A randomized controlled trial combining house screening and insecticide-treated nets reduces malaria transmission in northwestern ethiopia
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT House screening (HS) of doors, eaves, and windows using wire-mesh has demonstrated potential in the integrated vector management of malaria. However, limited epidemiological data
are available to guide its implementation across different ecological settings. In a 16-month randomized controlled trial (follow-up period) conducted across three agroecological areas (dry
mountain, plateau highland, and semi-arid) in Jabi Tehnan district, northwestern Ethiopia, treatment houses were equipped with HS combined with insecticide-treated nets (ITNs), while control
houses received ITNs only. The intervention led to a significant 2.3-fold reduction in indoor malaria vector density, the primary entomologic outcome, largely influenced by _An. gambiae_
s.l. mosquitoes. Fewer blood-fed mosquitoes were found in screened houses, indicating reduced human bites, which translated to six-fold decline in malaria prevalence (0.7%), the primary
epidemiologic outcome, compared to control houses (4.3%). In contrast, _Plasmodium_ sporozoite infection rates showed no differences between screened and control houses or agroecological
zones, with _An. arabiensis_ and _An. funestus_ s.l. identified as the primary vectors. A modest protective effectiveness (22.6%) was observed, based on the estimated entomological
inoculation rate of 0.24 and 0.31 infectious bites/person/night in screened and control houses, respectively, with no variation by agroecology. Despite the synergistic impact of HS with
existing ITNs in reducing vector densities, human bite rates, and household malaria prevalence, sustained transmission persisted, partly due to the presence of highly competent vectors such
as _An. funestus_ s.l. which had an overall sporozoite rate of 68%. Future research should explore the interactions between vector behavioral adaptations, ecological and social factors
contributing to residual transmission, even with seemingly effective control measures. SIMILAR CONTENT BEING VIEWED BY OTHERS EXPLORING ALTERNATIVE INSECTICIDE DELIVERY OPTIONS IN A “LETHAL
HOUSE LURE” FOR MALARIA VECTOR CONTROL Article Open access 24 March 2023 PARTIAL INDOOR RESIDUAL SPRAYING WITH PIRIMIPHOS-METHYL AS AN EFFECTIVE AND COST-SAVING MEASURE FOR THE CONTROL OF
_ANOPHELES GAMBIAE_ S.L. IN NORTHERN GHANA Article Open access 10 September 2021 A RANDOMIZED, DOUBLE-BLIND PLACEBO-CONTROL STUDY ASSESSING THE PROTECTIVE EFFICACY OF AN ODOUR-BASED
‘PUSH–PULL’ MALARIA VECTOR CONTROL STRATEGY IN REDUCING HUMAN-VECTOR CONTACT Article Open access 11 July 2023 INTRODUCTION Malaria is a deadly public health challenge, particularly in
sub-Saharan Africa (SSA), which bears the brunt of the global malaria burden (94%); the disease contributes to a vicious cycle of poverty, underdevelopment, illness, and death1. According to
the WHO, globally, nearly 233 million people are affected by malaria of which 580 000 die, each year, mostly children under the age of five years1. Conventional preventive strategies using
insecticide-treated nets (ITNs) and indoor residual spraying (IRS) have been instrumental in controlling malaria worldwide, leading to a 60% reduction in the number of deaths, from 2000 to
2015 together2. However, since 2015, the progress in malaria control has stalled and gains made are now slowly being reversed, with increased disease prevalence which is evident in some
malaria-endemic areas of SSA. Increasing insecticide resistance, changes in the behavior of primary vectors, the spread of cryptic vectors3,4,5 and invasive species (e.g., _Anopheles
stephensi_)6, and climate change and variation are among the major threats to effective malaria control and barriers to elimination efforts7. Additional vector control measures are needed to
combat the persistent malaria problem, which can be implemented within the framework of integrated vector management (IVM), as highlighted in the WHO Global Technical Strategy for Malaria
2016–308. House screening (HS) ─the use of fine wire-mesh screens to cover house doors, windows, and eaves, is among the promising approaches that can be adapted at the local level for
effective and sustainable management of malaria9,10. Historically, house screening contributed to the eradication of malaria outside Africa11,12, yet it remains an under-utilized approach to
control contemporary malaria13. The use of HS reduces man–vector contact by preventing people from the bites of malaria vectors that mainly occur indoors at night when in or before they go
to bed. It represents a cheaper version or practical shortcut to house improvements to limit malaria infection14. Recent studies have demonstrated the reduction in vector densities and
_Plasmodium_ infection rates, and the epidemiologic impact (e.g. decline in malaria parasite prevalence and anaemia) of HS on malaria15,16,17. These studies highlight the importance of HS as
a supplementary tool for IVM, with added environmental value11. Indeed, untreated screens are environmentally friendly and do not require the use of chemicals that can induce resistance or
behavioral changes in disease vectors. Additional benefits include less frequent replacements, ease of adoption, provision of protection to the entire household, and even during the day
(when people are indoors but not in bed). Despite the proven effectiveness of HS against malaria, there is a lack of data to define eco-epidemiological settings where HS can be most
effectively deployed. The WHO malaria prevention guidelines emphasize the need for further research on the impact of HS across different vector species, as variations in their behavioral
divergence can significantly influence intervention outcomes18. Additionally, given the potential influence of local vector dynamics, variations in performance are expected if other local
and environmental conditions affect the trial. In this study, we hypothesize that HS can significantly reduce human-vector contact and mosquito entry, leading to decreased asymptomatic
parasite prevalence and residual malaria transmission intensity among district inhabitants. By supplementing existing vector control measures, HS may prove effective across three
agroecological zones with distinct malaria transmission dynamics and varying vector species compositions. To test this hypothesis, we conducted a randomized control trial (RCT) in the Jabi
Tehnan district, northwestern Ethiopia, using empirical data to evaluate the entomologic and epidemiologic impacts on malaria prevalence and transmission. Ethiopia is among the five
countries with the highest malaria burden globally, recording more than 1.3 million cases between 2021 and 20221. The RCT compared house screening on doors, windows and eaves, combined with
ITNs (intervention) to control houses that received only ITNs. The trial spanned three agroecological areas — dry mountain, plateau highland and semi-arid, to assess whether the
effectiveness of HS varies by agroecology. Ultimately, our study aimed to evaluate the role of HS in mitigating residual malaria transmission in the district and to provide insights into its
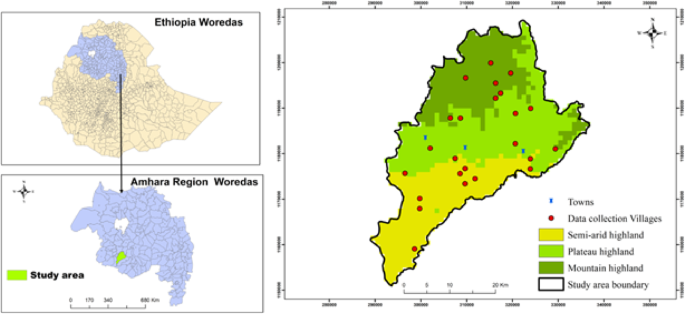
potential contribution to strengthening existing vector control strategies. METHODOLOGY STUDY AREA The study was carried out in the Jabi Tehnan district (coordinates: 37.074° − 37.508°E;
10.405° − 10.945°N), situated within the West Gojjam Zone, Amhara Regional State of Northwestern Ethiopia (Fig. 1). The altitude of this subtropical dry area (semi-arid) is between 1300 and
2330 m above sea level (asl), and its topography is largely composed of plain (65%) and mountainous (15%) highlands. Mean daily temperatures range from 15 to 20 °C, and a long rainfall
season of about three months (mid-June to mid-September), with an annual precipitation of 1356–1720 mm19. There are 41 villages in the district, with an estimated human population of over
228,351, distributed in 45,827 rural and 7,927 urban households20. The primary economic activity of the local population in the district is small-scale mixed farming, comprised of crop and
livestock production9,19. Malaria transmission is bimodal- the peak season occurs after the heavy rains (June to September) and harvest period (Autumn), and it lasts between September and
December; the second peak occurs during the short rainy season (Spring), between April to June21. There are about six streams that flow from north to south, dividing the district
longitudinally at different distances from east to west. The plateau highland and semi-arid areas have partly irrigated arable land during the dry season which fuels the malaria incidence
and is sustained throughout the dry months of January and February9. The district is divided into three zones, the major ones being semi-arid, plateau highlands, and dry mountain highlands
(Fig. 1), classified using QGIS Geographic Information System (V3.30.0) software with a 30-meter resolution digital elevation model which was obtained from the United States Geological
Survey. STUDY DESIGN This interventional study which was conducted in Jabi-Tehnan district, Northwestern Ethiopia, was part of the larger project work for a Randomized Controlled Trial (RCT)
on the combined impact of ITNs (DuraNet® impregnated with the pyrethroid alpha-cypermethrin, Shobikaa Impex Pvt Ltd., Karur, Tamil Nadu 639,006, India), house screening, and pull-push
technology for improved malaria control and livelihoods in rural Ethiopia9,22. The trial registration number Trial ID = 11,101, has previously been documented at the Pan African Clinical
Trials Registry, PACTR202006878245287 on 24/06/20209. The reconnaissance assessment found that 50% of households had at least one child aged ≤ 14 years in each treatment9,22. We first
prepared a list of 246 households for this study which included households that were similar, typically with mud-plastered walls, doors and windows made of furnished wood or metal sheet, and
corrugated iron roofs. Then, we randomly selected 123 homes to receive ITNs plus screened their houses with fine wire mesh (doors, eaves, and windows). The remaining 123 households were
assigned to the control group and received ITNs only. Notably, in this study, all the selected households received ITNs. The randomization study also considered differences in agroecological
zones: dry mountain, plateau highland, and semi-arid areas. To enhance the randomization process, we stratified households by agroecological zone (dry mountain, plateau highland, and
semi-arid) to ensure balanced allocation of intervention and control groups. Within each zone, households were further randomized to receive either ITNs plus house screening (_n_ = 123) or
ITNs only (_n_ = 123). Given the potential influence of household characteristics on malaria risk, we ensured that selected homes were similar in structure (mud-plastered walls, wooden or
metal doors, and corrugated iron roofs). Malaria prevalence was estimated by employing active case detection during the following malaria transmission season post-interventions from
asymptomatic house occupants. We conducted baseline epidemiological assessment before the intervention as presented in Asale et al.23. Most respondents (> 60%) kept cattle in a separate
area from humans dwellings, whereas others shared it with cattle9. DATA COLLECTION The Centers for Disease Control and Prevention (CDC) miniature light traps (Model 512, John W. Hock,
Gainesville, FL, USA), were used to collect indoor host-seeking mosquitoes bi-monthly, targeting two sampling rounds per season (autumn, dry, short and long rainy seasons) from July 2020 to
October 2021, for a total of 8 rounds (sessions). Mosquito collection was performed for one night per household per sampling session. Each sampling session included control and intervention
households in each of the three agroecological zones, providing comprehensive ecological coverage. Efforts were made to equilibrate the number of households in the control and intervention
for trap setting. In each house, traps were positioned close to the sleeping quarters, approximately one meter above the floor. During each trapping session, sets of randomly selected
residences were targeted to reduce the bias influenced by external factors. These included environmental conditions and human activities that might be unique to certain houses.
Epidemiological assessments for _Plasmodium_ infection were performed ten-months post-intervention using Rapid Diagnostic Tests (RDTs; The CareStart™ Malaria HRP2/pLDH COMBO Test, Access Bio
Inc., USA) on selected asymptomatic household members, including children, school-aged individuals, and adults. Household member selection followed a predefined approach primarily focused
on children, and if unavailable, a school-age or adult family member from the selected household was included. The intervention was assessed over a 16-month follow-up period after the
construction of house screening (HS). The primary entomologic outcome variable was indoor anopheline mosquito density. Secondary outcome variables including _Plasmodium_ infection rates were
used to estimate the entomological inoculation rates (EIRs), as a measure of the protective effectiveness of the HS. Other entomologic variables compared between screened and control houses
included mosquito richness and host feeding patterns. _Plasmodium_ parasite prevalence, as the primary epidemiologic outcome data, was estimated at the household across different age groups
using RDTs. MOSQUITO IDENTIFICATION AND PROCESSING Established taxonomic keys were used to identify mosquitoes microscopically (Coetzee, 2020). They were then grouped as unfed, blood fed,
half gravid, or gravid24. The head/thorax of individual adult female mosquitoes were extracted using Genomic DNA (gDNA) based on the Genomic DNA ISOLATE II Kit (Bioline, Meridian Bioscience,
Germany). We used PCR (Polymerase Chain Reaction) to detect _Plasmodium_ DNA in the individual mosquito samples, as described previously by Belay et al.3. This encompassed multiple gene
regions for _Plasmodium_ infection screening, including markers targeting mitochondrial and ribosomal regions, as well as single copy gene glutathione reductase25. Positive mosquitoes were
scored as positive for sporozoites. Further confirmation of a representative detected isolates was ensured by Sanger sequencing. We identified the sibling species in the _An. gambiae_
complex using PCR26. We then processed the DNA which was extracted from the abdomen of individually engorged anophelines by PCR and sequenced them to identify vertebrate blood meal
sources3,27. DATA ANALYSIS Female anopheline per trap was counted (i.e., mosquito densities). Densities for total anopheline and the predominant species, _An. gambiae_ s.l. and _An.
funestus_ s.l. were analysed separately using Generalized linear mixed models (GLMMs), with negative binomial distribution. Agroecological areas, season (autumn, dry, short and long rainy
seasons) and treatment houses (intervention vs. control) were included in the model as fixed effects and collection months (i.e. collection rounds) as random effect. The effect of the
intervention on the captures of blood-fed mosquitoes were similarly compared using GLMM with negative binomial distribution. GLMMs were implemented with the _lme_ package. We computed the
pair-wise comparisons of mean densities between the agroecological areas and seasons using the package _emmeans_ with an adjusted _p_-value based on Tukey test28. Further, we run a logistic
binomial regression model to determine whether intervention effectiveness affected malaria prevalence by age group and agroecology. A similar model was performed to investigate the effect of
the intervention on _Anopheles Plasmodium_ infection prevalence; treatment and agroecology were included as explanatory variables with quasibinomial error distribution to adjust for
overdispersion of the data. Bovine blood index (BBI) and human blood index (HBI) were estimated as the number of positive specimens of the total number analysed for a chosen species. The EIR
(total sporozoite-positive mosquitoes by PCR/total samples screened) × (total anopheline catch/total CDC trapping efforts) was estimated as described previously29. All statistical analyses
were carried out in R v. 4.2.128 and results considered significant at _p_ ≤ 0.05. RESULTS HOUSE SCREENING SUPPRESSES INDOOR _ANOPHELES_ DENSITY AND SPECIES RICHNESS A total of 1,672
anopheline mosquitoes were collected throughout the 16-month follow-up survey, comprising 504 and 1,168 anophelines in screened and control households, respectively. The predominant mosquito
species found in the traps was _Anopheles gambiae_ s.l. (_n_ = 1,224, 73.2%), out of 21 species collected, followed by _An. funestus_ s.l. (_n_ = 130, 7.8%). There was a two-fold
significant reduction in indoor densities of total anophelines in screened relative to control houses (z =−5.95, _p_ < 0.0001). A similar trend was observed for _An. gambiae_ s.l. The
intervention had no impact on the densities of _An. funestus_ s.l., which was recorded in low numbers (Table 1; Fig. 2). There was no significant interaction between agroecology and
intervention in influencing the densities of total anophelines (F value = 2.60, _p_ > 0.05), _An. gambiae_ s.l. (F value = 2.25, _p_ > 0.05) and _An. funestus_ s.l. (F value = 0.23,
_p_ > 0.05). This suggests that agroecology had no measurable impact on HS on mosquito densities. However, there was a discernible decline in the anopheline species richness, with only 13
species identified in treatment houses, and 19 species identified from the traps in the control houses (Supplementary Fig. S1 online). The densities of total anophelines, _An. gambiae_ s.l.
and _An. funestus_ s.l. varied by season and agroecology (Table 1; Fig. 3). Total anopheline densities were significantly higher in the semi-arid (3 mosquitoes/trap) than in the dry
mountain (1.5 mosquito/trap) (_p_ = 0.04) and plateau highlands (1.9 mosquito/trap) (_p_ = 0.02), with no significant difference between dry mountain and plateau highland (_p_ = 0.99). A
similar trend was mirrored by _An. gambiae_ s.l. The occurrence of _An. funestus_ s.l. was generally low, with variations in capture rates influenced by both season and agroecology.
Seasonally, total anopheline abundance differed significantly between autumn and the long rains (_p_ = 0.02) and short rains (_p_ < 0.0001) but not the dry season (_p_ = 0.99).
Significant differences were also observed between the dry season and the long rains (_p_ = 0.045) as well as the short rains (_p_ = 0.0005), though no difference was found between the long
and short rain seasons (_p_ = 0.75). The abundance of _An. funestus_ s.l. varied seasonally, with significant differences between autumn and the short rains (_p_ < 0.0001) and between the
long and short rain seasons (_p_ = 0.003). HOUSE SCREENING IMPACTS BLOOD-FEEDING RATES AND HOST RANGE OF _ANOPHELES_ MOSQUITOES The mosquito captures recorded 339 blood-fed anophelines,
representing 20.3% (339/1672) of total captures. Of these, 83.5% (_n_ = 283) were from the control houses, with 16.5% (_n_ = 56) recorded from the screened houses. PCR analysis revealed weak
or no band on the gel for 35 samples which could not be processed further. On the other hand, 253 (74.6%) engorged _Anopheles_ were successfully genotyped for host blood DNA out of the
remaining 304 samples. Of the sequenced samples, 211 (83.4%) were from the control houses and 42 (16.6%) from the intervention houses. The HS intervention significantly reduced blood feeding
based on number of blood-fed mosquito captures compared to those in control traps (56 vs. 283; z-value= −8.21, _p_ < 0.0001). Cattle emerged as the primary blood source for anophelines,
with BBI of 73.5% (186/253), followed by the human host (HHI = 12.6%, 32/253). In the control houses, the HBI and BBI were 10.9% (23/211) and 76.3% (161/211), respectively. Surprisingly,
screened houses had higher rates of HBI and BBI of 19.0% and 59.5%, respectively, than the control. _Anopheles gambiae_ s.l. had the largest proportion of blood-fed individuals (_n_ = 256,
76%) among all species. The number of blood-fed _An. gambiae_ s.l. was five times lower in the intervention households (_n_ = 41; 16.0%) compared to control households (_n_ = 215;83.9%).
Most of the engorged _An. gambiae_ s.l. (44%) were caught in the semi-arid (_n_ = 113), whereas 28% were found each in the dry mountain (_n_ = 73) and plateau highland (_n_ = 70) zones.
Regardless of the HS status, the HBI was higher in the semi-arid and plateau highlands, than in the dry mountain households in both groups. However, proportionally, human biting was higher
in the screened houses (HBI = 19.1%, 8/42) than in control houses (HBI = 10.9%, 23/211). On the other hand, the BBI in the intervention, 59.5% (25/42) was lower than in the control houses
76.3% (161/211). The intervention reduced the species richness of blood-fed anopheline, with only 8 species observed in intervention households compared to 19 species in the control
households (Fig. 4). _ANOPHELES_ INFECTION PREVALENCE AND ENTOMOLOGICAL INOCULATION RATE The detection of _Plasmodium_ sporozoite was performed on a subset of 428 mosquito samples,
comprising 253 blood-engorged and 175 gravid specimens. The control houses had 362 (engorged + gravid), whereas the intervention houses had 66 (engorged + gravid). A total of 11.2% (_n_ =
48) of these mosquitoes tested positive for _Plasmodium_ sporozoite infection. Of these, the intervention houses had a higher parasite rate (18.2%; 12/66) than the control houses (9.9%;
36/362). Among all examined anopheline species, 93.8% of infections were attributed to the two predominant species: _An. gambiae_ s.l. (_n_ = 27, all _An. arabiensis_) and _An. funestus_
s.l. (_n_ = 18). The remaining infections (_n_ = 3) were each found in a single specimen of _An. pharoensis_,_ An. cinereus_, and _An. natalensis_ (Table 2). The intervention did not
significantly reduce the mosquitoes infected with sporozoites (F-test = 3.3, df = 1, _p_ = 0.09), and there was no significant variation induced by agroecological area (F-test = 1.74, df =
2, _p_ = 0.18). The most frequent parasite detected was _Plasmodium falciparum_, infecting 95.8% (_n_ = 46) of mosquitoes, with only two mosquitoes infected with _P. vivax_. The
entomological inoculation rate (EIR) was 0.31 infectious bites per person per night (ib/p/night) in control houses and 0.24 ib/p/night in intervention houses, representing a 22.6% reduction
in infectious bites (0.24/0.31 = 77.4% protection) (Table 3). The control and intervention EIRs of 0.29 and 0.31 ib/p/night, respectively by different agroecological zones were not
significantly different between dry mountain and semi-arid environments. In contrast, the intervention provided complete protection in the plateau highland agroecology, where EIR was 0.32
ib/p/night in the control houses but zero in intervention houses. EPIDEMIOLOGICAL ASSESSMENT OF HS INTERVENTION Epidemiological assessment was conducted on a total of 1,490 individuals from
control (_n_ = 1053) and intervention (_n_ = 437) households using RDTs. The overall malaria prevalence in control households was 4.3% (45/1053), 58.3% _P. falciparum_ (_n_ = 27/45), 39.5%
_P. vivax_ (_n_ = 17/45), and 2.2% mixed infections (_n_ = 1/45), and was significantly higher than that found in the intervention households (0.7%, 3/437; χ2 = 8.4, df = 1, _p <_ 0.004).
The prevalence rate for _P. falciparum_ was 2.6% vs. 0.5% and 1.61% vs. 0.5% for _P. vivax_ in the control and intervention houses, respectively. The intervention significantly reduced (~
6-fold) the malaria prevalence among household members (OR = 0.16;95% CI:0.04–0.46; _p_ = 0.003). This suggests an 84% reduction in the odds of malaria in intervention households compared to
control households. The intervention had a significant impact on individuals older than 14 years, with ~ 3-fold reduction in malaria risk (Table 4). This age group comprised 70.8% of the
surveyed population (control = 720, intervention = 336). Gender was not a significant predictor of malaria risk (Table 4), and malaria prevalence did not vary by agroecology (Table 4).
DISCUSSION Our results demonstrate that HS, when combined with ITNs, has both entomological and epidemiological impacts on malaria transmission. Notably, the intervention resulted in a
significant two-fold reduction in indoor densities of host-seeking anophelines, accompanied by a decline in anopheline species richness. Much of this reduction was driven by _An.
arabiensis_, the primary malaria vector in Ethiopia3,30,31. The protective effectiveness of HS showed an overall 22.6% reduction in estimated EIR. Additionally, there was a notable decrease
in the number of blood fed mosquitoes, indicating reduced biting activity. Collectively, these effects translated into a six-fold reduction in malaria prevalence in screened houses compared
to control houses. Previous studies have shown that HS is associated with a decrease in indoor malaria vector abundance and/or malaria transmission rates, as measured by EIR, in rural
southeastern Zambia32, western Kenya33, and coastal Tanzania12. Furthermore, the positive impact of HS has been documented in reducing human infection prevalence12,33, malaria incidence15,
and anaemia in children16. Our study provides additional evidence of the synergistic effect of HS with existing ITN-based control strategies, highlighting its potential to enhance malaria
elimination efforts. Importantly, using insecticide-free screens, as in the current study, offers a sustainable malaria control strategy that is not susceptible to the physiological
resistance observed in insecticide-based interventions such as ITNs and IRS. Our findings suggest that the observed reduction in vector densities may reflect decreased mosquito-human contact
or reduced biting rates. Few bites resulted in a low rate of engorged mosquitoes, which may explain the overall lower infection prevalence in humans. While this may provide strong support
for HS as an effective household-level intervention18, it had only minimal effect on mosquito _Plasmodium_ positivity rates. This indicates a non-linear relationship between human parasite
prevalence and estimated EIR. Several factors may contribute to the observed lack of effect on parasite transmission. First, mosquito infectivity likely represents an indirect,
community-level effect of HS, which may only be accurately assessed with broader intervention coverage34. Second, human or household factors could influence vector exposure risk. For
instance, the higher mosquito parasite rate observed may be linked to the substantial proportion of human blood meals in both control and intervention houses, particularly if a small number
of parasite reservoirs disproportionately drive human-to-mosquito transmission34,35,36. Additionally, both _An. arabiensis_ and _An. funestus_ s.l., the dominant vectors in our study are
highly competent malaria transmitters3,30,37. Further research is needed to identify the human, environmental and mosquito-related factors contributing to residual transmission despite
seemingly successful control measures. Understanding the interplay of these factors could help better define spatial risk and transmission hotspots, ultimately guiding more targeting malaria
control strategies38. The intervention significantly reduced overall malaria prevalence among household members, with a particularly pronounced impact in adults aged > 14 years. This
difference may partially reflect a bias, although recent data suggests that this age group is now the most at risk for malaria. These results align with baseline studies, which found that
adults (> 15 years) accounted for 50% malaria cases, followed by school-aged children (5–14 years) at 31%, and children under 5 at 10%23. The consistent reduction in malaria prevalence
across age groups highlights the effectiveness of HS, which can be widely applied in malaria-endemic areas to provide significant protection to key population groups, including school-aged
children and adults. Additionally, these findings suggest that HS offers broader protection for all household members indoors if used effectively. We estimated the EIR as a measure of the
protective effectiveness of the house-screening intervention32 using CDC light trap data. The CDC light trap is considered less prone to human bias, and EIR estimates from these traps offer
an alternative to human landing collections32,39. Previous studies have shown a strong correlation between light traps and landing collection estimates39. The sporozoite rates were estimated
from mosquito cohorts that were engorged, representing those that had previously bitten a host, including humans. Testing gravid and blood-fed cohorts increases the likelihood of detecting
parasites, potentially introducing bias into the infection data. However, this cohort was consistently lower in intervention houses compared to their control counterparts. Since these
mosquitoes are potentially older and more dangerous, the intervention may have indirectly impacted the longevity/survival of anophelines in our study. Further analyses incorporating age
structure would be valuable for assessing future trials. Mosquito survival is a critical metric closely tied to the vectorial capacity of disease vectors40. One of the limitations of our
study is that intervention and control houses were not stratified by the presence of cattle near sleeping quarters, which may have influenced estimates of bovine blood-feeding rates.
Additionally, light traps may not capture all anophelines with equal efficiency. The EIR was aggregated over the entire 16 month trapping period (post-intervention) due to the total number
of captures and those analysed for infection presence, which limited the ability to explore seasonal variations. Passive prevalence data was collected after 8 months of intervention, but no
clear relationship with entomologic data could be identified. Our analysis precluded accounting for potential household effect on the mosquito data. While we enforced a random design, a
repeat of some households no more than twice occurred throughout the vector survey period. It is conceivable that this limited number of repeated measures per household likely provides
insufficient data to reliably estimate household-level variance components. Accurate scoring for _Plasmodium_ sporozoite detection could be affected by specific types of genomic DNA markers.
To limit this potential issue, our data focused on analysis of head/thorax region and combining a suit of markers. Unequal trapping across agroecological zones was another unexpected issue,
attributed to failed batteries whose data were excluded from the analysis. Overall, the data revealed low densities, consistent with previous reports in these ecological settings3,41.
CONCLUSIONS Our study demonstrates the benefits of HS of doors/windows/eaves, in reducing indoor host-seeking abundance of malaria vectors, dominated by _An. arabiensis_. This reduction
reflects the proportion of mosquitoes engaged in blood feeding, indicating decreased biting activity, which ultimately leads to a decline in malaria prevalence. Agroecology had no effect on
the performance of the intervention. The protective effectiveness of HS based on estimated EIR, was modest (22.6%), suggesting sustained transmission due to competent vectors such as _An.
arabiensis_ and _An. funestus_ s.l. mosquitoes. Additional studies are needed to explore the interactions between vector behavioral adaptations and ecological and social factors that
contribute to residual transmission, even with seemingly effective control measures. DATA AVAILABILITY Data is provided within the manuscript or supplementary information files.
ABBREVIATIONS * HS: House Screening * ITNs: Insecticide Treated Nets * CDC: Centre for Disease Control * EIR: Entomological Inoculation Rate * SSA: Sub-Saharan Africa * WHO: World Health
Organization * IRS: Indoor Residual Spray * IVM: Integrated Vector Management * RCT: Randomized Control Trial * RDT: Rapid Diagnostic Test * PCR: Polymerase Chain Reaction * DNA:
Deoxyribonucleic Acid * GLM: Generalised Linear Model * BBI: Bovine Blood Index * HBI: Human Blood Index REFERENCES * World Malaria Report. Geneva: World Health Organization; 2023. Licence:
CC BY-NC-SA 3.0 IGO. (2023). * The PMI VectorLink Project Ethiopia Final Entomological Report, April 2022-March 2023. Rockville, MD. Abt Associates Inc. (2023). * Belay, A. K. et al.
Vectorial drivers of malaria transmission in Jabi Tehnan district, Amhara regional State, Ethiopia. _Sci. Rep._ 14, 13669 (2024). Article ADS CAS PubMed PubMed Central Google Scholar *
Kinya, F. et al. Outdoor malaria vector species profile in dryland ecosystems of Kenya. _Sci. Rep._ 12, 7131 (2022). Article ADS CAS PubMed PubMed Central Google Scholar * Ogola, E.
O. et al. Insights into malaria transmission among _Anopheles funestus_ mosquitoes, Kenya. _Parasit. Vectors_. 11, 577 (2018). Article PubMed PubMed Central Google Scholar * Balkew, M.
et al. An update on the distribution, bionomics, and insecticide susceptibility of _Anopheles stephensi_ in Ethiopia, 2018–2020. _Malar. J._ 20, 263 (2021). Article PubMed PubMed Central
Google Scholar * Torto, B. & Tchouassi, D. P. Grand challenges in vector-borne disease control targeting vectors. _Front. Trop. Dis._ 1, 635356 (2021). Article Google Scholar * World
Health Organization. _Global Technical Strategy for Malaria 2016–2030_ (World Health Organization, 2015). * Asale, A. et al. The combined impact of LLINs, house screening, and pull-push
technology for improved malaria control and livelihoods in rural Ethiopia: study protocol for household randomised controlled trial. _BMC Public. Health_. 22, 930 (2022). Article PubMed
PubMed Central Google Scholar * Chisanga, B. et al. The economic impacts of house screening against malaria transmission: experimental evidence from Eastern Zambia. _Soc. Sci. Med._ 321,
115778 (2023). Article PubMed Google Scholar * Chanda, E. Exploring the effect of house screening: are we making gains? _Lancet Planet. Health_. 3, e105–e106 (2019). Article PubMed
Google Scholar * Killeen, G. F., Govella, N. J., Mlacha, Y. P. & Chaki, P. P. Suppression of malaria vector densities and human infection prevalence associated with scale-up of
mosquito-proofed housing in Dar Es Salaam, Tanzania: re-analysis of an observational series of parasitological and entomological surveys. _Lancet Planet. Health_. 3, e132–e143 (2019).
Article PubMed Google Scholar * Tusting, L. S. et al. The evidence for improving housing to reduce malaria: a systematic review and meta-analysis. _Malar. J._ 14, 209 (2015). Article
PubMed PubMed Central Google Scholar * Fox, T., Furnival-Adams, J., Chaplin, M., Napier, M. & Olanga, E. A. House modifications for preventing malaria. _Cochrane Database Syst. Rev._
10, CD013398 (2022). PubMed Google Scholar * Getawen, S. K., Ashine, T., Massebo, F., Woldeyes, D. & Lindtjørn, B. Exploring the impact of house screening intervention on entomological
indices and incidence of malaria in Arba minch town, Southwest Ethiopia: A randomized control trial. _Acta Trop._ 181, 84–94 (2018). Article PubMed Google Scholar * Kirby, M. J. et al.
Effect of two different house screening interventions on exposure to malaria vectors and on anaemia in children in the Gambia: a randomised controlled trial. _Lancet_ 374, 998–1009 (2009).
Article PubMed PubMed Central Google Scholar * Pinder, M. et al. Improved housing versus usual practice for additional protection against clinical malaria in the Gambia (RooPfs): a
household-randomised controlled trial. _Lancet Planet. Health_. 5, e220–e229 (2021). Article PubMed PubMed Central Google Scholar * Guidelines for malaria vector control. _Licence: CC
BY-NC-SA 3.0 IGO_ (World Health Organization, 2019). * Tafere, M. et al. Participatory rural appraisal report: Jabi Tehnan District. Bahir Dar University -CASCAPE Working Paper 4. (2017). *
Central Statistics Agency. Population projection of Ethiopia for all regions at wereda level from 2014–2017. Vol. 3, Central Statistics Agency. Addis Ababa. ; p. 28. (2013). * U.S.
President’s Malaria Initiative Ethiopia Malaria Operational Plan FY 2022. * Balew, S., Bulte, E., Abro, Z. & Kassie, M. Incentivizing and nudging farmers to spread information:
experimental evidence from Ethiopia. _Am. J. Agric. Econ._ 105, 994–1010 (2023). Article Google Scholar * Asale, A. et al. Community knowledge, perceptions, and practices regarding malaria
and its control in Jabi Tehnan district, Amhara region, Northwest Ethiopia. _Malar. J._ 20, 459 (2021). Article PubMed PubMed Central Google Scholar * World Health Organization. _Manual
on Practical Entomology in Malaria. Part II. Methods and Techniques_13 (Geneva, World Health Organization, 1975). * Nyasembe, V. O. et al. Adipokinetic hormone signaling in the malaria
vector _Anopheles gambiae_ facilitates _Plasmodium falciparum_ sporogony. _Commun. Biol._ 6, 171 (2023). Article CAS PubMed PubMed Central Google Scholar * Zianni, M. R., Nikbakhtzadeh,
M. R., Jackson, B. T., Panescu, J. & Foster, W. A. Rapid discrimination between _Anopheles gambiae_ S.s. And _Anopheles arabiensis_ by High-Resolution melt (HRM) analysis. _J. Biomol.
Tech._ 24, 1–7 (2013). PubMed PubMed Central Google Scholar * Kamau, W. W. et al. Patterns of _Aedes aegypti_ abundance, survival, human-blood feeding and relationship with dengue risk,
Kenya. _Front. Trop. Dis._ 4, 1113531 (2023). Article Google Scholar * R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna,
Austria. https://www.R-project.org/ (2024). * Briët, O. J. et al. Applications and limitations of centers for disease control and prevention miniature light traps for measuring biting
densities of African malaria vector populations: a pooled-analysis of 13 comparisons with human landing catches. _Malar. J._ 14, 247 (2015). Article PubMed PubMed Central Google Scholar
* Aschale, Y. et al. Systematic review of sporozoite infection rate of _Anopheles_ mosquitoes in Ethiopia, 2001–2021. _Parasit. Vectors_. 16, 437 (2023). Article CAS PubMed PubMed Central
Google Scholar * Debebe, Y. et al. Mosquito odour-baited mass trapping reduced malaria transmission intensity: a result from a controlled before-and-after intervention study. _BMC Med._
22, 41 (2024). Article CAS PubMed PubMed Central Google Scholar * Saili, K. et al. House screening reduces exposure to indoor host-seeking and biting malaria vectors: evidence from
rural South-East Zambia. _Trop. Med. Infect. Dis._ 9, 20 (2024). Article ADS PubMed PubMed Central Google Scholar * Ng’ang’a, P. N., Okoyo, C., Mbogo, C. & Mutero, C. M. Evaluating
effectiveness of screening house eaves as a potential intervention for reducing indoor vector densities and malaria prevalence in Nyabondo, Western Kenya. _Malar. J._ 19, 1–2 (2020). Article
Google Scholar * Ko, Y. K. et al. Unraveling the ‘community effects’ of interventions against malaria endemicity: a systematic scoping review. _BMJ Public. Health_. 2, e001557 (2024).
Article PubMed PubMed Central Google Scholar * Mbewe, R. B. et al. Genotyping of _Anopheles_ mosquito blood meals reveals nonrandom human host selection: implications for
human-to-mosquito _Plasmodium falciparum_ transmission. _Malar. J._ 22, 115 (2023). Article CAS PubMed PubMed Central Google Scholar * Markwalter, C. F. et al. _Plasmodium falciparum_
infection in humans and mosquitoes influence natural anopheline biting behavior and transmission. _Nat. Commun._ 15, 4626 (2024). Article ADS CAS PubMed PubMed Central Google Scholar *
Hemming-Schroeder, E. et al. Ecological drivers of genetic connectivity for African malaria vectors _Anopheles gambiae_ and _An. arabiensis_. _Sci. Rep._ 10, 19946 (2020). Article ADS CAS
PubMed PubMed Central Google Scholar * Gul, D. et al. Investigating differences in village-level heterogeneity of malaria infection and household risk factors in Papua new Guinea. _Sci.
Rep._ 11, 16540 (2021). Article ADS CAS PubMed PubMed Central Google Scholar * Massebo, F., Balkew, M., Gebre-Michael, T. & Lindtjørn, B. Entomologic inoculation rates of
_Anopheles arabiensis_ in Southwestern Ethiopia. _Am. J. Trop. Med. Hyg._ 89, 466–473 (2013). Article PubMed PubMed Central Google Scholar * Tchouassi, D. P. et al. Characterization of
malaria transmission by vector populations for improved interventions during the dry season in the Kpone-on-Sea area of coastal Ghana. _Parasit. Vectors_. 5, 212 (2012). Article PubMed
PubMed Central Google Scholar * Belay, A. K. et al. Feeding habits and malaria parasite infection of _Anopheles_ mosquitoes in selected agroecological areas of Northwestern Ethiopia.
_Parasit. Vectors_. 17, 412 (2024). Article PubMed PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS The authors would like to acknowledge all personnel including the
study household owners, the district health office team and the health extension workers who participate in field sampling and facilitating the study. We are also grateful to Getachew Kebede
for preparing the study area map also Janet Kosgei and Masese Shadrack, at icipe Duduville Campus, Nairobi, for technical support. FUNDING AKB was supported by the German Academic Exchange
Service (DAAD) In-Region Postgraduate Scholarship under the African Regional Postgraduate Programme in Insect Science (ARPPIS) tenable at the International Centre of Insect Physiology and
Ecology (_icipe_), Nairobi, Kenya, and a bursary award from the University of Pretoria as well. This study, conducted under the project Combatting Arthropod Pests for Better Health, Food,
and Climate Resilience (CAP-Africa), was funded by the Norwegian Agency for Development Cooperation (Norad) (Grant number: RAF-3058 KEN-18/0005). DPT is supported by a Wellcome Trust
International Intermediate Fellowship (222005/Z/20/Z). The authors gratefully acknowledge the financial support for this research by the following organizations and agencies: the Swedish
International Development Cooperation Agency (Sida); the Swiss Agency for Development and Cooperation (SDC); the Australian Centre for International Agricultural Research (ACIAR); the
Government of Norway; the German Federal Ministry for Economic Cooperation and Development (BMZ); and the Government of the Republic of Kenya. The views expressed herein do not necessarily
reflect the official opinion of the donors. The views expressed herein do not necessarily reflect the official opinion of the donors. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS *
International Centre of Insect Physiology and Ecology, P.O. Box 30772-00100, Nairobi, Kenya Aklilu K. Belay, Baldwyn Torto, Zewdu Abro, Menale Kassie, Clifford M. Mutero & David P.
Tchouassi * International Centre of Insect Physiology and Ecology, P.O. Box 5689, Addis Ababa, Ethiopia Abebe Asale * Department of Zoology and Entomology, University of Pretoria, Private
Bag X0028, Pretoria, South Africa Aklilu K. Belay, Catherine L. Sole, Abdullahi A. Yusuf & Baldwyn Torto * School of Health Systems and Public Health, University of Pretoria, Private Bag
X0028, Pretoria, South Africa Clifford M. Mutero Authors * Aklilu K. Belay View author publications You can also search for this author inPubMed Google Scholar * Abebe Asale View author
publications You can also search for this author inPubMed Google Scholar * Catherine L. Sole View author publications You can also search for this author inPubMed Google Scholar * Abdullahi
A. Yusuf View author publications You can also search for this author inPubMed Google Scholar * Baldwyn Torto View author publications You can also search for this author inPubMed Google
Scholar * Zewdu Abro View author publications You can also search for this author inPubMed Google Scholar * Menale Kassie View author publications You can also search for this author
inPubMed Google Scholar * Clifford M. Mutero View author publications You can also search for this author inPubMed Google Scholar * David P. Tchouassi View author publications You can also
search for this author inPubMed Google Scholar CONTRIBUTIONS AKB AA ZA MK CMM DPT conceived and designed the experiments. AKB and AA implemented mosquito surveys. AKB conducted the
experiments. AKB, DPT analysed the data. CMM, BT, MK, DPT contributed to resources and funding acquisition. AA CLS AAY BT CMM DPT supervised the student (AKB). AKB, BT, DPT wrote the draft
manuscript. All authors reviewed subsequent versions of the manuscript and approved the final submitted version. CORRESPONDING AUTHOR Correspondence to David P. Tchouassi. ETHICS
DECLARATIONS ETHICS APPROVAL AND CONSENT TO PARTICIPATE A review of the study protocol and ethical approval were given by the Regional Public Health Research Ethics Review
Committee/RERC/(Ref. No: H/R/T/T/D/5/3) from Amhara Public Health Research Institute, Ethiopia, and confirm that all methods were performed in accordance with the relevant guidelines and
regulations. The research activity was started by providing information about the whole objective of the study to the community and consensus obtained from household owners for the
project-related activities done with the directions given by local stakeholders. Further written informed consent was obtained from all subjects and/or their legal guardian(s). COMPETING
INTERESTS The authors declare no competing interests. TRIAL REGISTRATION The study protocol was registered online on May 28, 2020, with the Pan African Clinical Trials Registry (PACTR) at
www.pactr.org. The registration number assigned to the protocol is PACTR202006878245287. The Trial’s methodology was also discussed by Asale et al.9. ADDITIONAL INFORMATION PUBLISHER’S NOTE
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. ELECTRONIC SUPPLEMENTARY MATERIAL Below is the link to the electronic
supplementary material. SUPPLEMENTARY MATERIAL 1 RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits
use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the
Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated
otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds
the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints and
permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Belay, A.K., Asale, A., Sole, C.L. _et al._ A randomized controlled trial combining house screening and insecticide-treated nets reduces
malaria transmission in northwestern Ethiopia. _Sci Rep_ 15, 17709 (2025). https://doi.org/10.1038/s41598-025-02943-7 Download citation * Received: 18 December 2024 * Accepted: 16 May 2025 *
Published: 21 May 2025 * DOI: https://doi.org/10.1038/s41598-025-02943-7 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link
Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * Randomized controlled
trial * Malaria * _Plasmodium_ infection * Entomology * Epidemiology