Paracrine and endocrine actions of bone-the functions of secretory proteins from osteoblasts, osteocytes, and osteoclasts. _Bone Res._ 6, 16
(2018). Article PubMed PubMed Central CAS Google Scholar * Dallas, S. L., Prideaux, M. & Bonewald, L. F. The osteocyte: an endocrine cell… and more. _Endocr. Rev._ 34, 658–690
(2013). Article CAS PubMed PubMed Central Google Scholar * Uda, Y., Azab, E., Sun, N., Shi, C. & Pajevic, P. D. Osteocyte mechanobiology. _Curr. Osteoporos. Rep._ 15, 318–325
(2017). Article PubMed PubMed Central Google Scholar * Yavropoulou, M. P. & Yovos, J. G. The molecular basis of bone mechanotransduction. _J. Musculoskelet. Neuronal Interact._ 16,
221–236 (2016). CAS PubMed PubMed Central Google Scholar * Hemmatian, H., Bakker, A. D., Klein-Nulend, J. & van Lenthe, G. H. Aging, osteocytes, and mechanotransduction. _Curr.
Osteoporos. Rep._ 15, 401–411 (2017). Article PubMed PubMed Central Google Scholar * Qin, L., Liu, W., Cao, H. & Xiao, G. Molecular mechanosensors in osteocytes. _Bone Res._ 8, 23
(2020). Article CAS PubMed PubMed Central Google Scholar * Thompson, W. R., Rubin, C. T. & Rubin, J. Mechanical regulation of signaling pathways in bone. _Gene_ 503, 179–193 (2012).
Article CAS PubMed PubMed Central Google Scholar * Geoghegan, I. P., Hoey, D. A. & McNamara, L. M. Integrins in osteocyte biology and mechanotransduction. _Curr. Osteoporos. Rep._
17, 195–206 (2019). Article PubMed Google Scholar * Zaidel-Bar, R., Itzkovitz, S., Ma’ayan, A., Iyengar, R. & Geiger, B. Functional atlas of the integrin adhesome. _Nat. Cell Biol._
9, 858–867 (2007). Article CAS PubMed PubMed Central Google Scholar * Zaidel-Bar, R. & Geiger, B. The switchable integrin adhesome. _J. Cell Sci._ 123, 1385–1388 (2010). Article
CAS PubMed PubMed Central Google Scholar * Geiger, B., Spatz, J. P. & Bershadsky, A. D. Environmental sensing through focal adhesions. _Nat. Rev. Mol. Cell Biol._ 10, 21–33 (2009).
Article CAS PubMed Google Scholar * McNamara, L. M., Majeska, R. J., Weinbaum, S., Friedrich, V. & Schaffler, M. B. Attachment of osteocyte cell processes to the bone matrix. _Anat.
Rec._ 292, 355–363 (2009). Article CAS Google Scholar * Xie, J. et al. Compliant substratum changes osteocyte functions: the role of ITGB3/FAK/β-catenin signaling matters. _ACS Appl. Bio
Mater._ 1, 792–801 (2018). Article CAS Google Scholar * Tu, Y., Wu, S., Shi, X., Chen, K. & Wu, C. Migfilin and Mig-2 link focal adhesions to filamin and the actin cytoskeleton and
function in cell shape modulation. _Cell_ 113, 37–47 (2003). Article CAS PubMed Google Scholar * Montanez, E. et al. Kindlin-2 controls bidirectional signaling of integrins. _Genes Dev._
22, 1325–1330 (2008). Article CAS PubMed PubMed Central Google Scholar * Shi, X. et al. The MIG-2/integrin interaction strengthens cell-matrix adhesion and modulates cell motility. _J.
Biol. Chem._ 282, 20455–20466 (2007). Article CAS PubMed Google Scholar * Ma, Y.-Q., Qin, J., Wu, C. & Plow, E. F. Kindlin-2 (Mig-2): a co-activator of beta3 integrins. _J. Cell
Biol._ 181, 439–446 (2008). Article CAS PubMed PubMed Central Google Scholar * Bledzka, K. et al. Spatial coordination of kindlin-2 with talin head domain in interaction with integrin β
cytoplasmic tails. _J. Biol. Chem._ 287, 24585–24594 (2012). Article CAS PubMed PubMed Central Google Scholar * Hirbawi, J. et al. The extreme C-terminal region of kindlin-2 is
critical to its regulation of integrin activation. _J. Biol. Chem._ 292, 14258–14269 (2017). Article CAS PubMed PubMed Central Google Scholar * Kadry, Y. A., Huet-Calderwood, C., Simon,
B. & Calderwood, D. A. Kindlin-2 interacts with a highly conserved surface of ILK to regulate focal adhesion localization and cell spreading. _J. Cell Sci._ 131, jcs221184 (2018). *
Theodosiou, M. et al. Kindlin-2 cooperates with talin to activate integrins and induces cell spreading by directly binding paxillin. _Elife_ 5, e10130 (2016). Article PubMed PubMed Central
Google Scholar * Bledzka, K. et al. Kindlin-2 directly binds actin and regulates integrin outside-in signaling. _J. Cell Biol._ 213, 97–108 (2016). Article CAS PubMed PubMed Central
Google Scholar * Yasuda-Yamahara, M. et al. FERMT2 links cortical actin structures, plasma membrane tension and focal adhesion function to stabilize podocyte morphology. _Matrix Biol._
68–69, 263–279 (2018). Article PubMed CAS Google Scholar * Böttcher, R. T. et al. Kindlin-2 recruits paxillin and Arp2/3 to promote membrane protrusions during initial cell spreading.
_J. Cell Biol._ 216, 3785–3798 (2017). Article PubMed PubMed Central CAS Google Scholar * Sun, Y. et al. Kindlin-2 association with Rho GDP-dissociation inhibitor α suppresses Rac1
activation and podocyte injury. _J. Am. Soc. Nephrol._ 28, 3545–3562 (2017). Article CAS PubMed PubMed Central Google Scholar * Jahed, Z., Shams, H., Mehrbod, M. & Mofrad, M. R. K.
in _International Review of Cell and Molecular Biology_, Vol. 310 (ed. Jeon, K. W.) 171–220 (Academic Press, 2014). * Siegel, D. H. et al. Loss of kindlin-1, a human homolog of the
Caenorhabditis elegans actin-extracellular-matrix linker protein UNC-112, causes Kindler syndrome. _Am. J. Hum. Genet._ 73, 174–187 (2003). Article CAS PubMed PubMed Central Google
Scholar * Ussar, S., Wang, H.-V., Linder, S., Fässler, R. & Moser, M. The kindlins: subcellular localization and expression during murine development. _Exp. Cell Res._ 312, 3142–3151
(2006). Article CAS PubMed Google Scholar * Guo, L. et al. Kindlin-2 regulates mesenchymal stem cell differentiation through control of YAP1/TAZ. _J. Cell Biol._ 217, 1431–1451 (2018).
Article CAS PubMed PubMed Central Google Scholar * Wu, C. et al. Kindlin-2 controls TGF-β signalling and Sox9 expression to regulate chondrogenesis. _Nat. Commun._ 6, 7531 (2015).
Article PubMed PubMed Central Google Scholar * Dowling, J. J., Vreede, A. P., Kim, S., Golden, J. & Feldman, E. L. Kindlin-2 is required for myocyte elongation and is essential for
myogenesis. _BMC Cell Biol._ 9, 36–36 (2008). Article PubMed PubMed Central CAS Google Scholar * Dowling James, J. et al. Kindlin-2 is an essential component of intercalated discs and
is required for vertebrate cardiac structure and function. _Circ. Res._ 102, 423–431 (2008). Article CAS PubMed Google Scholar * Zhang, Z. et al. Kindlin-2 is essential for preserving
integrity of the developing heart and preventing ventricular rupture. _Circulation_ 139, 1554–1556 (2019). Article PubMed PubMed Central Google Scholar * Zhang, Z. et al. Postnatal loss
of kindlin-2 leads to progressive heart failure. _Circ. Heart Fail._ 9, e003129 (2016). Article CAS PubMed Google Scholar * Zhu, K. et al. Kindlin-2 modulates MafA and β-catenin
expression to regulate β-cell function and mass in mice. _Nat. Commun._ 11, 484 (2020). Article CAS PubMed PubMed Central Google Scholar * Gao, H. et al. Lipoatrophy and metabolic
disturbance in mice with adipose-specific deletion of kindlin-2. _Jci Insight_ 4, e128405 (2019). * Cao, H. et al. Focal adhesion protein Kindlin-2 regulates bone homeostasis in mice. _Bone
Res._ 8, 2–2 (2020). Article CAS PubMed PubMed Central Google Scholar * Fu, X. et al. Kindlin-2 regulates skeletal homeostasis by modulating PTH1R in mice. _Signal Transduct. Target
Ther._ 5, 297 (2020). Article CAS PubMed PubMed Central Google Scholar * Sebastian, A. & Loots, G. G. Transcriptional control of Sost in bone. _Bone_ 96, 76–84 (2017). Article CAS
PubMed Google Scholar * Robling, A. G. et al. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. _J. Biol. Chem._ 283, 5866–5875 (2008). Article CAS
PubMed Google Scholar * Tu, X. et al. Sost downregulation and local Wnt signaling are required for the osteogenic response to mechanical loading. _Bone_ 50, 209–217 (2012). Article CAS
PubMed Google Scholar * Gaudio, A. et al. Increased sclerostin serum levels associated with bone formation and resorption markers in patients with immobilization-induced bone loss. _J.
Clin. Endocrinol. Metab._ 95, 2248–2253 (2010). Article CAS PubMed Google Scholar * Spatz, J. M. et al. Serum sclerostin increases in healthy adult men during bed rest. _J. Clin.
Endocrinol. Metab._ 97, E1736–E1740 (2012). Article CAS PubMed PubMed Central Google Scholar * Belavý, D. L. et al. Serum sclerostin and DKK1 in relation to exercise against bone loss
in experimental bed rest. _J. Bone Miner. Metab._ 34, 354–365 (2016). Article PubMed CAS Google Scholar * Kouvelioti, R. et al. Response of sclerostin and bone turnover markers to high
intensity interval exercise in young women: does impact matter? _Biomed. Res. Int._ 2018, 4864952 (2018). Article CAS PubMed PubMed Central Google Scholar * Hinton, P. S., Nigh, P.
& Thyfault, J. Serum sclerostin decreases following 12months of resistance- or jump-training in men with low bone mass. _Bone_ 96, 85–90 (2017). Article CAS PubMed Google Scholar *
Lu, Y. et al. DMP1-targeted Cre expression in odontoblasts and osteocytes. _J. Dent. Res._ 86, 320–325 (2007). Article CAS PubMed Google Scholar * Wang, D. et al. Calvarial versus long
bone: implications for tailoring skeletal tissue engineering. _Tissue Eng. Part B Rev._ 26, 46–63 (2020). Article CAS PubMed Google Scholar * Vatsa, A. et al. Osteocyte morphology in
fibula and calvaria - is there a role for mechanosensing? _Bone_ 43, 452–458 (2008). Article PubMed Google Scholar * Hillam, R. A., Goodship, A. E. & Skerry, T. M. Peak strain
magnitudes and rates in the tibia exceed greatly those in the skull: An in vivo study in a human subject. _J. Biomech._ 48, 3292–3298 (2015). Article PubMed PubMed Central Google Scholar
* Lu, Y., Thiagarajan, G., Nicolella, D. P. & Johnson, M. L. Load/strain distribution between ulna and radius in the mouse forearm compression loading model. _Med. Eng. Phys._ 34,
350–356 (2012). Article PubMed Google Scholar * Kato, Y., Windle, J. J., Koop, B. A., Mundy, G. R. & Bonewald, L. F. Establishment of an osteocyte-like cell line, MLO-Y4. _J. Bone
Miner. Res._ 12, 2014–2023 (1997). Article CAS PubMed Google Scholar * Qu, H., Tu, Y., Guan, J. L., Xiao, G. & Wu, C. Kindlin-2 tyrosine phosphorylation and interaction with Src
serve as a regulatable switch in the integrin outside-in signaling circuit. _J. Biol. Chem._ 289, 31001–31013 (2014). Article CAS PubMed PubMed Central Google Scholar * Mattei, B.,
Lira, R. B., Perez, K. R. & Riske, K. A. Membrane permeabilization induced by Triton X-100: the role of membrane phase state and edge tension. _Chem. Phys. Lipids_ 202, 28–37 (2017).
Article CAS PubMed Google Scholar * Tanaka-Kamioka, K., Kamioka, H., Ris, H. & Lim, S. S. Osteocyte shape is dependent on actin filaments and osteocyte processes are unique
actin-rich projections. _J. Bone Miner. Res._ 13, 1555–1568 (1998). Article CAS PubMed Google Scholar * Viguet-Carrin, S., Garnero, P. & Delmas, P. D. The role of collagen in bone
strength. _Osteoporos. Int._ 17, 319–336 (2006). Article CAS PubMed Google Scholar * Marie, P. J., Haÿ, E. & Saidak, Z. Integrin and cadherin signaling in bone: role and potential
therapeutic targets. _Trends Endocrinol. Metab._ 25, 567–575 (2014). Article CAS PubMed Google Scholar * Klein-Nulend, J., Bacabac, R. G. & Bakker, A. D. Mechanical loading and how
it affects bone cells: the role of the osteocyte cytoskeleton in maintaining our skeleton. _Eur. Cell Mater._ 24, 278–291 (2012). Article CAS PubMed Google Scholar * van Oers, R. F.,
Wang, H. & Bacabac, R. G. Osteocyte shape and mechanical loading. _Curr. Osteoporos. Rep._ 13, 61–66 (2015). Article PubMed PubMed Central Google Scholar * Bonewald, L. F.
Mechanosensation and transduction in osteocytes. _Bonekey Osteovision_ 3, 7–15 (2006). Article PubMed PubMed Central Google Scholar * Spatz, J. M. et al. The Wnt Inhibitor Sclerostin Is
Up-regulated by Mechanical Unloading in Osteocytes in Vitro. _J. Biol. Chem._ 290, 16744–16758 (2015). Article CAS PubMed PubMed Central Google Scholar * Loots, G. G. et al. TGF-β
regulates sclerostin expression via the ECR5 enhancer. _Bone_ 50, 663–669 (2012). Article CAS PubMed Google Scholar * Collette, N. M. et al. Targeted deletion of Sost distal enhancer
increases bone formation and bone mass. _Proc. Natl Acad. Sci. USA_ 109, 14092–14097 (2012). Article CAS PubMed Google Scholar * D’Souza, R. N. et al. Gene expression patterns of murine
dentin matrix protein 1 (Dmp1) and dentin sialophosphoprotein (DSPP) suggest distinct developmental functions in vivo. _J. Bone Miner. Res._ 12, 2040–2049 (1997). Article PubMed Google
Scholar * Rawlinson, S. C., Mosley, J. R., Suswillo, R. F., Pitsillides, A. A. & Lanyon, L. E. Calvarial and limb bone cells in organ and monolayer culture do not show the same early
responses to dynamic mechanical strain. _J. Bone Miner. Res._ 10, 1225–1232 (1995). Article CAS PubMed Google Scholar * Kingsmill, V. J., McKay, I. J., Ryan, P., Ogden, M. R. &
Rawlinson, S. C. Gene expression profiles of mandible reveal features of both calvarial and ulnar bones in the adult rat. _J. Dent._ 41, 258–264 (2013). Article CAS PubMed Google Scholar
* Zhang, J. Z. & Link, D. C. Targeting of mesenchymal stromal cells by cre-recombinase transgenes commonly used to target osteoblast lineage cells. _J. Bone Miner. Res._ 31, 2001–2007
(2016). Article CAS PubMed PubMed Central Google Scholar * Lim, J., Burclaff, J., He, G. X., Mills, J. C. & Long, F. X. Unintended targeting of Dmp1-Cre reveals a critical role for
Bmpr1a signaling in the gastrointestinal mesenchyme of adult mice. _Bone Res._ 5, 16049 (2017). * Manolagas, S. C. Birth and death of bone cells: Basic regulatory mechanisms and implications
for the pathogenesis and treatment of osteoporosis. _J. Aging Phys. Act._ 8, 248–248 (2000). Google Scholar * Robling, A. G. & Turner, C. H. Mechanical signaling for bone modeling and
remodeling. _Crit. Rev. Eukaryot. Gene Expr._ 19, 319–338 (2009). Article CAS PubMed PubMed Central Google Scholar * Shekaran, A. et al. The effect of conditional inactivation of beta 1
integrins using twist 2 Cre, Osterix Cre and osteocalcin Cre lines on skeletal phenotype. _Bone_ 68, 131–141 (2014). Article CAS PubMed PubMed Central Google Scholar * Litzenberger, J.
B., Tang, W. J., Castillo, A. B. & Jacobs, C. R. Deletion of β1 integrins from cortical osteocytes reduces load-induced bone formation. _Cell. Mol. Bioeng._ 2, 416–424 (2009). Article
CAS Google Scholar * Sato, T. et al. A FAK/HDAC5 signaling axis controls osteocyte mechanotransduction. _Nat. Commun._ 11, 3282 (2020). Article CAS PubMed PubMed Central Google Scholar
* Wang, Y. S. et al. Focal adhesion proteins Pinch1 and Pinch2 regulate bone homeostasis in mice. _JCI Insight_ 4, e131692 (2019). * Lei, Y. M. et al. LIM domain proteins Pinch1/2 regulate
chondrogenesis and bone mass in mice. _Bone Res._ 8, 37 (2020). * Larjava, H., Plow, E. F. & Wu, C. Kindlins: essential regulators of integrin signalling and cell-matrix adhesion. _EMBO
Rep._ 9, 1203–1208 (2008). Article CAS PubMed PubMed Central Google Scholar * Sugiyama, T. et al. Risedronate does not reduce mechanical loading-related increases in cortical and
trabecular bone mass in mice. _Bone_ 49, 133–139 (2011). Article CAS PubMed PubMed Central Google Scholar * Sugiyama, T. et al. Mechanical loading enhances the anabolic effects of
intermittent parathyroid hormone (1-34) on trabecular and cortical bone in mice. _Bone_ 43, 238–248 (2008). Article CAS PubMed Google Scholar * Zhao, L. et al. Inactivation of Lrp5 in
osteocytes reduces young’s modulus and responsiveness to the mechanical loading. _Bone_ 54, 35–43 (2013). Article CAS PubMed PubMed Central Google Scholar * Fu, X. et al. Runx2/osterix
and zinc uptake synergize to orchestrate osteogenic differentiation and citrate containing bone apatite formation. _Adv. Sci._ 5, 1700755 (2018). Article CAS Google Scholar * Chen, S. et
al. Moderate fluid shear stress regulates heme oxygenase-1 expression to promote autophagy and ECM homeostasis in the nucleus pulposus cells. _Front. Cell Dev. Biol._ 8, 127–127 (2020).
Article PubMed PubMed Central Google Scholar * Murtey, M. D. & Ramasamy, P. _Modern Electron Microscopy in Physical and Life Sciences_ (IntechOpen, 2016). * Rho, J.-Y., Tsui, T. Y.
& Pharr, G. M. Elastic properties of human cortical and trabecular lamellar bone measured by nanoindentation. _Biomaterials_ 18, 1325–1330 (1997). Article CAS PubMed Google Scholar
Download references ACKNOWLEDGEMENTS The authors acknowledge the assistance of Core Research Facilities of Southern University of Science and Technology (SUSTech) and the Pico Center at
SUSTech. This work was supported, in part, by the National Key Research and Development Program of China Grant (2019YFA0906004), the National Natural Science Foundation of China Grants
(81991513, 82022047, 81630066, 81870532, and 81972100), the Guangdong Provincial Science and Technology Innovation Council Grant (2017B030301018), and the Science and Technology Innovation
Commission of Shenzhen Municipal Government Grants (JCYJ20180302174117738, JCYJ20180302174246105, KQJSCX20180319114434843, and JSGG20180503182321166) and the China Postdoctoral Science
Foundation (2019M651641). AUTHOR INFORMATION Author notes * These authors contributed equally: Lei Qin, Xuekun Fu. AUTHORS AND AFFILIATIONS * Department of Biochemistry, School of Medicine,
Guangdong Provincial Key Laboratory of Cell Microenvironment and Disease Research, Shenzhen Key Laboratory of Cell Microenvironment, Southern University of Science and Technology, Shenzhen,
China Lei Qin, Xuekun Fu, Manxia Lin, Peijun Zhang, Yishu Wang, Qinnan Yan, Chu Tao, Wen Liu, Huiling Cao & Guozhi Xiao * Department of Biomedical Engineering, Southern University of
Science and Technology, Shenzhen, China Jing Ma & Bin Tang * Research Center for Human Tissues and Organs Degeneration, Shenzhen Institutes of Advanced Technology, Chinese Academy of
Sciences, Shenzhen, China Di Chen * Department of Cell Biology, School of Basic Medical Sciences, Southern Medical University, Guangzhou, China Xiaochun Bai Authors * Lei Qin View author
publications You can also search for this author inPubMed Google Scholar * Xuekun Fu View author publications You can also search for this author inPubMed Google Scholar * Jing Ma View
author publications You can also search for this author inPubMed Google Scholar * Manxia Lin View author publications You can also search for this author inPubMed Google Scholar * Peijun
Zhang View author publications You can also search for this author inPubMed Google Scholar * Yishu Wang View author publications You can also search for this author inPubMed Google Scholar *
Qinnan Yan View author publications You can also search for this author inPubMed Google Scholar * Chu Tao View author publications You can also search for this author inPubMed Google
Scholar * Wen Liu View author publications You can also search for this author inPubMed Google Scholar * Bin Tang View author publications You can also search for this author inPubMed Google
Scholar * Di Chen View author publications You can also search for this author inPubMed Google Scholar * Xiaochun Bai View author publications You can also search for this author inPubMed
Google Scholar * Huiling Cao View author publications You can also search for this author inPubMed Google Scholar * Guozhi Xiao View author publications You can also search for this author
inPubMed Google Scholar CONTRIBUTIONS Study design by G.X., H.C., and L.Q. Study conducts and data collection and analysis by L.Q., X.F., J.M., M.L., P.Z., Y.W., Q.Y., C.T., and W.L. Data
interpretation by L.Q., B.T., X.B., D.C., H.C., and G.X. Drafting the manuscript by L.Q., H.C., and G.X.; L.Q., X.F., H.C., and G.X. take the responsibility for the integrity of the data
analysis. CORRESPONDING AUTHORS Correspondence to Huiling Cao or Guozhi Xiao. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION
PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION
DESCRIPTION OF ADDITIONAL SUPPLEMENTARY FILES SUPPLEMENTARY MOVIE 1 SUPPLEMENTARY MOVIE 2 SUPPLEMENTARY DATA 1 REPORTING SUMMARY RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed
under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate
credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article
are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and
your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this
license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Qin, L., Fu, X., Ma, J. _et al._ Kindlin-2 mediates
mechanotransduction in bone by regulating expression of Sclerostin in osteocytes. _Commun Biol_ 4, 402 (2021). https://doi.org/10.1038/s42003-021-01950-4 Download citation * Received: 29
August 2020 * Accepted: 03 March 2021 * Published: 25 March 2021 * DOI: https://doi.org/10.1038/s42003-021-01950-4 SHARE THIS ARTICLE Anyone you share the following link with will be able to
read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing
initiative