A corset function of exoskeletal ECM promotes body elongation in Drosophila
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
Body elongation is a general feature of development. Postembryonically, the body needs to be framed and protected by extracellular materials, such as the skeleton, the skin and the shell,
which have greater strength than cells. Thus, body elongation after embryogenesis must be reconciled with those rigid extracellular materials. Here we show that the exoskeleton (cuticle)
coating the Drosophila larval body has a mechanical property to expand less efficiently along the body circumference than along the anteroposterior axis. This “corset” property of the
cuticle directs a change in body shape during body growth from a relatively round shape to an elongated one. Furthermore, the corset property depends on the functions of Cuticular protein 11
A and Tubby, protein components of a sub-surface layer of the larval cuticle. Thus, constructing a stretchable cuticle and supplying it with components that confer circumferential stiffness
is the fly’s strategy for executing postembryonic body elongation.
Transformation of a relatively round-shaped body into an elongated one is a general feature of development. Embryonic axis elongation in a wide range of animals has been commonly explained
by active and coordinated cell behaviors1, such as oriented cell division2,3, cell intercalation4,5,6,7,8 and cell migration9,10. Postembryonic body elongation requires a different
consideration: because materials that frame the body during postembryonic life, such as the skeleton, the skin and the shell, are mainly shaped by extracellular matrix (ECM), body shape
change must involve shape change of those materials outside of cells.
The insect body is coated by cuticle, a seamless sheet of ECM composed of proteins and chitin (a linear polymer of N-acetyl-beta-D-glucosamine (GlcNAc)) that are deposited by the
epidermis11. The cuticle is continuously required throughout postembryonic life as an external skeleton that gives the insect its shape, supports movement, and protects against injury and
desiccation11,12. In the developmental context, the textbook view of the cuticle is that it restricts changes in body shape and size, and that it needs to be replaced through molting to
allow for those changes11,13,14. Nonetheless, stretching of cuticle between molts has been shown to accompany body size increase in some insects15,16,17,18. Furthermore, ECM dynamics has
emerged as a general mechanism of insect morphogenesis19. For example, deformation of the cuticle covering the larval body of Drosophila drives body shape change without molting20, and
elongation of wings and legs in Drosophila is controlled by ECM remodeling21,22. These findings suggest that body shaping during postembryonic development in insects depends not only on
cuticle replacement but also on dynamic properties of the existing cuticle.
Here we demonstrate a “corset” property of the Drosophila larval cuticle that permits less efficient expansion of the cuticle along the body circumference than along the anteroposterior
axis. During larval growth, this cuticle property directs a progressive change from a relatively round-shaped body to an elongated one. We further show that the corset property depends on
the functions of two proteins that localize to a sub-surface layer of the larval cuticle, Cuticular protein 11 A (Cpr11A) and Tubby (Tb). The stretchable cuticle on which circumferential
stiffness is conferred by its components allows the fly to execute postembryonic body elongation.
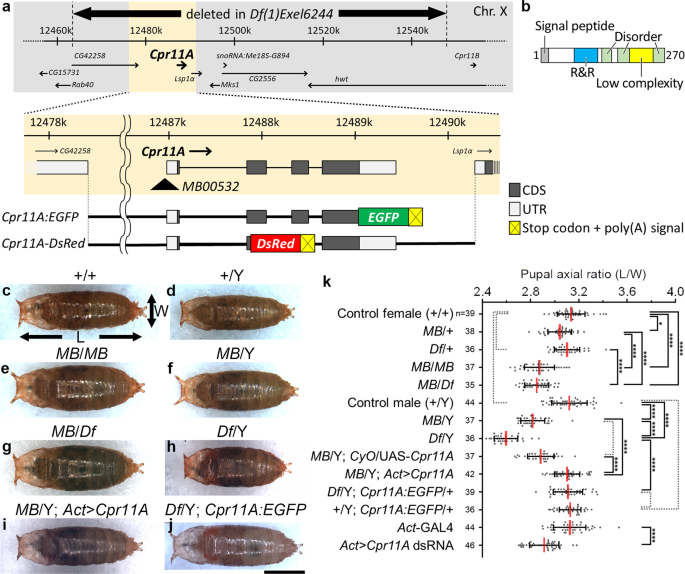
We found that the pupae of MB00532 homozygotes or hemizygotes, which harbor a transposon insertion immediately upstream of Cpr11A (Fig. 1a), appeared laterally wider and anteroposteriorly
shorter than the wild-type pupae (Fig. 1c–f). The abnormal shapes of MB00532 pupae were represented by their body axial ratios (length/width ratios), which were significantly smaller than
those of the wild-type (Fig. 1k; compare + /+ with MB/MB and + /Y with MB/Y). Df(1)Exel6244, a deficiency uncovering Cpr11A along with six neighboring genes (Fig. 1a), failed to compliment
the tubby phenotype of MB00532 (Fig. 1g, k; compare MB/Df with MB/MB). Df(1)Exel6244 hemizygotes were viable and exhibited even tubbier pupal shapes (represented by smaller axial ratios)
compared with the MB00532 hemizygotes (Fig. 1h, k; compare Df/Y with MB/Y). Df(1)Exel6244 homozygous females were larval lethal. The tubby phenotype was recapitulated by whole-body
expression of dsRNA against Cpr11A in the wild-type background (Fig. 1k; compare Act-GAL4 with Act > Cpr11A dsRNA). Conversely, the tubby phenotype of MB00532 or Df(1)Exel6244 was rescued by
inducing whole-body expression of Cpr11A cDNA (Fig. 1i, k; MB/Y; Act > Cpr11A), or by introduction of Cpr11A:EGFP, a rescue construct containing the genomic sequence of Cpr11A into which an
EGFP-coding sequence was inserted immediately upstream of the Cpr11A stop codon (Fig. 1a, j, k; Df/Y; Cpr11A:EGFP/ + ). These results indicated that loss of Cpr11A function was responsible
for the tubby body shape phenotype. Df(1)Exel6244 hemizygotes are hereafter referred to as Cpr11ADf.
a Schematic representation of the Cpr11A locus (X chromosome), and mutations and transgenic constructs used in this study. The full-length Cpr11A protein fused at its C-terminus with EGFP is
expressed from the Cpr11A:EGFP transgene. Nonsecretory DsRed is expressed from the Cpr11A-DsRed transgene. b Domain structure of Cpr11A. The signal peptide predicted by SignalP5.046, the
R&R domain (PF00379) by Pfam49, the low complexity region and the disorder regions by MobiDB50 are shown. c–j Pupae of the indicated genotypes, viewed from the dorsal side. Anterior is to
the left. The length (L) and the width (W) of a pupa were measured as indicated by arrows. MB, MB005322; Df, Df(1)Exel6244; Act > Cpr11A, Act-GAL4/UAS-Cpr11A; Act > Cpr11A dsRNA,
Act-GAL4/UAS-Cpr11A dsRNA. Bar, 1 mm. k Mean ± S.D. of pupal axial ratios (L/W) of individual genotypes. n, the number of pupae measured for each genotype. Significance was assessed using
one-way ANOVA (p