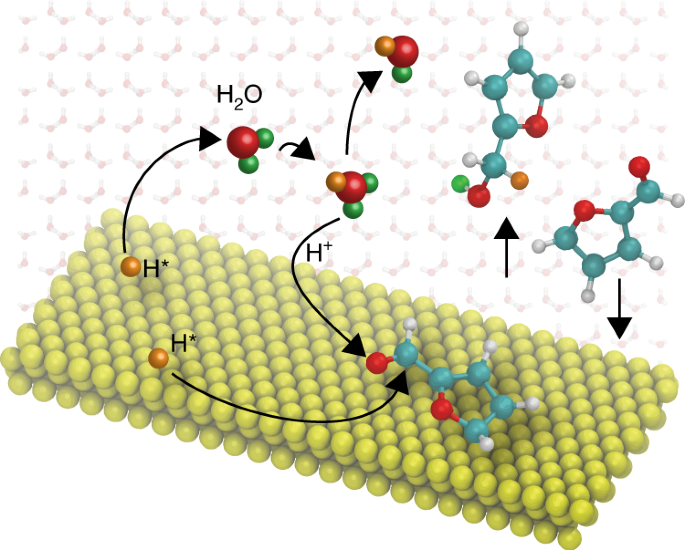
Solvent-mediated charge separation drives alternative hydrogenation path of furanics in liquid water
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Compared to the vapour phase, liquid-phase heterogeneous catalysis provides additional degrees of freedom for reaction engineering, but the multifaceted solvent effects complicate
analysis of the reaction mechanism. Here, using furfural as an example, we reveal the important role of water-mediated protonation in a typical hydrogenation reaction over a supported Pd
catalyst. Depending on the solvent, we have observed different reaction orders with respect to the partial pressure of H2, as well as distinct selectivity towards hydrogenation of the
conjugated C=O and C=C double bonds. Free energy calculations show that H2O participates directly in the kinetically relevant reaction step and provides an additional channel for
hydrogenation of the aldehyde group, in which hydrogen bypasses the direct surface reaction via a hydrogen-bonded water network. This solution-mediated reaction pathway shows the potential
role of the solvent for tuning the selectivity of metal-catalysed hydrogenation when charge separation on the metal surface is feasible. Access through your institution Buy or subscribe This
is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Access Nature and 54 other Nature Portfolio journals Get Nature+, our
best-value online-access subscription $32.99 / 30 days cancel any time Learn more Subscribe to this journal Receive 12 digital issues and online access to articles $119.00 per year only
$9.92 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout
ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS CRITICAL ROLE OF
SOLVENT-MODULATED HYDROGEN-BINDING STRENGTH IN THE CATALYTIC HYDROGENATION OF BENZALDEHYDE ON PALLADIUM Article 18 November 2021 CATALYST-FREE SELECTIVE OXIDATION OF C(SP3)-H BONDS IN
TOLUENE ON WATER Article Open access 20 July 2024 IMPACT OF HYDRONIUM IONS ON THE PD-CATALYZED FURFURAL HYDROGENATION Article Open access 22 November 2022 DATA AVAILABILITY Any data that
support the plots within this paper and other findings of the study are available from the corresponding author upon reasonable request. The following files are available in the
Supplementary Information: catalyst particle size calculations, FAL conversion and product yields in water at varying times and H2 pressures, H/D exchange experiment, derivation of rate
equations, AIMD calculations of FAL in water, atomic structures along the reaction pathway, free energy diagram for furanyl ring hydrogenation and maximum rate analysis data. REFERENCES *
Carpenter, B. K., Harvey, J. N. & Orr-Ewing, A. J. The study of reactive intermediates in condensed phases. _J. Am. Chem. Soc._ 138, 4695–4705 (2016). Article CAS Google Scholar *
Chheda, J. N., Huber, G. W. & Dumesic, J. A. Liquid-phase catalytic processing of biomass-derived oxygenated hydrocarbons to fuels and chemicals. _Angew. Chem. Int. Ed._ 46, 7164–7183
(2007). Article CAS Google Scholar * Struebing, H. et al. Computer-aided molecular design of solvents for accelerated reaction kinetics. _Nat. Chem._ 5, 952–957 (2013). Article CAS
Google Scholar * Mellmer, M. A. et al. Solvent-enabled control of reactivity for liquid-phase reactions of biomass-derived compounds. _Nat. Catal._ 1, 199–207 (2018). Article Google
Scholar * Crossley, S., Faria, J., Shen, M. & Resasco, D. E. Solid nanoparticles that catalyze biofuel upgrade reactions at the water/oil. _Science_ 327, 68–72 (2010). Article CAS
Google Scholar * Franck, J. & Rabinowitsch, E. Some remarks about free radicals and the photochemistry of solutions. _Trans. Faraday Soc._ 30, 120–130 (1934). Article CAS Google
Scholar * Madon, R. J. & Iglesia, E. Catalytic reaction rates in thermodynamically non-ideal systems. _J. Mol. Catal. A_ 163, 189–204 (2000). Article CAS Google Scholar * Mellmer, M.
A. et al. Solvent effects in acid-catalyzed biomass conversion reactions. _Angew. Chem. Int. Ed._ 53, 11872–11875 (2014). Article CAS Google Scholar * Sicinska, D., Truhlar, D. G. &
Paneth, P. Solvent-dependent transition states for decarboxylations. _J. Am. Chem. Soc._ 123, 7683–7686 (2001). Article CAS Google Scholar * Hibbitts, D. D., Loveless, B. T., Neurock, M.
& Iglesia, E. Mechanistic role of water on the rate and selectivity of Fischer–Tropsch synthesis on ruthenium catalysts. _Angew. Chem. Int. Ed._ 52, 12273–12278 (2013). Article CAS
Google Scholar * Saavedra, J. et al. Controlling activity and selectivity using water in the Au-catalysed preferential oxidation of CO in H2. _Nat. Chem._ 8, 585–590 (2016). Article Google
Scholar * Saavedra, J., Doan, H. A., Pursell, C. J., Grabow, L. C. & Chandler, B. D. The critical role of water at the gold–titania interface in catalytic CO oxidation. _Science_ 345,
1599–1602 (2014). Article CAS Google Scholar * Yoon, Y., Rousseau, R., Weber, R. S., Mei, D. H. & Lercher, J. A. First-principles study of phenol hydrogenation on Pt and Ni catalysts
in aqueous phase. _J. Am. Chem. Soc._ 136, 10287–10298 (2014). Article CAS Google Scholar * Resasco, D. E., Sitthisa, S., Faria, J., Prasomsri, T. & Ruiz, M. P. in _Solid_ _Waste_ _as
a Renewable Resource: Methodologies_ (eds Albanese, J. A. F. & Pilar Ruiz, M.) 103 (CRC Press, 2015). * Lange, J. P., van der Heide, E., van Buijtenen, J. & Price, R. Furfural—a
promising platform for lignocellulosic biofuels. _ChemSusChem_ 5, 150–166 (2012). Article CAS Google Scholar * Resasco, D. E., Wang, B. & Sabatini, D. Distributed processes for
biomass conversion could aid UN sustainable development goals. _Nat. Catal._ 1, 731–735 (2018). Article Google Scholar * Sitthisa, S. & Resasco, D. E. Hydrodeoxygenation of furfural
over supported metal catalysts: a comparative study of Cu, Pd and Ni. _Catal. Lett._ 141, 784–791 (2011). Article CAS Google Scholar * Panagiotopoulou, P., Martin, N. & Vlachos, D. G.
Effect of hydrogen donor on liquid phase catalytic transfer hydrogenation of furfural over a Ru/RuO2/C catalyst. _J. Mol. Catal. A_ 392, 223–228 (2014). Article CAS Google Scholar *
Maldonado, G. M. G., Assary, R. S., Dumesic, J. & Curtiss, L. A. Experimental and theoretical studies of the acid-catalyzed conversion of furfuryl alcohol to levulinic acid in aqueous
solution. _Energy Environ. Sci._ 5, 6981–6989 (2012). Article Google Scholar * Corma, A., Iborra, S. & Velty, A. Chemical routes for the transformation of biomass into chemicals.
_Chem. Rev._ 107, 2411–2502 (2007). Article CAS Google Scholar * Serrano-Ruiz, J. C., Luque, R. & Sepulveda-Escribano, A. Transformations of biomass-derived platform molecules: from
high added-value chemicals to fuels via aqueous-phase processing. _Chem. Soc. Rev._ 40, 5266–5281 (2011). Article CAS Google Scholar * Vorotnikov, V., Mpourmpakis, G. & Vlachos, D. G.
DFT study of furfural conversion to furan, furfuryl alcohol, and 2-methylfuran on Pd(111). _ACS Catal._ 2, 2496–2504 (2012). Article CAS Google Scholar * Pang, S. H. & Medlin, J. W.
Adsorption and reaction of furfural and furfuryl alcohol on Pd(111): unique reaction pathways for multifunctional reagents. _ACS Catal._ 1, 1272–1283 (2011). Article CAS Google Scholar *
Wang, S. G., Vorotnikov, V. & Vlachos, D. G. Coverage-induced conformational effects on activity and selectivity: hydrogenation and decarbonylation of furfural on Pd(111). _ACS Catal._
5, 104–112 (2015). Article CAS Google Scholar * Pang, S. H., Schoenbaum, C. A., Schwartz, D. K. & Medlin, J. W. Effects of thiol modifiers on the kinetics of furfural hydrogenation
over Pd catalysts. _ACS Catal._ 4, 3123–3131 (2014). Article CAS Google Scholar * Pang, S. H., Schoenbaum, C. A., Schwartz, D. K. & Medlin, J. W. Directing reaction pathways by
catalyst active-site selection using self-assembled monolayers. _Nat. Commun._ 4, 2448 (2013). Article Google Scholar * Sitthisa, S. et al. Conversion of furfural and 2-methylpentanal on
Pd/SiO2 and Pd-Cu/SiO2 catalysts. _J. Catal._ 280, 17–27 (2011). Article CAS Google Scholar * Sitthisa, S., An, W. & Resasco, D. E. Selective conversion of furfural to methylfuran
over silica-supported Ni–Fe bimetallic catalysts. _J. Catal._ 284, 90–101 (2011). Article CAS Google Scholar * Fulajtarova, K. et al. Aqueous phase hydrogenation of furfural to furfuryl
alcohol over Pd–Cu catalysts. _Appl. Catal. A_ 502, 78–85 (2015). Article CAS Google Scholar * Merlo, A. B., Vetere, V., Ruggera, J. F. & Casella, M. L. Bimetallic PtSn catalyst for
the selective hydrogenation of furfural to furfuryl alcohol in liquid-phase. _Catal. Commun._ 10, 1665–1669 (2009). Article CAS Google Scholar * Chen, X. F., Zhang, L. G., Zhang, B., Guo,
X. C. & Mu, X. D. Highly selective hydrogenation of furfural to furfuryl alcohol over Pt nanoparticles supported on g-C3N4 nanosheets catalysts in water. _Sci. Rep._ 6, 28558 (2016).
Article Google Scholar * Vaidya, P. D. & Mahajani, V. V. Kinetics of liquid-phase hydrogenation of furfuraldehyde to furfuryl alcohol over a Pt/C catalyst. _Ind. Eng. Chem. Res._ 42,
3881–3885 (2003). Article CAS Google Scholar * Lee, J. C., Xu, Y. & Huber, G. W. High-throughput screening of monometallic catalysts for aqueous-phase hydrogenation of biomass-derived
oxygenates. _Appl. Catal. B_ 140, 98–107 (2013). Google Scholar * Frainier, L. J. & Fineberg, H. H. Copper chromite catalyst for preparation of furfuryl alcohol from furfural. US
patent 4,251,396A (1979). * Villaverde, M. M., Bertero, N. M., Garetto, T. F. & Marchi, A. J. Selective liquid-phase hydrogenation of furfural to furfuryl alcohol over Cu-based
catalysts. _Catal. Today_ 213, 87–92 (2013). Article CAS Google Scholar * Singh, U. K. & Vannice, M. A. Kinetics of liquid-phase hydrogenation reactions over supported metal
catalysts—a review. _Appl. Catal. A_ 213, 1–24 (2001). Article CAS Google Scholar * Nakagawa, Y., Takada, K., Tamura, M. & Tomishige, K. Total hydrogenation of furfural and
5-hydroxymethylfurfural over supported Pd–Ir alloy catalyst. _ACS Catal._ 4, 2718–2726 (2014). Article CAS Google Scholar * Dumesic, J. A., Rudd, D. F., Aparicio, L. M., Rekoske, J. E.
& Trevino, A. A. _The Microkinetics of Heterogeneous Catalysis_ (American Chemical Society, Washington DC, 1993). * Loffreda, D., Delbecq, F., Vigne, F. & Sautet, P.
Chemo-regioselectivity in heterogeneous catalysis: competitive routes for C=O and C=C hydrogenations from a theoretical approach. _J. Am. Chem. Soc._ 128, 1316–1323 (2006). Article CAS
Google Scholar * Maroncelli, M., MacInnis, J. & Fleming, G. R. Polar solvent dynamics and electron-transfer reactions. _Science_ 243, 1674–1681 (1989). Article CAS Google Scholar *
Henriksen, N. E. & Hansen, F. Y. _Theories of Molecular Reaction Dynamics: The Microscopic Foundation of Chemical Kinetics_ (Oxford University Press, Oxford, 2018). * Kibler, L. A.
Hydrogen electrocatalysis. _Chemphyschem_ 7, 985–991 (2006). Article CAS Google Scholar * Agmon, N. The Grotthuss mechanism. _Chem. Phys. Lett._ 244, 456–462 (1995). Article CAS Google
Scholar * Cukier, R. I. & Nocera, D. G. Proton-coupled electron transfer. _Annu. Rev. Phys. Chem._ 49, 337–369 (1998). Article CAS Google Scholar * Farberow, C. A., Dumesic, J. A.
& Mavrikakis, M. Density functional theory calculations and analysis of reaction pathways for reduction of nitric oxide by hydrogen on Pt(111). _ACS Catal._ 4, 3307–3319 (2014). Article
CAS Google Scholar * Mukherjee, S. & Vannice, M. A. Solvent effects in liquid-phase reactions II. Kinetic modeling for citral hydrogenation. _J. Catal._ 243, 131–148 (2006). Article
CAS Google Scholar * Norskov, J. K., Bligaard, T., Rossmeisl, J. & Christensen, C. H. Towards the computational design of solid catalysts. _Nat. Chem._ 1, 37–46 (2009). Article CAS
Google Scholar * Zhang, L., Pham, T. N., Faria, J. & Resasco, D. E. Improving the selectivity to C4 products in the aldol condensation of acetaldehyde in ethanol over faujasite
zeolites. _Appl. Catal. A_ 504, 119–129 (2015). Article CAS Google Scholar * Kresse, G. & Furthmuller, J. Efficient iterative schemes for ab initio total-energy calculations using a
plane-wave basis set. _Phys. Rev. B_ 54, 11169–11186 (1996). Article CAS Google Scholar * Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple.
_Phys. Rev. Lett._ 77, 3865–3868 (1996). Article CAS Google Scholar * Blochl, P. E. Projector augmented-wave method. _Phys. Rev. B_ 50, 17953–17979 (1994). Article CAS Google Scholar *
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. _Phys. Rev. B_ 59, 1758–1775 (1999). Article CAS Google Scholar * Grimme, S., Antony,
J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H–Pu. _J. Chem. Phys._ 132,
154104 (2010). Article Google Scholar * Henkelman, G., Uberuaga, B. P. & Jonsson, H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. _J.
Chem. Phys._ 113, 9901–9904 (2000). Article CAS Google Scholar * Henkelman, G. & Jonsson, H. Improved tangent estimate in the nudged elastic band method for finding minimum energy
paths and saddle points. _J. Chem. Phys._ 113, 9978–9985 (2000). Article CAS Google Scholar * Henkelman, G. & Jonsson, H. A dimer method for finding saddle points on high dimensional
potential surfaces using only first derivatives. _J. Chem. Phys._ 111, 7010–7022 (1999). Article CAS Google Scholar * Campbell, C. T. & Sellers, J. R. V. The entropies of adsorbed
molecules. _J. Am. Chem. Soc._ 134, 18109–18115 (2012). Article CAS Google Scholar Download references ACKNOWLEDGEMENTS This work was supported by the US Department of Energy, Basic
Energy Sciences (grant no. DE-SC0018284). The computational research used the supercomputer resources of the National Energy Research Scientific Computing Centre (NERSC), the OU
Supercomputing Centre for Education & Research (OSCER) at the University of Oklahoma and the Tandy Supercomputing Centre (TSC). The authors thank T. Sooknoi (King Mongkut’s Institute of
Technology Ladkrabang, Thailand) for valuable discussions. AUTHOR INFORMATION Author notes * These authors contributed equally: Zheng Zhao, Reda Bababrik, Wenhua Xue. AUTHORS AND
AFFILIATIONS * Center for Interfacial Reaction Engineering and School of Chemical, Biological and Materials Engineering, The University of Oklahoma, Norman, OK, USA Zheng Zhao, Reda
Bababrik, Nicholas M. Briggs, Dieu-Thy Nguyen, Umi Nguyen, Steven P. Crossley, Bin Wang & Daniel E. Resasco * Department of Physics and Engineering Physics, The University of Tulsa,
Tulsa, OK, USA Wenhua Xue, Yaping Li & Sanwu Wang Authors * Zheng Zhao View author publications You can also search for this author inPubMed Google Scholar * Reda Bababrik View author
publications You can also search for this author inPubMed Google Scholar * Wenhua Xue View author publications You can also search for this author inPubMed Google Scholar * Yaping Li View
author publications You can also search for this author inPubMed Google Scholar * Nicholas M. Briggs View author publications You can also search for this author inPubMed Google Scholar *
Dieu-Thy Nguyen View author publications You can also search for this author inPubMed Google Scholar * Umi Nguyen View author publications You can also search for this author inPubMed Google
Scholar * Steven P. Crossley View author publications You can also search for this author inPubMed Google Scholar * Sanwu Wang View author publications You can also search for this author
inPubMed Google Scholar * Bin Wang View author publications You can also search for this author inPubMed Google Scholar * Daniel E. Resasco View author publications You can also search for
this author inPubMed Google Scholar CONTRIBUTIONS Z.Z. conducted material synthesis, reaction tests and the H/D exchange experiment. R.B. completed the DFT calculations, the free energy
calculations and the micro kinetic analysis. W.X., Y.L. and S.W. performed the DFT calculations. N.M.B. and S.P.C. conducted the catalyst characterization and analysed the data. D.-T.N. and
U.N. performed the AIMD calculations. All authors discussed the results and commented on the manuscript. B.W. and D.E.R supervised the project. CORRESPONDING AUTHORS Correspondence to Bin
Wang or Daniel E. Resasco. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE: Springer Nature remains neutral with
regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION Supplementary Figures 1–17, Supplementary Table 1,
Supplementary Methods, Supplementary Notes 1–4, Supplementary References SUPPLEMENTARY DATA 1 DFT structure of FAL*+H* on Pd in H2O SUPPLEMENTARY DATA 2 AIMD simulation of FAL at the
water/Pd interface RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Zhao, Z., Bababrik, R., Xue, W. _et al._ Solvent-mediated charge separation drives
alternative hydrogenation path of furanics in liquid water. _Nat Catal_ 2, 431–436 (2019). https://doi.org/10.1038/s41929-019-0257-z Download citation * Received: 26 June 2017 * Accepted: 20
February 2019 * Published: 01 April 2019 * Issue Date: May 2019 * DOI: https://doi.org/10.1038/s41929-019-0257-z SHARE THIS ARTICLE Anyone you share the following link with will be able to
read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing
initiative