Balancing accuracy and cost in machine learning models for detecting medial vascular calcification in chronic kidney disease: a pilot study
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Machine learning algorithms that integrate multiple biomarkers are increasingly used in disease detection, yet economic considerations are often overlooked. Medial vascular
calcification (mVC), a pathology associated with elevated cardiovascular risk in chronic kidney disease (CKD), requires cost-effective diagnostic approaches. This pilot study evaluated the
cost-effectiveness of machine learning models for mVC detection using traditional risk markers and circulating biomarkers in 152 CKD patients undergoing living donor kidney transplantation.
Patients were classified as having no/minimal (_n_ = 93) or moderate/extensive (_n_ = 59) mVC. Five classification frameworks with automatic variable selection identified predictors of mVC.
Age and copeptin were selected by all algorithms, while diabetes, male sex, choline, and osteoprotegerin were chosen by four methods. The number of features selected ranged from 5 to 21.
Although accuracy differences among classifiers were limited to 3%, models using more features nearly tripled the procedure’s cost. By incorporating the incremental cost-effectiveness ratio,
the study highlighted significant disparities in performance versus cost among classifiers. The present findings suggest that machine learning has the potential to complement imaging
techniques for mVC detection and uncover novel biomarkers. However, modest performance improvements may not justify higher costs, underscoring the importance of considering
cost-effectiveness when selecting classification models. SIMILAR CONTENT BEING VIEWED BY OTHERS UNRAVELING THE IMPACT OF ABDOMINAL ARTERIAL CALCIFICATIONS ON KIDNEY TRANSPLANT WAITLIST
MORTALITY THROUGH ENSEMBLE MACHINE LEARNING Article Open access 16 October 2024 AN INDEPENDENT VALIDATION OF THE KIDNEY FAILURE RISK EQUATION IN AN ASIAN POPULATION Article Open access 31
July 2020 MACHINE LEARNING TO PREDICT END STAGE KIDNEY DISEASE IN CHRONIC KIDNEY DISEASE Article Open access 19 May 2022 INTRODUCTION Medial vascular calcification (mVC) is a pathological
condition, with estimated prevalence rates ranging from 27 to 80% in the chronic kidney disease (CKD) population1,2,3. The pathology contributes to the high cardiovascular morbidity and
mortality in this group of patients4,5. Moreover, a recent study has revealed that it is associated with the progression of CKD6. While the pathogenesis of mVC is not fully understood and a
causal therapy is not available as of today, new therapeutic possibilities are currently being studied7,8,9,10. Moreover, the feasibility of slowing down mVC progression in patients with CKD
has been demonstrated11,12. Therefore, improved methods for mVC detection, especially at early stages, is highly warranted. At present, there is a lack of a dedicated and reliable method of
mVC assessment in clinical practice2,13,14. Invasive techniques such as artery biopsy15 or transcutaneous ultrasound16 are rarely performed and cannot be considered as screening procedures.
Both direct semi-quantitative methods such as computed tomography, plain X-rays, or ultrasound13, and indirect methods such as measurement of pulse wave velocity that reflects increased
arterial stiffness in calcified arteries17,18, are not always available and not easy to perform; therefore, the presence of mVC is likely underestimated. Moreover, currently used tools
struggle to differentiate between the two types of vascular calcification: medial and intimal19,20,21; this is clinically significant, as these types have distinct implications and require
different patient care strategies. Recently, a method enabling this differentiation, involving the identification of mVC patterns on PET-CT scans, has been introduced22. Nonetheless, the
expense and limited availability of PET-CT scans highlight the need for an approach that can indicate the presence of mVC and readily determine which patients truly require this imaging
technique. Machine learning algorithms, which are designed to detect patterns in data, are thought to have the potential to radically improve our ability to diagnose and treat diseases. The
large number of potential mVC markers complicates mVC diagnosis and statistical feature selection procedures may therefore play a crucial role in establishing future diagnostics. In previous
studies, numerous biomarkers have been linked with vascular calcification including serum biomarkers23, vitamin-K dependent proteins24, various phenotypic features25,26 and risk factors
such as high age, male sex, and diabetes mellitus20. While models for mVC detection have demonstrated promising performance quality25, the variability in their cost-effectiveness across
different frameworks remains unexplored. In a clinical setting, besides evaluating the statistical performance of the newly introduced methods, their overall applicability is a crucial
consideration. This covers factors such as the procedure’s availability, safety, and the overall expense of the diagnostic procedure. One of the indicators that can characterize the latter
is the incremental cost-effectiveness ratio (ICER) which provides insights into the method’s cost in relation to the potential benefits for patients27. The objective of this pilot study was
to investigate the cost-effectiveness of various machine learning frameworks for mVC detection in the chronic kidney disease population. For each of the tested models, in addition to
conventional classification correctness metrics, ICER was calculated to incorporate both performance and cost considerations into evaluation. The most favorable model in terms of ICER was
further investigated to showcase its possible clinical utility. Finally, we discussed possible pathophysiological associations between mVC and the variables selected by the applied
algorithms. METHODS Data investigation, model building process, and performance evaluation were implemented in R version 4.0.5 and Python version 3.7. PATIENTS AND STUDY DESIGN In this
retrospective study, a cohort of patients with advanced CKD undergoing living donor kidney transplantation at Karolinska University Hospital was included. The study’s eligibility criteria
aligned with those established for patients eligible for kidney transplantation. Exclusion criteria were age under 18 years and unwillingness to participate in the study. The clinical
procedures and protocol of measurements were described previously28. The patients gave their informed consent for all performed procedures. The study was approved by the regional ethical
review board in Stockholm and adhered to the Declaration of Helsinki. The participants were classified into two groups according to the extent of medial calcification in inferior epigastric
artery biopsies assessed by an experienced pathologist. ‘_Group 0’_ included patients with _no_ and _minimal_ signs of VC (_n_ = 93), whereas patients having _moderate_ and _extensive_ signs
of VC were classified into ‘_Group 1’_ (n = 59). The procedure of histological mVC examination was presented in detail in25,28. The dataset consisted of 60 features in 152 patients. All 60
features were available in 71% of the patients; in total, 8.3% of the data were missing. The full data flow is described in25. The dataset included demographic and clinical data, circulating
biomarkers, body composition and anthropometric measurements, and skin content of advanced glycation end products measured by autofluorescence. The investigated features are presented in
Table 1. DATA PREPROCESSING First, we standardized the predictors proportionally within the range from 0 to 1. Missing values imputation was performed using the k-nearest neighbors algorithm
with k = 3 and Euclidean distance measure between the patients. mVC, as an outcome variable, was not involved in the imputation process. The distributions of the imputed and non-imputed
variables did not exhibit statistical differences (Kolmogorov-Smirnov and Chi-square test for continuous and discrete distributions, respectively). Feature selection and patient
classification were performed on the complete, standardized set of variables, while the univariable analysis was performed on the raw data. DATA INVESTIGATION To choose the appropriate
feature selection and classification algorithms, a preliminary data investigation was conducted. Firstly, the Spearman rank correlation coefficient was used to reveal interdependencies
between the analyzed features. Categorical variables (sex, smoking, and diabetes mellitus) were excluded from the analysis. Secondly, logistic regression was carried out to assess the
interrelationship between a single feature and mVC. To account for multiple comparisons, p-values were adjusted using Benjamini-Hochberg correction29. METHODS OF FEATURE SELECTION AND
PATIENT CLASSIFICATION In the process of feature selection and patient classification, the following methods were applied: logistic regression with forward Akaike feature elimination process
(LR)30, support vector machine with recursive feature elimination (SVM)31, random forest with permutation importance (RF)32, logistic regression with elastic net penalty (EN)33,34, and,
less explored, relaxed linear separability method (RLS)35. Each of the methods was applied in its standard configuration, with algorithm-specific hyperparameter optimization conducted where
appropriate. For feature selection, we opted for well-established algorithms commonly used within the applied classification frameworks. A brief description of the chosen methods can be
found in the supplementary material. LR, EN, RF and SVM models were built using R _caret_ package, for training RLS we used our own MATLAB implementation. PERFORMANCE EVALUATION All methods
were validated in the leave-one-out cross-validation (LOOCV) process. In the algorithms where hyperparameter tuning was required, a nested 5-fold cross-validation was incorporated aiming to
maximize accuracy as the primary optimization criterion. The metrics used to evaluate the predictions were accuracy, area under the receiver operating characteristic curve (AUC), precision,
recall, and F-score, which are discussed in the supplementary material. Additionally, confidence intervals for the LOOCV AUC values were estimated using the bootstrap method with 1,000
resamples. INCREMENTAL COST-EFFECTIVENESS RATIO The incremental cost-effectiveness ratio (ICER)27,36 represents the additional cost incurred for achieving an additional unit of health
outcome, usually measured in quality-adjusted life years (QALYs). It allows decision-makers to ensure that limited healthcare resources are directed towards treatments that provide the most
substantial health benefits relative to their associated costs. Thus, the evaluation of ICER facilitates informed decisions about the adoption and funding of medical interventions. In our
study, ICER was calculated as:
$$\:ICER\:=\:\frac{measure\_cost+\:\left(prevalence\text{*}TPR\:+\:\left(1-prevalence\right)\text{*}FPR\right)\text{*}ct\_price}{prevalence\text{*}TPR\text{*}years\_gained}$$ Where: *
_measure_cost_ – expense associated with evaluating the biomarkers. For certain biomarkers, their costs are considered hyperparameters (parameters with unknown true value) since they are not
routinely measured - see Supplementary Table S2 for a list. For the biomarkers with unknown costs, where only the kit price is available, we introduce an additional factor called the
_unavailability weight_ which used to scale the kit prices accordingly. * _prevalence_ – a hyperparameter indicating true prevalence of mVC among the advanced CKD population. * _TPR_ – the
rate of correctly identified true positive cases by the evaluated method. * _FPR_ – the rate at which the evaluated method incorrectly identifies cases as positive when they are actually
negative. * _ct_price_ – the price of a PET-CT scan to confirm mVC presence; sourced from a polish laboratory in June 2023 and converted from PLN to USD at a rate of 0.23, was assumed to be
1127 USD. * _years_gained_ – quality of life years gained due to mVC detection. A hyperparameter. The pricing details for the biomarkers, sourced from Polish laboratories in June 2023 are
presented in Table 2. The prices were converted from PLN to USD for clarity using an exchange rate of 0.23. Biomarkers denoted with an asterisk (*) represent hyperparameters. In addition, we
performed a sensitivity analysis to assess how the assumed prices influence the results; see supplementary material. We decided to incorporate the cost of a PET-CT scan in the equation as
we presume that, irrespective of how well the classifiers perform, cases with a certain likelihood of being positive would be additionally verified using a more direct method. RESULTS DATA
INVESTIGATION Spearman correlation analysis revealed the presence of collinearity among certain feature pairs. Associations are presented as a heat map in Fig. S1. Using a univariable
logistic regression model, we identified age, male sex, angiopoietin 2, choline, copeptin, duMGP, hsCRP, IgM anti-PC, insulin-like growth factor 1, osteoprotegerin, sclerostin, troponin T,
and body mass index as factors associated with mVC (Table 1). However, after adjusting for multiple comparisons, only age, male sex, copeptin, IGF1, osteoprotegerin, and BMI remained
statistically significant (Table 1). CLASSIFICATION FRAMEWORKS In a multivariable analysis, we applied five classification frameworks with appropriate variable selection methods. To
fine-tune SVM, RF, and EN, we conducted hyperparameter optimization. Table S1 in the supplementary material presents the calculated optimal values and short parameter descriptions. The
algorithms applied to the data differed regarding features identified as being potentially associated with mVC (Table 2). Only age and copeptin were chosen by all five methods (Table 2). The
number of selected features varied between the methods with 21 features being selected by SVM, 16 by RLS, 11 by EN, 6 by RF, and 5 features chosen by LR. The classification ability of the
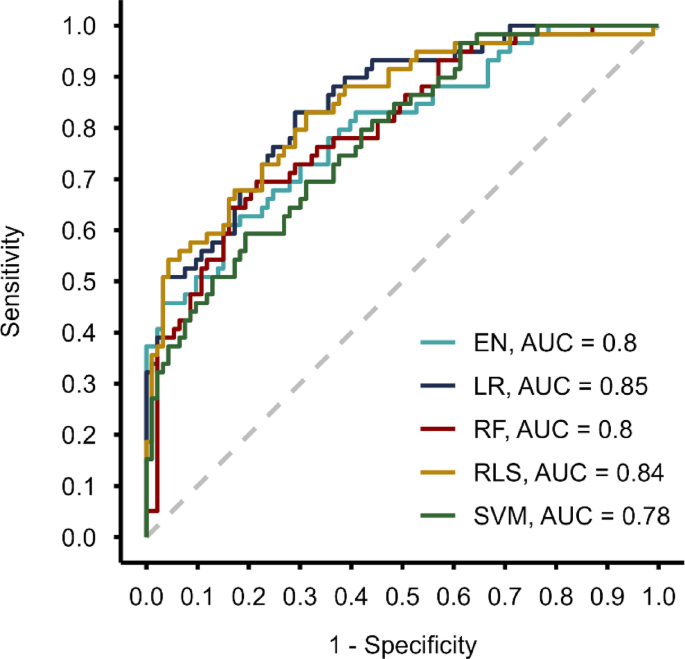
applied methods was measured, among others, by the area under the receiver operating characteristic curve (AUC). In the cross-validation evaluation process, the highest AUC was achieved by
LR (0.85 [0.78–0.90]), followed by RLS (0.84 [0.77–0.90]), EN (0.80 [0.72–0.87]), RF (0.80 [0.73–0.86]), and SVM (0.78 [0.70–0.85]) (Fig. 1). The values in square brackets represent
bootstrapped 95% confidence intervals. All computed performance evaluation metrics are summarized in Table 3. None of the applied methods outperformed the others across all the assessed
measures. INCREMENTAL COST-EFFECTIVENESS RATIO Figure 2 illustrates the Incremental Cost-Effectiveness ratio for the built models across three unknown parameters: unavailability weights (1,
10, 20, 30), reflecting the possible increase in procedure costs caused by the biomarkers with the unknown prices; true mVC prevalence in CKD population (0.4, 0.6, 0.8); and Quality Adjusted
Life Years gained. In general, ICER decreases as QUALYs gained increase, indicating better cost-effectiveness with more QUALYs. Higher unavailability weights lead to higher ICER values for
models relying on features with unknown costs (all except LR). Additionally, higher disease prevalence tends to result in lower ICER values suggesting better cost-effectiveness of the
models. Moreover, the examination of the ICER indicate that irrespective of the model employed, the procedural costs remain notably low37 when compared with the potential gain in
quality-adjusted life years (Fig. 2). Logistic Regression (LR), a model requiring only five input features, of which only one incurs a substantial cost, remains the cheapest procedure, while
SVM, which takes 21 features as an input, remains the most expensive (Fig. 2). When sticking to the current state of knowledge about the prices, i.e., taking into account kit price for the
features unavailable to examine in a laboratory, the order of ICER follows the order of the number of features. However, when considering the scenario where features not currently routinely
measured are presumed to be significantly more expensive than the kit price, which is much more plausible, there is a shift in ICER outcomes among the methods evaluated. Averaged over
prevalence, QUALYs, and unavailability weights, LR emerges as the most cost-effective option with mean ICER equal to $278, followed by RF ($412), RLS ($445), EN ($608), and SVM ($769).
Sensitivity analysis revealed that the presented results are consistent regardless of the established feature prices. The only exception is sclerostin; assuming a 50% increase in its cost,
RLS is favored over RF. In the supplementary material we explored the LR model’s coefficients and showcase its possible clinical utility by calculating ICER for various probability
thresholds. DISCUSSION In our research, based on the data from 152 participants, we demonstrated the cost-effectiveness of five machine learning frameworks for detecting medial vascular
calcification in CKD patients, a group susceptible to mVC. The algorithms were assessed in terms of statistical performance (Table 3) and cost-effectiveness assessed by the incremental
cost-effectiveness ratio, ICER (Fig. 2). Whereas the tested methods had similar predictive power with AUC values between 0.80 and 0.84 and most of them identified traditional risk factors
including age, diabetes, male sex, and body mass index (BMI) as important predictors of mVC in patients with CKD, they yielded different results regarding mVC-related features (Table 2).
However, the cost differs significantly between the frameworks with LR working on 5 features appearing as the most efficient option. The accuracies of the models were not perfect,
underscoring that there is still much to uncover regarding the biomarkers associated with mVC and that machine-learning-based algorithms cannot serve as a standalone method for assessing mVC
presence in CKD patients. However, they can help reduce the frequency of performing unnecessary CT scans for individuals who are found to be less likely to have the pathology, based on the
initial assessment of the biomarkers. This reduction can lead to significant savings in healthcare costs, limit radiation exposure, and decrease the time required for diagnostic procedures.
In the supplementary material, we provide a detailed example using logistic regression to illustrate how model outputs can be translated into clinical decision-making. Lowering the cut-off
threshold for recommending scans increases diagnostic accuracy but reduces potential savings from avoiding unnecessary imaging. The final choice of threshold should be guided by clinical
context and resource availability, allowing practitioners to balance diagnostic performance with operational constraints. In this pilot study, logistic regression emerged as the most
effective method. Besides favorable cost-effectiveness, as well as simplicity, and interpretability of the coefficients, it offered another advantage over the other built classifiers: it
required only 5 easily obtainable features (Table 2). This minimizes the likelihood of encountering missing values, a situation more common in complex models. However, this interpretation is
possible only after looking at the models’ cost-effectiveness and the sets of their required features. Solely examining performance evaluation metrics (Table 3) makes determining the best
of the built models much more complex. Furthermore, examining a panel of different outcomes of the applied feature selection frameworks may provide valuable insights into biomarkers
potentially related to mVC. A predictor that emerged as particularly important in our analysis is copeptin that was chosen by all utilized algorithms (Table 2). This confirms findings from a
previous study on this topic38. Osteoprotegerin and sclerostin, chosen by 4 and 3 models, respectively, have also been demonstrated to be associated with mVC presence15,39. Hence, it would
be worthwhile to perform a longitudinal study to assess whether it is justified to incorporate one or more of these three biomarkers into regular clinical practice. Finally, we highlight
some of the well-established or plausible underlying pathophysiological links between the selected variables and mVC (Table S4). This may reinforce the rationale for including some of the
identified predictors when designing studies aiming at detecting mVC in future investigations. In the context of applied biomedicine, it is increasingly recognized that the criteria for
assessing a successful statistical model should extend beyond the predictive power of the classifiers; they ought to also be tailored to align with the medical facilities’ condition and
capabilities. Thus, the cost of the procedure, the availability, and interpretability of the utilized features, should be also considered. Our findings demonstrated that, given certain
conditions, a framework employing less expensive variables can outperform another that relies on fewer but costly ones. This was exemplified by RLS, which produced better results in terms of
ICER when compared to EN despite utilizing 5 additional features (Fig. 2b–d) and obtaining far worse precision. Moreover, it produced equivalent results when compared to RF which employed
10 additional features (Fig. 2c, d). Although effective therapies specifically targeting mVC are currently lacking, there are interventions available that can slow its progression11,12. This
supports the inclusion of _years_gained_ in the ICER calculation, as early detection of mVC followed by appropriate clinical management may lead to gains in quality-adjusted life years. In
the future, the development of therapies capable of reversing mVC would likely increase the expected _years_gained_, thereby reducing the relative cost of using biomarkers as a pre-screening
tool, as illustrated in Fig. 2. A major strength of our study is the comprehensiveness of the performed analysis and that it is based on a unique clinical material with histological
identification of mVC in artery specimens. To the best of our knowledge, this represents one of the most extensive clinical datasets of arterial biopsies gathered from chronic kidney disease
patients. The collected database includes, inter alia, an evaluation of several factors with documented involvement in the disturbed mineral metabolism in CKD and plausible involvement in
the etiology of mVC such as sclerostin38 osteoprotegerin39, calciprotein particles40, FGF2341, klotho41, and parathyroid hormone42. We showed the interdependencies between features (Spearman
rho, Fig. S1), univariable associations between mVC and each one of the 60 investigated features (Table 1) and performed a multivariable analysis that allowed us to select subsets of
features associated with mVC, which entered classification models (Table 2). To the best of our knowledge, no previous studies on mVC detection analyzed ICER or any other price-related
metrics of the evaluated procedures. Our study has several limitations which should be considered when interpreting the results. First, the database includes missing values. Whereas their
imputation can change the original dataset, including only complete cases may result in a considerable reduction of the number of included patients and features and therefore, a loss of
statistical power. Additionally, many statistical tools and algorithms require a complete dataset; for this reason, and considering the relatively small sample size, we decided to fill in
the missing data and ensured that the variable distribution did not alter significantly post-imputation. It should also be noted that imputation may interfere with the stability of feature
selection. Furthermore, the lack of external validation is a key limitation, as it prevents us from fully assessing the generalizability and robustness of the developed models. Moreover, due
to the retrospective nature of this long-lasting study, some potentially relevant features were not analysed which may limit the comprehensiveness of our findings. Missing features include,
for example, N-terminal pro b-type natriuretic peptide (NT-proBNP) and Gla-rich protein, a vitamin K dependent calcification inhibitor43,44. Another issue is that the costs related to the
measurements needed for ICER analysis can vary significantly between countries, laboratories, and over time. While the sensitivity analysis revealed the consistency of the presented results,
it is important to emphasize that the conducted investigation is only a rough estimation of the potential costs associated with each procedure. Before implementation of such a detection
method, medical facilities should estimate the costs based on their resources and capabilities. Lastly, it is important to note that mVC distribution varies across different vascular
beds45,46. In the past, mVC presence assessed in the inferior epigastric artery was linked with higher values of coronary artery calcification (CAC) score15, which altogether demonstrates
the complexity and variability of the condition. However, further studies are needed to assess the impact of the selected features on calcification in different vascular beds, as the current
findings may not be universally applicable. CONCLUSION Our findings showcase the importance of employing analysis that considers not only statistical accuracy but also economic implications
of proposed machine learning frameworks. In the present study, the incremental cost-effectiveness ratio (ICER), was found to provide a suitable criterion for model selection, as analysis
using ICER is where the difference between the models becomes evident. This highlights the importance of considering cost-effectiveness when selecting the final classifier, as a minor
increase in model performance might not balance the costs related to measuring model-required inputs. While the findings from this pilot study warrant validation on a larger dataset, we
believe that it may encourage other researchers using machine learning algorithms for detection of medial vascular calcification to seek optimal solutions that consider not only predictive
capabilities but also the applicability of the implemented methods. DATA AVAILABILITY The data that support the findings of this study are not openly available due to reasons of sensitivity
and are available from the corresponding author upon reasonable request. Data are located in controlled access data storage at Karolinska Institutet. REFERENCES * London, G. M. et al.
Arterial media calcification in end-stage renal disease: impact on all-cause and cardiovascular mortality. _Nephrol. Dialysis Transplantation_. https://doi.org/10.1093/ndt/gfg414 (2003).
Article Google Scholar * Nelson, A. J. et al. Targeting vascular calcification in chronic kidney disease. _JACC Basic. Transl Sci._ https://doi.org/10.1016/j.jacbts.2020.02.002 (2020).
Article PubMed PubMed Central Google Scholar * Duhn, V. et al. Breast arterial calcification: A marker of medial vascular calcification in chronic kidney disease. _Clin. J. Am. Soc.
Nephrol._ https://doi.org/10.2215/CJN.07190810 (2011). Article PubMed PubMed Central Google Scholar * London, G. M. et al. Arterial media calcification in end-stage renal disease: impact
on all-cause and cardiovascular mortality. _Nephrol. Dialysis Transplantation_. 18, 1731–1740. https://doi.org/10.1093/ndt/gfg414 (2003). Article Google Scholar * Erlandsson, H. et al.
Scoring of medial arterial calcification predicts cardiovascular events and mortality after kidney transplantation. _J. Intern. Med._ 291, 813–823. https://doi.org/10.1111/joim.13459 (2022).
Article CAS PubMed PubMed Central Google Scholar * Park, S. et al. Vascular calcification as a novel risk factor for kidney function deterioration in the nonelderly. _J. Am. Heart
Assoc._ https://doi.org/10.1161/JAHA.120.019300 (2021). Article PubMed PubMed Central Google Scholar * Lin, Y-L. & Hsu, B-G. Vitamin K and vascular calcification in chronic kidney
disease: an update of current evidence. _Tzu Chi Med. J._ 35, 44. https://doi.org/10.4103/tcmj.tcmj_100_22 (2023). Article PubMed Google Scholar * Bao, W. H. et al. Relationship between
gut microbiota and vascular calcification in Hemodialysis patients. _Ren. Fail._ https://doi.org/10.1080/0886022X.2022.2148538 (2023). Article PubMed PubMed Central Google Scholar *
Düsing, P. et al. Vascular pathologies in chronic kidney disease: pathophysiological mechanisms and novel therapeutic approaches. _J. Mol. Med._ https://doi.org/10.1007/s00109-021-02037-7
(2021). Article PubMed Google Scholar * Xu, C., Smith, E. R., Tiong, M. K., Ruderman, I. & Toussaint, N. D. Interventions to attenuate vascular calcification progression in chronic
kidney disease: A systematic review of clinical trials. _J. Am. Soc. Nephrol._ https://doi.org/10.1681/ASN.2021101327 (2022). Article PubMed PubMed Central Google Scholar * Raggi, P. et
al. The ADVANCE study: A randomized study to evaluate the effects of Cinacalcet plus low-dose vitamin D on vascular calcification in patients on Hemodialysis. _Nephrol. Dialysis
Transplantation_. https://doi.org/10.1093/ndt/gfq725 (2011). Article Google Scholar * Chen, N. C., Hsu, C. Y. & Chen, C. L. The strategy to prevent and regress the vascular
calcification in dialysis patients. _Biomed. Res. Int._ https://doi.org/10.1155/2017/9035193 (2017). Article PubMed PubMed Central Google Scholar * Marreiros, C., Viegas, C. & Simes,
D. Targeting a silent disease: vascular calcification in chronic kidney disease. _Int. J. Mol. Sci._ https://doi.org/10.3390/ijms232416114 (2022). Article PubMed PubMed Central Google
Scholar * Raggi, P. & O’Neill, W. C. Imaging for vascular calcification. _Semin Dial_. https://doi.org/10.1111/sdi.12596 (2017). Article PubMed Google Scholar * Qureshi, A. R. et al.
Increased circulating sclerostin levels in end-stage renal disease predict biopsy-verified vascular medial calcification and coronary artery calcification. _Kidney Int._
https://doi.org/10.1038/ki.2015.194 (2015). Article PubMed Google Scholar * Lanzer, P. et al. Medial arterial calcification: JACC state-of-the-art review. _J. Am. Coll. Cardiol._
https://doi.org/10.1016/j.jacc.2021.06.049 (2021). Article PubMed PubMed Central Google Scholar * Ren, S. C. et al. Vascular calcification in chronic kidney disease: an update and
perspective. _Aging Dis._ 13, 673–697. https://doi.org/10.14336/AD.2021.1024 (2022). Article PubMed PubMed Central Google Scholar * Lanzer, P. et al. Medial vascular calcification
revisited: review and perspectives. _Eur. Heart J._ 35, 1515–1525. https://doi.org/10.1093/eurheartj/ehu163 (2014). Article PubMed PubMed Central Google Scholar * Hjortnaes, J., New, S.
E. P. & Aikawa, E. Visualizing novel concepts of cardiovascular calcification. _Trends Cardiovasc. Med._ https://doi.org/10.1016/j.tcm.2012.09.003 (2013). Article PubMed PubMed Central
Google Scholar * Lanzer, P. et al. Medial vascular calcification revisited: review and perspectives. _Eur. Heart J._ https://doi.org/10.1093/eurheartj/ehu163 (2014). Article PubMed
PubMed Central Google Scholar * Jinnouchi, H. et al. Intravascular imaging and histological correlates of medial and intimal calcification in peripheral artery disease. _EuroIntervention_.
https://doi.org/10.4244/EIJ-D-20-01336 (2021). * Konijn, L. C. D. et al. CT calcification patterns of peripheral arteries in patients without known peripheral arterial disease. _Eur. J.
Radiol._ https://doi.org/10.1016/j.ejrad.2020.108973 (2020). Article PubMed Google Scholar * Golüke, N. M. S. et al. Serum biomarkers for arterial calcification in humans: A systematic
review. _Bone Rep._ https://doi.org/10.1016/j.bonr.2022.101599 (2022). Article PubMed PubMed Central Google Scholar * Wen, L., Chen, J., Duan, L. & Li, S. Vitamin K-dependent
proteins involved in bone and cardiovascular health (Review). _Mol. Med. Rep._ https://doi.org/10.3892/mmr.2018.8940 (2018). * Dai, L. et al. Phenotypic features of vascular calcification in
chronic kidney disease. _J. Intern. Med._ https://doi.org/10.1111/joim.13012 (2020). Article PubMed Google Scholar * Lyu, B. et al. Vascular calcification markers and hemodialysis
vascular access complications. _Am. J. Nephrol._ https://doi.org/10.1159/000493549 (2018). Article PubMed Google Scholar * Bambha, K. & Kim, W. R. Cost-effectiveness analysis and
incremental cost-effectiveness ratios: uses and pitfalls. _Eur. J. Gastroenterol. Hepatol._ 16, 519–526. https://doi.org/10.1097/00042737-200406000-00003 (2004). Article PubMed Google
Scholar * Qureshi, A. R. et al. Increased circulating sclerostin levels in end-stage renal disease predict biopsy-verified vascular medial calcification and coronary artery calcification.
_Kidney Int._ 88, 1356–1364. https://doi.org/10.1038/ki.2015.194 (2015). Article CAS PubMed Google Scholar * Benjamini, Y. & Hochberg, Y. On the adaptive control of the false
discovery rate in multiple testing with independent statistics. _J. Educational Behav. Stat._ 25, 60–83. https://doi.org/10.3102/10769986025001060 (2000). Article Google Scholar * Hastie,
T., Tibshirani, R. & Friedman, J. The Elements of Statistical Learning Data Mining, Inference, and Prediction (12th printing). (2017). * Ben-Hur, A. & Weston, J. A user’s guide to
support vector machines. _Methods Mol. Biol._ https://doi.org/10.1007/978-1-60327-241-4_13 (2010). Article PubMed Google Scholar * James, G., Witten, D., Hastie, T. & Tibshirani, R.
_An Introduction To Statistical Learning with Applications in R_ 8th edn https://doi.org/10.1201/9781315120256 (Springer, 2017). * Freijeiro-González, L., Febrero-Bande, M. &
González-Manteiga, W. A critical review of LASSO and its derivatives for variable selection under dependence among covariates. _Int. Stat. Rev._ 90, 118–145.
https://doi.org/10.1111/insr.12469 (2022). Article MathSciNet Google Scholar * Hastie, T., Tibshirani, R., James, G. & Witten, D. An introduction to statistical learning (2nd ed.).
_Springer Texts_ 102. (2021). * Bobrowski, L. et al. Selection of genetic and phenotypic features associated with inflammatory status of patients on dialysis using relaxed linear
separability method. _PLoS ONE_. 9, e86630. https://doi.org/10.1371/journal.pone.0086630 (2014). Article ADS CAS PubMed PubMed Central Google Scholar * Weinstein, M. C. & Stason,
W. B. Foundations of cost-effectiveness analysis for health and medical practices. _N. Engl. J. Med._ 296, 716–721. https://doi.org/10.1056/nejm197703312961304 (1977). Article CAS PubMed
Google Scholar * Appleby, J., Devlin, N. & Parkin, D. NICE’S cost effectiveness threshold. _Br. Med. J._ https://doi.org/10.1136/bmj.39308.560069.BE (2007). Article Google Scholar *
Golembiewska, E. et al. Copeptin is independently associated with vascular calcification in chronic kidney disease stage 5. _BMC Nephrol._ https://doi.org/10.1186/s12882-020-1710-6 (2020).
Article PubMed PubMed Central Google Scholar * Makarović, S., Makarović, Z., Steiner, R., Mihaljević, I. & Milas-Ahić, J. Osteoprotegerin and vascular calcification: clinical and
prognostic relevance. _Coll. Antropol_. 39. (2015). * ter Braake, A. D. et al. Calciprotein particle Inhibition explains magnesium-mediated protection against vascular calcification.
_Nephrol. Dialysis Transplantation_. https://doi.org/10.1093/ndt/gfz190 (2020). Article Google Scholar * Yamada, S. & Giachelli, C. M. Vascular calcification in CKD-MBD: roles for
phosphate, FGF23, and Klotho. _Bone_ 100, 87–93. https://doi.org/10.1016/j.bone.2016.11.012 (2017). Article CAS PubMed Google Scholar * Fujii, H. Association between parathyroid hormone
and cardiovascular disease. _Therapeutic Apheresis Dialysis_. https://doi.org/10.1111/1744-9987.12679 (2018). Article PubMed Google Scholar * Silva, A. P. et al. Gla-rich protein (GRP) as
an early and novel marker of vascular calcification and kidney dysfunction in diabetic patients with CKD: A pilot cross-sectional study. _J. Clin. Med._ https://doi.org/10.3390/jcm9030635
(2020). Article PubMed PubMed Central Google Scholar * Jouni, H., Rodeheffer, R. J. & Kullo, I. J. Increased serum N-terminal pro-B-type natriuretic peptide levels in patients with
medial arterial calcification and poorly compressible leg arteries. _Arterioscler. Thromb. Vasc Biol._ https://doi.org/10.1161/ATVBAHA.110.216770 (2011). Article PubMed Google Scholar *
Sinha, S. & Santoro, M. M. New models to study vascular mural cell embryonic origin: implications in vascular diseases. _Cardiovasc. Res._ https://doi.org/10.1093/cvr/cvy005 (2018).
Article PubMed Google Scholar * Muyor, K. et al. Vascular calcification in different arterial beds in ex vivo ring culture and in vivo rat model. _Sci. Rep._
https://doi.org/10.1038/s41598-022-15739-w (2022). Article PubMed PubMed Central Google Scholar Download references FUNDING Baxter Novum is the result of a grant from Baxter Healthcare
Corporation to Karolinska Institutet. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Nalecz Institute of Biocybernetics and Biomedical Engineering, Polish Academy of Sciences, Warsaw, Poland
Urszula Bialonczyk, Malgorzata Debowska, Leon Bobrowski & Jan Poleszczuk * Aging Research Center, Department of Neurobiology, Care Sciences and Society, Karolinska Institutet and
Stockholm University, Stockholm, Sweden Lu Dai * Renal Medicine and Baxter Novum, Department of Clinical Science, Intervention and Technology, Karolinska Institutet, Stockholm, Sweden Abdul
Rashid Qureshi, Bengt Lindholm & Peter Stenvinkel * Faculty of Computer Science, Bialystok University of Technology, Białystok, Poland Leon Bobrowski & Tomasz Lukaszuk * Pathology,
Clinical Pharmacology and Safety Sciences, AstraZeneca R&D, Gothenburg, Sweden Magnus Soderberg Authors * Urszula Bialonczyk View author publications You can also search for this author
inPubMed Google Scholar * Malgorzata Debowska View author publications You can also search for this author inPubMed Google Scholar * Lu Dai View author publications You can also search for
this author inPubMed Google Scholar * Abdul Rashid Qureshi View author publications You can also search for this author inPubMed Google Scholar * Leon Bobrowski View author publications You
can also search for this author inPubMed Google Scholar * Magnus Soderberg View author publications You can also search for this author inPubMed Google Scholar * Bengt Lindholm View author
publications You can also search for this author inPubMed Google Scholar * Peter Stenvinkel View author publications You can also search for this author inPubMed Google Scholar * Tomasz
Lukaszuk View author publications You can also search for this author inPubMed Google Scholar * Jan Poleszczuk View author publications You can also search for this author inPubMed Google
Scholar CONTRIBUTIONS UB – conceptualization, methodology, software, visualization, writing – original draft; MD – conceptualization, writing – review & editing; LD – data curation,
writing – review & editing; AQ – data curation; LB – software; MS – supervision; BL – supervision, writing – review & editing; PS – supervision; TL – software; JP –
conceptualization, supervision, writing – review & editing. CORRESPONDING AUTHOR Correspondence to Urszula Bialonczyk. ETHICS DECLARATIONS COMPETING INTERESTS Peter Stenvinkel has a
support from Bayer for conducting a randomized trial on testosterone supplementation in dialysis patients, participates in scientific advisory boards: Astra Zeneca, Glaxo, Vifor, Baxter,
Fresenius, Invizius, and has payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Reata, Astra Zeneca, Baxter, Fresenius, Novo
Nordisk, Astellas, Pfizer, Bayer; Magnus Soderberg is a full-time employee of AstraZeneca; Malgorzata Debowska has grant no 2018/31/D/ST7/03472 from National Science Center (Poland); Jan
Poleszczuk has a grant grant No. 2018/31/D/ST7/03472 from National Science Center (Poland); Bengt Lindholm has a grant to Karolinska Institutet from Baxter Healthcare Corporation and was
previously employed by Baxter Healthcare Corporation. He also has received stock or stock options from Baxter Healthcare Corporation; Urszula Bialonczyk – none; Lu Dai – none; Abdul Rashid
Qureshi – none; Leon Bobrowski – none; Tomasz Lukaszuk – none. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps
and institutional affiliations. ELECTRONIC SUPPLEMENTARY MATERIAL Below is the link to the electronic supplementary material. SUPPLEMENTARY MATERIAL 1 RIGHTS AND PERMISSIONS OPEN ACCESS This
article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction
in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the
licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article
are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and
your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this
licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Bialonczyk, U., Debowska, M., Dai, L. _et al._ Balancing
accuracy and cost in machine learning models for detecting medial vascular calcification in chronic kidney disease: a pilot study. _Sci Rep_ 15, 17453 (2025).
https://doi.org/10.1038/s41598-025-02457-2 Download citation * Received: 07 January 2025 * Accepted: 13 May 2025 * Published: 20 May 2025 * DOI: https://doi.org/10.1038/s41598-025-02457-2
SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to
clipboard Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * Classification * Feature selection * Medial vascular calcification * Chronic kidney disease *
Incremental cost-effectiveness ratio