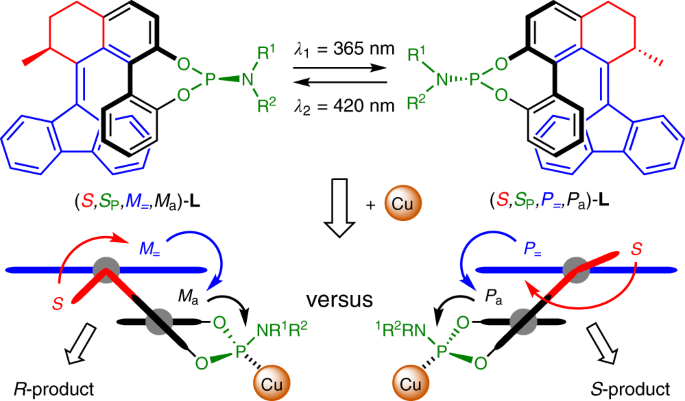
Phosphoramidite-based photoresponsive ligands displaying multifold transfer of chirality in dynamic enantioselective metal catalysis
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT The transfer and amplification of chirality in biological and artificial systems is a fundamental process that allows for a dynamic control of structure and function. Only a few
responsive systems harness the dynamic transfer of chirality and can act as photoswitchable chiral inductors. Here we demonstrate that photoresponsive phosphoramidite ligands based on a
chiral biaryl-substituted molecular switch can be used to alter the activity and invert the stereoselectivity of a copper-catalysed asymmetric conjugate addition. The phosphoramidites were
obtained as pairs of diastereoisomers, each displaying a distinct catalytic activity and stereoselectivity as a result of the light-controlled matched–mismatched interaction between the
fixed chirality at the phosphorus atom and the dynamic chirality of the switch. The result is an elegant balance of two competing catalysts, of which the complementary catalytic performance
is tunable via light, which takes advantage of the internal dynamic transfer of chirality on reversible alkene photoisomerization. This discovery paves the way for the future development of
more complex chirality-dependent photoresponsive and multitasking catalysts. Access through your institution Buy or subscribe This is a preview of subscription content, access via your
institution ACCESS OPTIONS Access through your institution Access Nature and 54 other Nature Portfolio journals Get Nature+, our best-value online-access subscription $32.99 / 30 days cancel
any time Learn more Subscribe to this journal Receive 12 digital issues and online access to articles $119.00 per year only $9.92 per issue Learn more Buy this article * Purchase on
SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about
institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS ENANTIODIVERGENT EPOXIDATION OF ALKENES WITH A PHOTOSWITCHABLE PHOSPHATE
MANGANESE-SALEN COMPLEX Article 22 September 2022 CHIRAL BRØNSTED ACID-CONTROLLED INTERMOLECULAR ASYMMETRIC [2 + 2] PHOTOCYCLOADDITIONS Article Open access 30 September 2021 PORPHYRIN AS A
VERSATILE VISIBLE-LIGHT-ACTIVATABLE ORGANIC/METAL HYBRID PHOTOREMOVABLE PROTECTING GROUP Article Open access 24 June 2022 DATA AVAILABILITY All the data generated or analysed during this
study are included in this article (and its Supplementary Information files). Crystallographic data for the structure L1 reported has been deposited at the Cambridge Crystallographic Data
Centre (CCDC) under the deposition number 1990520. This data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif. REFERENCES * Bentley, R. in _Encyclopedia of Molecular
Cell Biology and Molecular Medicine_ 2nd edn (ed. Mayers, R. A.) 579–618 (Wiley-VCH, 2006). * Browne, W. R. & Feringa, B. L. Making molecular machines work. _Nat. Nanotechnol._ 1, 25–35
(2006). Article CAS PubMed Google Scholar * Feringa, B. L. The art of building small: from molecular switches to motors (Nobel Lecture). _Angew. Chem. Int. Ed._ 56, 11060–11078 (2017).
Article CAS Google Scholar * Erbas-Cakmak, S., Leigh, D. A., McTernan, C. T. & Nussbaumer, A. L. Artificial molecular machines. _Chem. Rev._ 115, 10081–10206 (2015). Article CAS
PubMed PubMed Central Google Scholar * Kinbara, K. & Aida, T. Toward intelligent molecular machines: directed motions of biological and artificial molecules and assemblies. _Chem.
Rev._ 105, 1377–1400 (2005). Article CAS PubMed Google Scholar * Göstl, R., Senf, A. & Hecht, S. Remote-controlling chemical reactions by light: towards chemistry with high
spatio-temporal resolution. _Chem. Soc. Rev._ 43, 1982–1996 (2014). Article PubMed CAS Google Scholar * Jacobsen, E. N., Pfaltz, A. & Yamamoto, H. _Comprehensive Asymmetric
Catalysis_ including Supplements 1 and 2 (Springer, 2004). * Davie, E. A. C., Mennen, S. M., Xu, Y. & Miller, S. J. Asymmetric catalysis mediated by synthetic peptides. _Chem. Rev._ 107,
5759–5812 (2007). Article PubMed CAS Google Scholar * Yu, J., RajanBabu, T. V. & Parquette, J. R. Conformationally driven asymmetric induction of a catalytic cendrimer. _J. Am.
Chem. Soc._ 130, 7845–7847 (2008). Article CAS PubMed Google Scholar * Desmarchelier, A. et al. Correlation between the selectivity and the structure of an asymmetric catalyst built on a
chirally amplified supramolecular helical scaffold. _J. Am. Chem. Soc._ 138, 4908–4916 (2016). Article CAS PubMed Google Scholar * Stoll, R. S. & Hecht, S. Artificial light-gated
catalyst systems. _Angew. Chem. Int. Ed._ 49, 5054–5075 (2010). Article CAS Google Scholar * Blanco, V., Leigh, D. A. & Marcos, V. Artificial switchable catalysts. _Chem. Soc. Rev._
44, 5341–5370 (2015). Article CAS PubMed Google Scholar * Vlatković, M., Collins, B. S. L. & Feringa, B. L. Dynamic responsive systems for catalytic function. _Chem. Eur. J._ 22,
17080–17111 (2016). Article PubMed CAS Google Scholar * van Dijk, L. et al. Molecular machines for catalysis. _Nat. Rev. Chem._ 2, 0117 (2018). Article CAS Google Scholar * Dorel, R.
& Feringa, B. L. Photoswitchable catalysis based on the isomerisation of double bonds. _Chem. Commun._ 55, 6477–6486 (2019). Article CAS Google Scholar * Wang, J. & Feringa, B. L.
Dynamic control of chiral space in a catalytic asymmetric reaction using a molecular motor. _Science_ 331, 1429–1432 (2011). Article CAS PubMed Google Scholar * Vlatković, M., Bernardi,
L., Otten, E. & Feringa, B. L. Dual stereocontrol over the Henry reaction using a light- and heat-triggered organocatalyst. _Chem. Commun._ 50, 7773–7775 (2014). Article CAS Google
Scholar * Yamamoto, T., Yamada, T., Nagata, Y. & Suginome, M. High-molecular-weight polyquinoxaline-based helically chiral phosphine (PQXphos) as chirality-switchable, reusable, and
highly enantioselective monodentate ligand in catalytic asymmetric hydrosilylation of styrenes. _J. Am. Chem. Soc._ 132, 7899–7901 (2010). Article CAS PubMed Google Scholar * Mortezaei,
S., Catarineu, N. R. & Canary, J. W. A redox-reconfigurable, ambidextrous asymmetric catalyst. _J. Am. Chem. Soc._ 134, 8054–8057 (2012). Article CAS PubMed Google Scholar * Sud, D.,
Norsten, T. B. & Branda, N. R. Photoswitching of stereoselectivity in catalysis using a copper dithienylethene complex. _Angew. Chem. Int. Ed._ 44, 2019–2021 (2005). Article CAS
Google Scholar * Zhao, D., Neubauer, T. M. & Feringa, B. L. Dynamic control of chirality in phosphine ligands for enantioselective catalysis. _Nat. Commun._ 6, 6652 (2015). Article CAS
PubMed PubMed Central Google Scholar * Eigen, M. Wilkins, R. G. in _Mechanisms of Inorganic Reactions_ (eds Kleinberg, J., Murmann, R. K., Fraser, R. T. M. & Bauman, J.) 55–80
(American Chemical Society, 1965). * Otsuki, J., Akasaka, T. & Araki, K. Molecular switches for electron and energy transfer processes based on metal complexes. _Coord. Chem. Rev._ 252,
32–56 (2008). Article CAS Google Scholar * Shinoda, S. Dynamic cyclen–metal complexes for molecular sensing and chirality signaling. _Chem. Soc. Rev._ 42, 1825–1835 (2013). Article CAS
PubMed Google Scholar * Boiocchi, M. & Fabbrizzi, L. Double-stranded dimetallic helicates: assembling–disassembling driven by the Cu(i)/Cu(ii) redox change and the principle of
homochiral recognition. _Chem. Soc. Rev._ 43, 1835–1847 (2014). Article CAS PubMed Google Scholar * Zhao, D., van Leeuwen, T., Cheng, J. & Feringa, B. L. Dynamic control of chirality
and self-assembly of double-stranded helicates with light. _Nat. Chem._ 9, 250–256 (2017). Article CAS PubMed Google Scholar * Ousaka, N., Takeyama, Y., Iida, H. & Yashima, E.
Chiral information harvesting in dendritic metallopeptides. _Nat. Chem._ 3, 856–861 (2011). Article CAS PubMed Google Scholar * Miyake, H. & Tsukube, H. Coordination chemistry
strategies for dynamic helicates: time-programmable chirality switching with labile and inert metal helicates. _Chem. Soc. Rev._ 41, 6977–6991 (2012). Article CAS PubMed Google Scholar *
Koumura, N., Geertsema, E. M., Meetsma, A. & Feringa, B. L. Light-driven molecular rotor: unidirectional rotation controlled by a single stereogenic center. _J. Am. Chem. Soc._ 122,
12005–12006 (2000). Article CAS Google Scholar * Feringa, B. L. The art of building small: from molecular switches to molecular motors. _J. Org. Chem._ 72, 6635–6652 (2007). Article CAS
PubMed Google Scholar * Schliwa, M. _Molecular Motors_ (Wiley-VCH, 2006). * Koumura, N., Zijlstra, R. W. J., van Delden, R. A., Harada, N. & Feringa, B. L. Light-driven
monodirectional molecular rotor. _Nature_ 401, 152–155 (1999). Article CAS PubMed Google Scholar * Koumura, N., Geertsema, E. M., van Gelder, M. B., Meetsma, A. & Feringa, B. L.
Second generation light-driven molecular motors. Unidirectional rotation controlled by a single stereogenic center with near-perfect photoequilibria and acceleration of the speed of rotation
by structural modification. _J. Am. Chem. Soc._ 124, 5037–5051 (2002). Article CAS PubMed Google Scholar * Pizzolato, S. F. et al. Central-to-helical-to-axial-to-central transfer of
chirality with a photoresponsive catalyst. _J. Am. Chem. Soc._ 140, 17278–17289 (2018). Article CAS PubMed PubMed Central Google Scholar * Kistemaker, J. C. M., Pizzolato, S. F., van
Leeuwen, T., Pijper, T. C. & Feringa, B. L. Spectroscopic and theoretical identification of two thermal isomerization pathways for bistable chiral overcrowded alkenes. _Chem. Eur. J._
22, 13478–13487 (2016). Article CAS PubMed Google Scholar * Wezenberg, S. J., Vlatković, M., Kistemaker, J. C. M. & Feringa, B. L. Multi-state regulation of the dihydrogen phosphate
binding affinity to a light- and heat-responsive bis-urea receptor. _J. Am. Chem. Soc._ 136, 16784–16787 (2014). Article CAS PubMed Google Scholar * Greb, L. & Lehn, J.-M.
Light-driven molecular motors: imines as four-step or two-step unidirectional rotors. _J. Am. Chem. Soc._ 136, 13114–13117 (2014). Article CAS PubMed Google Scholar * Vlatković, M.,
Feringa, B. L. & Wezenberg, S. J. Dynamic inversion of stereoselective phosphate binding to a bisurea receptor controlled by light and heat. _Angew. Chem. Int. Ed._ 55, 1001–1004 (2016).
Article CAS Google Scholar * Teichert, J. F. & Feringa, B. L. Phosphoramidites: privileged ligands in asymmetric catalysis. _Angew. Chem. Int. Ed._ 49, 2486–2528 (2010). Article CAS
Google Scholar * Feringa, B. L. Phosphoramidites: marvellous ligands in catalytic asymmetric conjugate addition. _Acc. Chem. Res._ 33, 346–353 (2000). Article CAS PubMed Google Scholar
* Grabulosa, A. _P-Stereogenic Ligands in Enantioselective Catalysis_ 5–16 (Royal Society of Chemistry, 2011). * Imamoto, T., Kikuchi, S., Miura, T. & Wada, Y. Stereospecific reduction
of phosphine oxides to phosphines by the use of a methylation reagent and lithium aluminum hydride. _Org. Lett._ 3, 87–90 (2001). Article CAS PubMed Google Scholar * Cahn, R. S.,
Ingold, C. & Prelog, V. Specification of molecular chirality. _Angew. Chem. Int. Ed._ 5, 385–415 (1966). Article CAS Google Scholar * Prelog, V. & Helmchen, G. Basic principles of
the CIP-system and proposals for a revision. _Angew. Chem. Int. Ed._ 21, 567–583 (1982). Article Google Scholar * Reetz, M. T., Ma, J.-A. & Goddard, R. BINOL-derived monodentate
phosphites and phosphoramidites with phosphorus stereogenic centers: novel ligands for transition-metal catalysis. _Angew. Chem. Int. Ed._ 44, 412–415 (2005). Article CAS Google Scholar *
Gavrilov, K. N. et al. (_S_)-6-bromo-BINOL-based phosphoramidite ligand with _C_1 symmetry for enantioselective hydrogenation and allylic substitution. _Chirality_ 22, 844–848 (2010).
Article CAS PubMed Google Scholar * Trost, B. M., Bringley, D. A. & Silverman, S. M. Asymmetric synthesis of methylenetetrahydrofurans by palladium-catalyzed [3 + 2] cycloaddition of
trimethylenemethane with aldehydes—a novel ligand design. _J. Am. Chem. Soc._ 133, 7664–7667 (2011). Article CAS PubMed PubMed Central Google Scholar * González, A. Z. & Dean
Toste, F. Gold(i)-catalyzed enantioselective [4 + 2]-cycloaddition of allene-dienes. _Org. Lett._ 12, 200–203 (2010). Article PubMed PubMed Central CAS Google Scholar * Wiedbrauk, S.,
Bartelmann, T., Thumser, S., Mayer, P. & Dube, H. Simultaneous complementary photoswitching of hemithioindigo tweezers for dynamic guest relocalization. _Nat. Commun._ 9, 1456 (2018).
Article PubMed PubMed Central CAS Google Scholar * De Bo, G., Leigh, D. A., McTernan, C. T. & Wang, S. A complementary pair of enantioselective switchable organocatalysts. _Chem.
Sci._ 8, 7077–7081 (2017). Article CAS PubMed PubMed Central Google Scholar * Malda, H., van Zijl, A. W., Arnold, L. A. & Feringa, B. L. Enantioselective copper-catalyzed allylic
alkylation with dialkylzincs using phosphoramidite ligands. _Org. Lett._ 3, 1169–1171 (2001). Article CAS PubMed Google Scholar * van Zijl, A. W., Arnold, L. A., Minnaard, A. J. &
Feringa, B. L. Highly enantioselective copper-catalyzed allylic alkylation with phosphoramidite ligands. _Adv. Synth. Catal._ 346, 413–420 (2004). Article CAS Google Scholar Download
references ACKNOWLEDGEMENTS This work was supported financially by the Netherlands Organization for Scientific Research (NWO-CW), Foundation for Fundamental Research on Matter (FOM, a
subsidiary of NWO), the Zernike Institute for Advanced Materials, The Royal Netherlands Academy of Arts and Sciences (KNAW), The European Research Council (Advanced Investigator Grant no.
694345 to B.L.F.), Ministry of Education, Culture and Science (Gravitation program 024.001.035) and the University of Groningen. The authors thank E. Otten for the X-ray structure
experiments. AUTHOR INFORMATION Author notes * These authors contributed equally: Stefano F. Pizzolato and Peter Štacko. AUTHORS AND AFFILIATIONS * Centre for Systems Chemistry, Stratingh
Institute for Chemistry and Zernike Institute for Advanced Materials, Faculty of Mathematics and Natural Sciences, University of Groningen, AG Groningen, the Netherlands Stefano F.
Pizzolato, Peter Štacko, Jos C. M. Kistemaker, Thomas van Leeuwen & Ben L. Feringa Authors * Stefano F. Pizzolato View author publications You can also search for this author inPubMed
Google Scholar * Peter Štacko View author publications You can also search for this author inPubMed Google Scholar * Jos C. M. Kistemaker View author publications You can also search for
this author inPubMed Google Scholar * Thomas van Leeuwen View author publications You can also search for this author inPubMed Google Scholar * Ben L. Feringa View author publications You
can also search for this author inPubMed Google Scholar CONTRIBUTIONS S.F.P., P.S., J.C.M.K. and B.L.F. conceived the project. S.F.P. and P.S. carried out the experimental work. J.C.M.K. and
T.v.L. conducted the computational study. All the authors contributed to the design of the experiments, the analysis of the data and the writing of the paper. CORRESPONDING AUTHOR
Correspondence to Ben L. Feringa. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral
with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION Supplementary Methods, Figs. 1–18 and Tables 1 and
2. COMPUTATIONAL DATA 1 Cartesian coordinates. SUPPLEMENTARY DATA Crystallographic data for compound L1. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE
Pizzolato, S.F., Štacko, P., Kistemaker, J.C.M. _et al._ Phosphoramidite-based photoresponsive ligands displaying multifold transfer of chirality in dynamic enantioselective metal catalysis.
_Nat Catal_ 3, 488–496 (2020). https://doi.org/10.1038/s41929-020-0452-y Download citation * Received: 21 October 2019 * Accepted: 13 March 2020 * Published: 04 May 2020 * Issue Date: June
2020 * DOI: https://doi.org/10.1038/s41929-020-0452-y SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable
link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative