Nematode controlling effects and safety tests of Duddingtonia flagrans biological preparation in sheep
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
Duddingtonia flagrans is a nematode-trapping fungus that is widely used to control parasitic nematodes in livestock. After oral ingestion and passage through the digestive tract of animals,
this microorganism captures nematodes in feces. Although many researchers have examined the safety of this fungus for humans, animals, and the environment, few reports have discussed the
safety of nematode-trapping D. flagrans biologics for animals. In this study, D. flagrans safety was tested, while adverse effects and toxicities were examined in sheep. First, the nematode
killing effects in naturally parasitized sheep after administration of lyophilized D. flagrans preparations were tested.lyophilized D. flagrans preparations were administered to sheep at
various doses, followed by key blood factor monitoring and an examination of major tissues, organ lesions, and pathology. Lastly, lyophilized D. flagrans preparations were administered to
sheep at various doses, followed by key blood factor monitoring and an examination of major tissues, organ lesions, and pathology. the nematode killing effects of naturally parasitized sheep
after administration were tested. The results demonstrated that treatment with D. flagrans isolates significantly reduced developing larvae numbers in feces, with an efficiency of 92.99%.
Lyophilized preparations had no observable effects on physiological parameters in sheep, thus indicating a wide safety range in target animals, with potentially minimal risks in veterinary
clinical practice. Overall, D. flagrans freeze-dried biologics effectively helped to controlled parasitic infections, which are safe in animals like sheep, and thus may provide a practical
platform for nematode-trapping fungi in veterinary clinical settings.
Currently, to biologically control parasites in livestock, anthelmintic drugs are widely applied but the misuse of these drugs has led to serious resistance issues; therefore, new control
measures must be researched and developed1. One of the most important approaches is the exploitation of natural enemies, including predatory fungi that prevent and control parasites2.
Predatory fungi are widely distributed in soils and exist in many ecosystems, from tropical areas to cold Antarctica and from terrestrial to aquatic ecosystems3.
Based on their discovery, considerable research efforts have been directed toward predatory fungi, their predatory properties, biochemical mechanisms, and clinical applications4. For
example, the nematode-predatory fungus Duddingtonia flagransgenerates large numbers of thick-walled spores, which can pass through animal digestive tracts without inactivation in clinical
use, and then germinate in feces5. With recognized predatory roles, D. flagrans then targets a variety of parasitic nematodes, such as Osertagia spp., Nematodirus, and Strongyloides
stercoralis. More recently, Mendes et al. demonstrated that a solution of D. flagransconidia was effective in vitro against gastrointestinal nematodes in buffaloes when used alone and/or in
association with ivermectin6. In Voinot et al., the daily administration of nutritional pellets enriched with M. circinelloides and D. flagrans spores, for a prolonged interval (2 years),
prevented helminth infection (C. daubneyiand gastrointestinal nematodes) in dairy cattle under rotational grazing7. Critically, predatory activities increase with increased larvae numbers,
thus the fungus has broad application values8,9,10,11,12. However, there is a dearth of information on predatory fungi safety profiles in target animals13. In a recent study, edible gelatins
were desiccated and tested on captive bison maintained in a zoological garden under continuous pasturing. Lyophilized D. flagranspreparations had no effects on physiological parameters in
bison14. Recently, Braga et al.15 demonstrated the efficacy and safety of Bioverm®, a commercial product based on D. flagrans chlamydospores (AC001), available in Brazil for the integrated
control of helminth infections in farm animals. Despite the scientific efficiency and proven safety of D. flagrans(AC001) by oral administration, no reports have yet assessed possible
harmful effects during gastrointestinal transit. Long-term clinical and anatomical-pathological studies on heifers fed parasitic fungal pellets on a daily basis were performed and showed no
side-effects or specific lesions. Moreover, the risk of heifers being infected with trematodes was reduced and their health was improved16.
Duddingtonia flagrans has been positively developed as a biological agent in the United States, Sweden, and New Zealand for controlling parasitic nematodes15. Biological control with the
predacious fungi D. flagrans still remains a promising free-living parasite regulator alternative for use in livestock17. To control ruminant parasitic nematodes, several studies examining
D. flagrans as a biological control agent have shown excellent results18,19. The main advantages of D. flagrans include fewer resistance problems, a fully natural product, and
environmentally friendly. To characterize potential primary and clinical applications, predatory D. flagranssafety profiles were examined in our study in target animals, with a view to their
becoming widely accepted lyophilized biological agents1. Our study lays the foundation for future clinical applications and commercial production, and provides a reference for future
in-depth predatory fungi studies.
A test strain of D. flagrans strain CIM1 (NCBI bio-sample accession number: SAMN05504105), was obtained from the Veterinary Parasite Laboratory of the Inner Mongolia Agricultural University,
Hohhot, Inner Mongolia, China. D. flagrans was inoculated onto Potato Dextrose Agar (PDA) (Beijing Landbridge, China) medium until the mycelium covered the dish surface16, then it was cut
into 0.5 cm × 0.5 cm squares, added to a new dish, and incubated for 1 week at 25 °C. Next, the mycelium was cut into 0.5 cm × 0.5 cm squares and transferred to corn kernel medium at 25 °C
for 3 weeks. Spore eluate was prepared by adding 1 mL Tween-80 to 500 mL of water, and then heating it to 121℃ for 15 min. Spores were eluted several times from the medium using spore eluent
and then filtered on an ultra-clean bench. Samples then underwent vacuum freeze drying until a dry powder was obtained. We determined chlamydospore counts in the lyophilized formulation to
be 2.13 × 108 chlamydospores/g. The Bottle were then sealed with sealing film, and stored at 4℃.
Experimental sheep (n = 30) were naturally infected with gastrointestinal nematodes on farms in Siziwang Banner, Ulanqab, Inner Mongolia, China, and had not been previously dewormed within 6
months of the trial. The Animal Experimentation Ethics Committee followed Chinese National Standard Laboratory Animal-Guidelines for the ethical review of animal welfare (GB/T 35892 −
2018). All animal protocols were reviewed by the Animal Experiments Ethics Committee at Inner Mongolia Agricultural University.
The animal safety test site was located in the animal house of Inner Mongolia Agricultural University. 3–4 month old sheep were selected (n = 9). At 10 days before the trial, albendazole
(99% purity; Kangmu Animal Pharmaceutical, China) was orally administered once. Ivermectin (99% purity; Kangmu Animal Pharmaceutical, China). was also subcutaneously injected for 3
consecutive days according to body weight, to ensure the absence of helminth infections.
Thirty Small Tailed Han Sheep, 6 ~ 8 months old, and approximately 50 kg were randomly selected. Rectal feces were collected and examined for nematode infection. Ten sheep with a similar
number of EPG infections were divided into groups, including ivermectin, D. flagrans biologic, and blank control groups. The D. flagrans group dose was 1 × 106chlamydospores/kg body weight
(bw)20. Egg numbers per gram (EPG) and larvae per gram (LPG) of feces were measured and collected before and 1 week after the trial. Eggs in feces were counted using a modified McMaster
technique to calculate EPG. Fecal samples were incubated at a constant temperature of 25 °C for 15 days. After this period, third-stage non-predatory larvae (L3) were isolated using the
modified Baermann’s method21. Mean EPG and LPG values in groups were compared before and after dosing to determine D. flagrans effectiveness in controlling parasites.
Nine sheep with an average body weight = 18 kg were randomly divided into 5-fold dose (5 × 106 chlamydospores/kg bw), 10-fold dose (1 × 107 chlamydospores/ kg bw), and blank control groups.
Each group consisted of three sheep, and corresponding doses were applied based on bw. Suspensions were administered as a single dose on an empty stomach by perfusion with a syringe.
During trials, multiple key parameters were observed in sheep every day, including behavioral disorders, food and drink appetite, jaundice, respiratory rate, cough, neurological signs,
diarrhea, edema, and developmental status. Behavioral disorders referred to excitement, depression, restlessness, and other abnormalities. Defecation represented constipation and diarrhea.
During trials, sheep were fed high-quality alfalfa three times a day (morning, noon, and night).
Oral D. flagrans biological effects on body mass and weight gain were examined by weighing sheep before the trial and at days3, 7, 15, and 30 after dosing. Measurements were taken between 9
am and 10 am. on an empty stomach.
Blood samples were collected for routine blood tests at 3 days before the trial and then at days15 and 30 after dosing. The following biomarkers were measured using an automatic animal blood
cell analyzer: WBC, Lym, Mon, Neu, Eo, Ba, RBC, MCV, Hct, MCHC, Hb, and MPV.
At study end, all test sheep were euthanized according to ethical regulations. Euthanasia was conducted by intravenously injecting animals with phenobarbital (90 mg/kg body weight). This was
formulated in accordance with Laboratory Animal-Guidelines for Euthanasia by the People’s Republic of China (GB/T39760-2021). The sheep dissected, and the abdominal cavity cut open to check
for fluids or blood in the cavity. All soft ribs on the left side were cut using bone cutters, and ribs on left and right sides were broken by hand to expose the entire thoracic cavity to
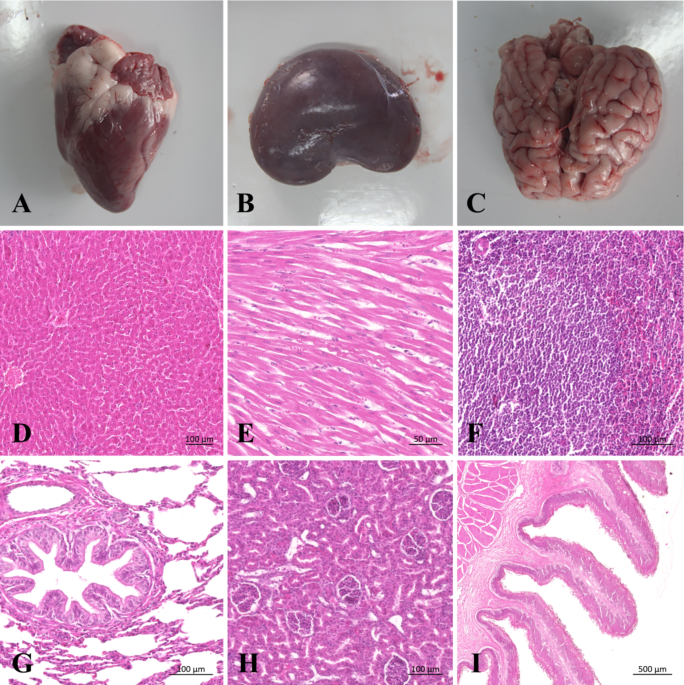
observe pleural colors and bleeding/adhesions. The following organs and tissues were checked by visual inspection: brain, cerebellum, kidney, heart, pancreas, liver, spleen, lung, stomach,
ileum, jejunum, colon, cecum, and lymph nodes. All gross pathological lesions were photographed and recorded. The heart, liver, spleen, lungs, and kidneys were then taken fur further
examinations. Excess connective tissue around organs was carefully peeled away and surface fluids blotted with filter paper before weighing and recording data. To calculate organ
coefficients, the following equation was used: organ weight (g)/body weight (g) × 100%.
Experimental data were processed as the mean ± standard deviation, and ANOVA was performed using IBM SPSS Statistics 22 software. Data were analyzed for normal distributions using
Kolmogorov–Smirnov and Levene’ s tests. If variance was unevenly distributed, equivalent non-parametric tests (primarily Kruskal–Wallis analysis of variance) were used. A P