Optimization of polyphenols extraction by deep eutectic solvent from broccoli stem and characterization of their composition and antioxidative effects
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Broccoli stem is known to possess abundant polyphenols. To efficiently extract polyphenols from broccoli stems, we herein describe an updated deep eutectic solvent extraction (DESE)
method. Response surface modeling was utilized for optimization of the DESE method, achieving the best yield of 5.10 ± 0.04 mg polyphenols/g broccoli stem powder under the following
conditions: solvent, choline chloride-urea (molar ratio 1:3); extraction temperature, 80 °C; extraction duration, 55 min; water content, 60%; and liquid-solid ratio, 41:1 mL/g. Ultra
Performance Liquid Chromatography-ElectrosprayIonization-Quadrupole Time-of-Flight/Mass Spectrometry (UPLC-ESI-QTOF/MS) was then employed for characterization of the main -composition, which
revealed sinapinic acid (5.32%), trans-cinnamic acid (88.8%), quercetin (3.06%) and isochlorogenic acid (2.88%) as the dominant polyphenol compounds, and the extraction mechanism of the
high efficiency of DES was dicussed. Moreover, the extracts from (ChCl-urea) DES exhibited remarkable antioxidant activity in vitro antioxidant test. These findings provide a viable and
practical approach for broccoli culm polyphenol extraction and analysis, providing a methodological and knowledge basis for recycling broccoli wastes in an eco-friendly fashion. SIMILAR
CONTENT BEING VIEWED BY OTHERS OPTIMIZATION OF MICROWAVE-ASSISTED EXTRACTION OF ANTIOXIDANT COMPOUNDS FROM SPRING ONION LEAVES USING BOX–BEHNKEN DESIGN Article Open access 10 September 2023
OPTIMIZATION OF COMBINED SUBCRITICAL WATER AND CO2 EXTRACTION FOR ENHANCED PHENOLICS AND ANTIOXIDANT ACTIVITY FROM COFFEE BYPRODUCTS Article Open access 15 March 2025 BOOSTING ANTIOXIDATIVE
POLYPHENOLS EXTRACTION EFFICIENCY VIA NANO SIZED POMEGRANATE PEEL PARTICLES Article Open access 07 May 2025 INTRODUCTION Broccoli (_Brassica oleracea_ var. _italica_) is a
globally-distributed annual plant of the Bassicaceae family related with cauliflower, cabbage, kale and brussels sprouts1. Broccoli enjoys popularity among consumers and is of great health
significance because it contains many nutrients (protein, fibre, minerals, vitamins) and phytochemicals (glucosinolates, phenolic compounds)2,3,4,5,6. However, the edible part of broccoli,
mainly the florets, accounts for less than half of the whole broccoli, while stems and leaves totally account for over 95% of the weight of the whole plant7,8,9. Numerous studies have showed
that broccoli stem and leaf waste is rich in nutrients and bioactive components with potential preventative and therapeutic effects against human disorders10,11. Polyphenols are a group of
bioactive compounds that are primarily synthesised by plants12. They possess multiple phenol structural units and are good antioxidants and free radical scavengers. Polyphenols removes free
radicals from the body, prevents and treats circulatory system diseases, and prevents the deterioration of physiological functions13,14, which can protect proteins, lipids, and carbohydrates
from oxidative damage, thereby decreasing the risk of various chronic illnesses15. Consequently, polyphenols are considered a valuable source of nutraceuticals in both food and organism
lines. Separation and extraction is an important part in the research of plant polyphenols. Solvents such as methanol and its aqueous solutions, ethyl acetate andacetone have gained
widespread application for plant polyphenol isolation16,17,18. However, these conventional solvents are not eco-friendly or safe, They also don’t extract products of different polarities
well and can not maintain their bioactivity19,20. Deep Eutectic Solvents (DESs) are a type of green and efficient extraction solvent developed in recent years. DESs are generally
custom-designed that possess the capacity for self-association frequently by means of hydrogen bond. interactions21. DESs are usually composed of biocompatible, eco-friendly, inexpensive and
recoverable substances with ideal ionic liquid attributes, including thermal and chemical inertia and superb solubility22,23. The utilization of DESs as solvents in polyphenol isolation
from natural resources has already been described24. For instance, Wang et al.25 employed DESs for extracting Ficus carica leave polyphenols; Wei et al.26 utilized maltose and choline
chloride as a pair of DES to extract compounds of different polarities, which was better than using conventional solvents. Ruan et al.27 extracted the main polyphenols in Tieguanyin oolong
tea using a DES made from lactic acid and betaine. DESs extraction agent provides a eco-friendly new extraction method with high efficiency. However, research on broccoli stem polyphenol
isolation with DESs remains scarce. Therefore, we herein developed a highly efficient and nonpolluting DES (ChCl-urea) extraction strategy for polyphenols within the discarded stems of
broccoli. Response surface modeling (RSM) with Box-Behnken design (BBD) was utilized for determining the optimal methodological parameters. The main polyphenol constituents were identified
by UPLC-ESI-Q-TOF/MS. Additionally, the extracted polyphenols were also tested in vitro for their capabilities of scavenging DPPH and ABTS free radicals, ORAC total antioxidant capacity and
ferric reducing antioxidant power (FRAP) to appraise their application prospects in food industry and clinical settings. MATERIALS AND METHODS REAGENTS AND PLANT SAMPLES Fresh broccoli
samples were acquired in a local supermarket (Ningbo, China). Standard gallic acid was obtained from Yuanye Biotechnology Co., Ltd. Chemical Corporation (Shanghai, China). Reagents
containing Folin-Ciocalteu reagent, citric acid, glucose, sucrose, urea, choline chloride, sodium carbonate, 2,2 -Azinobis-(3- ethylbenzthiazoline-6-sulphonate) (ABTS),
1,1-diphenyl-2-picrylhydrazyl (DPPH), ORAC and FRAP assay kits were procured from Sinopharm Group Chemical Reagent Co. Ltd. (Shanghai, China). All reagents utilized in this research were of
analytical-grade purity. OPTIMIZATION OF THE DES METHOD DES PREPARATION DESs were prepared according to literature methods28,29 Four types of DESs were generated by a heating protocol, which
started from stir-mixing two DES components in a glass flask with round bottom on a magnetic stirrer with constant temperature (80 °C). After 40–60 min, this step was stopped once the
formation of a homogeneous mixture was observed. After incubation 30 min at ambient temperature, a transparent and colourless concentrated solution was obtained. The composition, molar
ratios and codes of DESs utilized are summarized in Table 1. EXTRACTION OF POLYPHENOLS The stems of fresh broccoli were rinsed with tap water, processed into 1-cm pieces, and subjected to
oven-drying at 60 °C (DHG-9053 A, Electric Heating Constant Temperature Blast Drying Oven, Yilin Scientific Instrument Co., Ltd, Shanghai, China) until the weight became constant. The
samples were then mashed into powder using a food processor (Joyoung Corporation., Ltd, Zhejiang China), which was passed through a 60-mesh sieve. Polyphenols within the samples were
extracted according to the previously described method30,31 For all experiments, a quantity of 0.25 g of broccoli stem powder was placed with a certain amount of DES solution, water or 70%
ethanol, mixed by vortex shaking evenly, and water-bathed a certain period of time (5 min shaken by vortexing once) with the temperature maintained unchanged (QL861 Vortex Shaker, Qilin Bell
Instrument Manufacturing Co., Ltd.Haimen, China). After equilibrating the mixture transiently to room temperature, it was subjected to a 20-min centrifugation (LC-180 Centrifuge, Keda
Innovation Incorporation, Ltd. China) at 5,000 rpm for supernatant collection. TOTAL POLYPHENOLS CONTENT DETERMINATION Total polyphenol contents were determined based on the Folin-Ciocalteu
method31, with gallic acid being utilized for standard curve establishment. Gallic acid standard working solutions with concentrations of 0–50 µg/mL (with 10 µg/mL intervals) were prepared
respectively, distilled water (for blank control) into a 25 mL colorimetric tube, add 5.0 mL of Folin-Ciocalteu reagent, shake well. Within 3 min ~ 8 min of the reaction, add 4.0 mL of 7.5%
Na2CO3 solution, make up the volume with water and shake well. After a 1-h reaction in darkness at ambient temperature, the 765-nm absorbance (A) was determined with a spectrophotometer and
the A value was determined for 3 times in parallel. The standard curve for gallic acid is obtained as y = 10.8771x + 0.0032, R2 = 0.9991. The outcomes are presented in the form of milligram
of gallic acid equivalent (GAE) per gram of dry matter (mg GAE/g DM). $$\text{Total phenolic content(mg GAE/g)}= m1\times{\frac{V_{1}}{V}}\times{m}\times1000$$ where m1is Content of gallic
acid found in standard equation(mg/mL); V1 is Total volume of extraction(mL); V denotes the volume of sample utilized for measurement (mL); m represents sample amount (g). OPTIMIZATION
EXTRACTION ON THE YIELD OF POLYPHENOLS We primarily analyzed the effects of different solvents on polyphenol yield, the extraction solvent was selected, Following a single-factorial design,
Extraction Solvents we explored the effects of independent variables, namely liquid-solid ratio (10–70 (mL/g), with intervals of 10), extraction time (10–90 min, with 20-min intervals),
extraction temperature (40–90 °C, with intervals of 10 °C), and water content (10–100% (w/w), with intervals of 10%) on polyphenol yield as the dependent variable, for DES2 with molecular
ratios of 1:2, 1:1, 2:1, 3:1 and 4:1. Based on the results of the univariate experiments, the extraction parameters were then optimized by RSM based on Design-Expert 10.0.7. Table 2 displays
the designed levels and coding for RSM factors, with data representing values from three replicates of one assay. The BBD analysis data were subjected to regression analysis. The accuracy
of RSM was verified by repeating the extraction procedures thrice under the optimized conditions. The polyphenols isolated from broccoli stem utilizing the optimized parameters were further
characterized for their structures and biological effects. COMPOSITION ANALYSIS Chromatographic separation and detection in the samples were analysed using a Waters synpat G2
(UPLC-ESI-QTOF/MS) system. Samples were chromatographically separated utilizing a reverse-phase ACQUITY UPLC C18 column (100 mm × 2.1 mm × 1.7 μm), during which a 2-µL injection volume and a
0.2-mL/min flow rate were adopted. The mobile phase was prepared by mixing water (A) with acetonitrile (B), the proportions of which were adjusted in accordance with the following scheme
during the gradient elution procedure: 5% B, 0–1.0 min; 5–100% B, 1.0–12.0 min; 100% B, 12.0–13.0 min; 100 − 50% B, 13.0–15.0 min. The negative-ion ESI mode was adopted for sample analysis,
spanning a 50–1200 m/z range. The desolvation gas temperature and flow of 350 °C and 900 L/h were applied. Cone hole and capillary voltages of 49.0 and 3.0 kV were utilized for mass spectra
acquisition. MassLynx v.4.1 was operated for instrument control and acquisition of data. ANALYSIS OF ANTIOXIDATIVE CAPABILITIES OF EXTRACTED POLYPHENOL COMPONENTS DPPH-SCAVENGING CAPABILITY
Antioxidative abilities of extraction of broccoli stem sample were compared with water and 70% ethanol solution. The DPPH-scavenging ability was appraised in accordance with a
previously-described procedure32. First, a DPPH solution (50 µg/mL) was mixed thoroughly with 1 mL broccoli stem extract in a closely-capped tube. The mixture was subjected to a 0.5-h
incubation at ambient temperature, after which the 517-nm optical densities (OD517) of the samples were tested with anhydrous ethanol as the blank control. Three replicates were tested
concurrently for each sample. The following equation was employed for calculating the DPPH scavenging rate: $$\text{DPPH scavenging percentage}\left( \%
\right)=\bigg[1-\frac{(A_{2}-A_{1})}{A_{0}}\bigg] \times {\text{ 1}}00\%$$ In which A2, A1 and A0 respectively denote OD517 values of 1 mL extract + 3 mL DPPH solution, 1 mL extract + 3 mL
ethanol and 1 mL DES solvent + 3 mL DPPH solution mixtures. ABTS-SCAVENGING CAPABILITY The ABTS-scavenging ability evaluation protocol was modified based on a previously-described
procedure33. Briefly, a 4.5-mM K2S2O8 solution was mixed thoroughly with a 7.4-mM ABTS solution to produce an ABTS reserve solution, which was then subjected to a 12–16-h incubation at 25 °C
till the solution colour turned dark blue-green. The ABTS reserve solution was then diluted into a concentration of 2 mM with 95% ethanol, and 200 µL of this diluted solution was mixed with
an equal volume of antioxidant solutions with various concentrations. A control group was included in the assays, for which the antioxidants were not added. The following equation was
employed for calculating the ABTS scavenging rate: $$\text{ABTS scavenging percentage}\left( \% \right)=\bigg[1-\frac{(A_{2}-A_{1})}{A_{0}}\bigg] \times {\text{ 1}}00\%$$ In which A2
represents the absorbance at 734 nm of 200µL of ABTS solution after adding 200µLof extract; A1 is the absorbance at 734 nm of 200µL of extract and 200µL of 95% ethanol; A0 is the absorbance
at 734 nm of 200µL of ABTS solution and 200µL of solvent(DES). FRAP TOTAL ANTIOXIDANT CAPACITY Total antioxidant capacity was appraised by an FRAP-based Total Antioxidant Capacity
Colorimetric Assay Kit (Yuan Yea, Shanghai). The FRAP working solution with deionised water was prepared from the kit stock solution. 30 uL of ferrous ion standard solution with different
gradient concentrations were added to 264 uL of FRAP solution respectively. A blank control group with samples replaced by deionised water was also included in the assays. The reaction
mixtures were sequentially subjected to vortexing and a 30 min incubation at 37 °C under darkness. Finally, a pectrophotometer was employed for measuring 593 nm optical absorbance. ORAC
ASSAYS The Oxygen Radical Antioxidant Capacity(ORAC) of the extract was evaluated using the test kit (Yuan ye, Shanghai). The antioxidant effects of samples were determined based on their
abilities for scavenging oxygen radicals, which otherwise would reduce the fluorescence intensity of fluorescein probes. Trolox solutions with concentrations of 0–40 µM were utilized in
these assays to establish an antioxidant standard curve, based on which the antioxidant capabilities of test samples were quantitatively analyzed. The linear regression equation for the
antioxidant standard curve was y = 3.943x-2.003, with a high degree of fit (r2 = 0.979). COSMO-RS CALCULATION COSMOthermX based on the COSMO-RS theory was used to calculate the thermodynamic
properties of the systems containing deep eutectic solvents. The structures of the involved compounds were optimized using TmoleX18 to obtain the σ-profile and σ-potential at BP/def-TZVP
basis set. STATISTICAL ANALYSIS All determinations were performed ≥ 3 times. All results are presented as mean ± standard deviation (SD) Statistically significant variations between groups
were identified with analysis of variance (ANOVA) followed by Fisher’s least significant difference (LSD) method utilizing SPSS 19. P values less than 0.05 were deemed to signify differences
with statistical significance. Error bars in artworks correspond to the 95% confidence level. RESULTS AND DISCUSSION OPTIMIZATION OF THE EXTRACTION OF BROCCOLI STEM POLYPHENOLS EFFECTS OF
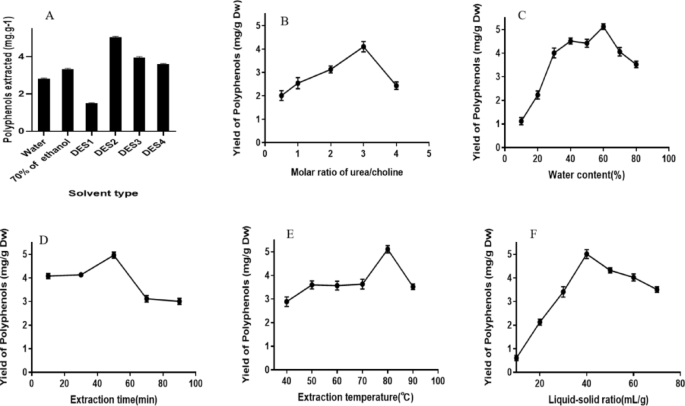
EXTRACTION SOLVENTS ON POLYPHENOL YIELD Extraction solvents are a crucial factor influencing the yield of final natural products. Considering that the extraction solvents’ permeability
within broccoli stem specimens may be largely affected by their physicochemical characteristics34, Table 1 summarizes the constituents and their molar ratios in analyzed DESs. We also
compared the polyphenol yields of using hot water and 70% ethanol as the solvents with those of DES-based methods. As shown in Fig. 1A, the yield of polyphenols differed notably among the
tested solvents, with that of the DES-1 group being markedly lower than those of others (_p_ < 0.05). Additionally, the DES-3 and DES-4 groups exhibited comparable polyphenol extraction
efficiencies (_p_ > 0.05). DES2 exhibited a significantly higher extraction efficiency than others, reaching 5.051 ± 0.04 mg.g−1. DW.This could be attributed to the low viscosity,
relatively large diffusion coefficient, good flowability, better permeability to the cell wall and the presence of a certain viscosity, which promoted the suspension and dispersion of
broccoli stem powder within the solvent and increased the solute-solvent contact surface, thereby facilitating polyphenol extraction. Therefore, we further optimized the extraction protocol
based on the DES system consisting of choline chloride and urea (DES-2). EFFECTS OF DES COMPOSITION RATIO ON POLYPHENOL YIELD As shown in Fig. 1B, adjusting the compostion ratio of DES-2
resulted in notable alterations in polyphenol extraction efficiency. Specifically, enhancing the proportion of urea within DES-2 elevated polyphenol yield, with the highest yield obtained at
the urea/choline ratio of 3:1. It may be due to urea improving DES-2’s polarity which facilitates the dissolution of polyphenols from broccoli. As the urea/ChCl ratio further increased,
made the viscosity of DESs larger, resulting in poor fluidity, which made the subsequent extraction process difficult35. Therefore, further analyses were carried out based on the urea/ChCl
ratio of 2:1. EFFECTS OF DES WATER CONTENT ON POLYPHENOL YIELD We next explored if DES water content could affect polyphenol yield. Indeed, distint polyphenol yields were noted under varied
DES water contents (Fig. 1C). Specifically, enhancing the water content of DES-2 from 10 to 60% boosted the yield from 13.82 ± 0.05% to 50.83 ± 0.04%. When the water content exceeded 70%,
however, the yield was markedly reduced by subsequent increases in water content. This might be attributed to the alterations in the viscosity, solubility, and relative polarity of the DES
extraction solvent, which are associated with the presence of water36. Additionally, the excessive addition of water can result in the destruction of DES37. Considering these findings,
further investigations were performed based on a DES-2 water content of 60%. EFFECTS OF EXTRACTION TIME ON POLYPHENOL YIELD Our findings revealed that extraction time exerted differential
impacts on polyphenol yield before and after an inflection point that appeared at the 50 th minute during a 90-min experimental procedure (Fig. 1D). Before the inflection point, the yield of
polyphenols rised from 4.16 to 4.87 mg/g with extraction time; after the inflection point, however, polyphenol yield declined from 4.87 to 3.03 mg/g (Fig. 1D). One feasible explanation is
that the prolongation of the extraction time may destruct the cell membrane, enhancing cell permeability and facilitating polyphenol extraction; whereas a long-term extraction at high
temperature may lead to the decomposition of the extracted polyphenolic compounds. Therefore, subsequent assays were performed based on an extraction time of 50 min. EFFECTS OF EXTRACTION
TEMPERATURE ON POLYPHENOL YIELD The effects of extraction temperature ranging from 40–90℃ on polyphenol yield were explored. As exhibited by Fig. 1E, extraction temperature affected
polyphenol yield differentially before and after an inflection point that was observed at an extraction temperature of 80℃. Before the inflection point, enhancing extraction temperature
elevated polyphenol yield; whereas after the inflection point, further elevating extraction temperature resulted in a significanly diminished polyphenol yield (_p_< 0.05). One possible
explanation for this observation is that the initial increase in extraction temperature simultaneously facilitated DES-2 dispersion and diminished its viscosity, while also expedited
polyphenol transportation38, thereby boosting polyphenol dissolution and augmenting its extraction yield. An extraction temperature exceeding 80℃, however, will damage the structure of the
polyphenols. Therefore, the following investigations were carried out with 80℃ as the optimized extraction temperature. EFFECTS OF LIQUID–SOLID RATIO ON POLYPHENOL YIELD The impacts of
liquid-solid ratio ranging from 10:1 to 70:1 (mL/g) on polyphenol yield were evaluated. According to our experimental data displayed in Fig. 1F, liquid-solid ratio was a crucial determining
factor for polyphenol extraction efficiency. The highest yield of 5.12 mg/g was observed at the liquid-solid ratio of 40:1, before which enhancing the liquid-solid ratio heightened
polyphenol yield. After this ratio, however, polyphenol yield seemed to exhibit a negative correlation with the liquid-solid ratio. It is reasonable to speculate that the initial increases
in DES-2 amount elevated solute-solvent contact surface and the concentration gradient, which is conducive to the dissolution of polyphenols, However, when the material-liquid ratio exceeds
the extraction equilibrium state or even saturates, it will increase impurity dissolution, compete for and hinder the dissolution of polyphenols. And the excessively high material-liquid
ratio increases the experimental cost37. Therefore, we selected 40:1 (mL/g) as the optimized liquid-solid ratio for this research. RSM-BASED OPTIMIZATION OF THE POLYPHENOL EXTRACTION
PROTOCOL We then carried out RSM-based optimisation to minimize the use of plant materials and solvents, save enery and time consumed during analyses, and identify an optimal factor
combination that could achieve the best polyphenol extraction efficiency. ANOVA and F-test analysis, as presented in Table 3, The regression model had an F-value of 74.61 (_p_ < 0.0001),
and the determination coefficient R² = 0.9897, together with the lack-of-fit term value was 0.0577 (_p_> 0.05) show that the established quadratic equation has high significance, and this
model can better reflect the relationship between each factor and the yield. The individual factors, the interaction terms AC and AB and the coefficients of the quadratic terms (A2, B2and
C2 were also found to be significant (_P_ < 0.05). According to the size of the F-value, the three factors A, B and C can be considered as influencing the yield of broccoli stem
polyphenols. The order from largest to smallest was C (liquid-solid ratio) > B (extraction time) > A (extraction temperature). Two factors were utilized each time to generate 2D
contour plots, representing a two-way interaction between extraction factors, to evaluate their collective effects on the response. From the contour plots as shown in Fig. 2, the trend of
significant factors and their interactions were investigated by referring to these plots. The findings revealed that the optimised polyphenol extraction parameters were an extraction time of
40–60 min, an extraction temperature of 70–90 ℃, and a liquid-solid ratio of 30–50:1. Compared to the interaction between other factors, the slope change of the response surface between
extraction temperature and liquid-solid ratio is steeper, which exhibited a great impact on the extraction amount of broccoli stem polyphenols, corroborating the ANOVA results. Multiple
regression analysis was employed for generating the following linear equation for predicting total polyphenol yield by extraction: $${\text{Y}} = {\text{5}}.0{\text{94}} -
0.{\text{1}}0{\text{13A}} + 0.{\text{1741B}} + 0.{\text{1879C}} + 0.{\text{14}}00{\text{AB}} + 0.{\text{5875AC}} - 0.0{\text{712BC}} - 0.{\text{5439A}}^{{\text{2}}} -
0.{\text{1891B}}^{{\text{2}}} - 0.{\text{7}}0{\text{87C}}^{{\text{2}}}$$ where Y was the yield of polyphenols (mg/g Dw), A was the extraction temperature (℃), B was the extraction time
(min), and C was the Liquid-solid ratio (mL/g). Design-Expert was then utilized for further optimizing the variables selected based on the abovementioned results, identifying the optimal
polyphenol extraction parameters of an extraction time (A) of 54.63 min, an extraction temperature (B) of 60℃, and a liquid-solid ratio (C) of 41:1, under which a highest estimated yield of
5.12 mg/g could be obtained. Under these extraction conditions, a polyphenol yield of 5.103 ± 0.03 mg/g was obtained by real experiments, a value that is close to the estimated yield,
thereby verifying the model’s validity. LC-MS ANALYSIS OF POLYPHENOLS UPLC-Q-TOF/MS was used to further characterize polyphenolic compounds. Table 4 summarizes the MS data of fragment ion,
molecular ion and polyphenol compound retention time. The exact molecular weight patterns was used for identification, These data were compared against the corresponding published results,
leading to the identification of four major polyphenol components, namely quercetin, isochlorogenic acid, transcinnamic acid and sinapinic acid.The total ion chromatogram of a broccoli stem
extract after (ChCl-Urea) DES and the representative SIM chromatograms of the main polyphenolic compounds are shown in Figs. 3 and 4. ANTIOXIDANT ACTIVITY OF BROCCOLI EXTRACTS BROCCOLI
EXTRACTS FROM DIFFERENT SOLVENTS Study of the effects of the in vitro antioxidant activity of different extract solvents (DES-2/H2O/70% EtOH). As shown in Fig. 5(A), the antioxidant capacity
of different solvent extracts is directly proportional to their concentration and increases with increasing concentration. At the mass concentration of 250 µg/ml, the DPPH radical
scavenging capacity of 70% EtoH, DES-2 and H2O was 79.48%, 78.12% and 34.39%, respectively. As shown in Fig. 5(B), when the different solvent extraction concentrations increased from 20
µg/mL to 250 ug/mL, the clearance of ABTS radical from DES-2 increased from 21.50 to 82.93%, from 70%EtOH increased from 20.37 to 79.63%, and from H2O extract increased from 12.37 to 34.36%,
the clearance of ABTS radical of all three extraction solvents were proportional to the concentration. As shown in Fig. 5 (C), the Trolox molar equivalence of reduced Fe(III) was achieved
as the different solvent extraction concentrations increased from 50 µg/mL to 250 ug/mL. The results showed that DES-2 extract was the best antioxidant, followed by 70% ethanol extract. The
water extract has the lowest oxidation, the TE content is only 105 mmol/L at the concentration of 250 u g/mL. As shown in Fig. 5 (D), the total antioxidant capacity (TRAC) of the three
Trolox at different solvent extraction concentrations increased from 50 µg/mL to 250 ug/mL. DES-2 extract was 3.45 ± 0.1; 3.21 ± 0.2umol TE/L for 70% ethanol and water 1.27 ± 0.2umol TE/L;
it is concluded that the antioxidant activity of DES-2 broccoli extract was better than that of the conventional extract.The efficient enrichment of polyphenols in broccoli stems based on
ChCl-urea has been further validated by these results. COSMO-RS CALCULATION RESULTS Infinite dilution activity coefficients (\(\gamma^\infty\)) reflects the strength of interaction between
solute and solvent molecules in the dissolved system, which is the solubility of solvent for solute. The larger the concentration of solute in the solvent, the smaller the value. The
calculated results of the two low eutectic solvents are shown in Table 5, DES2 (ChCl-Urea) are the lowest in both solutes, indicating that the solubility of DES2 (ChCl-Urea) for the two
solutes is best. The calculated results are consistent with the experimental results, it proves the reliability of the experimental data. Because of the molecular specificity, σ-profile and
σ-potential were used to analyze the intermolecular interactions, the results of which are shown in Figs. 6 and 7. From Fig. 6, it can be seen that the peaks of DES2 (ChCl-Urea) and
quercetin, trans-cinnamic acid overlapped more in the nonpolar region, which indicated the strong interaction between them. It can be seen from Fig. 7 that DES2 (ChCl-Urea) has the same
trend with quercetin, trans-cinnamic acid. They have higher negative values in the hydrogen bond donor region and their value tends to increase in the hydrogen bond acceptor region,
indicates that there is a strong affinity between solutes and solvents. The simulation results also reflect the reliability of the experimental results. CONCLUSION This study has
demonstrated the efficiency of deep eutectic solvents as green alternatives for the extraction of polyphenols from Broccoli Stem.ChCl-urea was selected from a series of solvents for the
extraction of total polyphenols from Broccoli Stem. Based on the results of the single factor experiment, RSM was used to identify the main parameters and optimise the extraction conditions.
The extraction from broccoli stem system possessed excellent antioxidant activity which was significantly higher than that from tranditonal solvent extraction methods. The COSMO-RS
calculation indicates that there is a strong affinity between solutes and solvents. Therefore, the extraction of polyphenols by DES not only enhanced the yield, but also better retained the
bioactivities of extracts. DES extraction of polyphenols from broccoli stems has the characteristics of simple synthesis, low cost, and good environmental protection extraction effect.
Overall, this study evidences that DES(ChCl-urea) provides theoretical support and technical support for its application and development in the extraction of polyphenols from broccoli waste,
valorizable for pharmaceutical, food, and cosmetic applications. DATA AVAILABILITY All data generated or analysed during this study are included in this published article [and its
supplementary information files].The authors declare that the data supporting the findings of this study are available within the paper and its Supplementary Information files. Should any
raw data files be needed in another format they are available from the corresponding author upon reasonable request. Source data are provided with this paper. REFERENCES * Ilahy, R. et al.
Pre- and post-harvest factors affecting glucosinolate content in broccoli. _Frontrs Nutr._ 10 (7), 147 (2020). Article Google Scholar * Hang, L. et al. Nutritional values, beneficial
effects, and food applications of broccoli (Brassica Oleracea Var. Italica Plenck). _Trends Food Sci. Technol._ 119, 288–308 (2021). Google Scholar * Takashi, W. et al. An approach of
shelf-life extension technology focusing on recovery lug of physiological responses: kinetic analysis for residual effect of modified atmosphere packaging on the color changes of broccoli
flower buds. _Postharvest Biol. Technol._ 206, 112579 (2023). Article Google Scholar * Miyahira, R. F. et al. Changes in phenolic compound and antioxidant activity of germinated broccoli,
wheat, and lentils during simulated Gastrointestinal digestion. _Plant Foods Hum. Nutr._ 77, 233–240 (2022). Article CAS PubMed Google Scholar * Li, C. et al. Effect of Choline-Based
deep eutectic solvent pretreatment on the structure of cellulose Malaeke and lignin in Bagasse. _Processes_ 9, 384 (2021). Article CAS Google Scholar * Thomas, M. et al. Characterization
of industrial broccoli discards (Brassica Oleracea Var. italica) for their glucosinolate, polyphenol and flavonoid contents using UPLC MS/MS and spectrophotometric methods. _Food Chem._ 245,
1204–1211 (2017). Article PubMed Google Scholar * Le, T. N. et al. Polyphenolic profile and varied bioactivities of processed Taiwanese grown broccoli: A comparative study of edible and
Non-Edible parts. _Pharmaceuticals_ 13, 82 (2020). Article CAS PubMed PubMed Central Google Scholar * Domínguez, P. R. et al. Composition and antioxidant capacity of a novel beverage
produced with green tea and minimally-processed byproducts of broccoli. _Innov. Food Sci. Emerg. Technol._ 12, 361–368 (2011). Article Google Scholar * Borja-Martínez, M. et al.
Revalorization of broccoli By-Products for cosmetic uses using supercritical fluid extraction. _Antioxidants_ 9, 1195 (2020). Article PubMed PubMed Central Google Scholar * Alejandra, G.
A. et al. Edible plant by-products as source of polyphenols: prebiotic effect and analytical methods. _Food Sci. Nutr._ 63 (31), 10814–10835 (2022). Google Scholar * Gudiño, A. et al.
Evaluation of broccoli (Brassica Oleracea Var. italica) crop by-products as sources of bioactive compounds. _Sci. Hort._ 304, 111284 (2022). Article Google Scholar * Zhang, L. Delivery of
synergistic polyphenol combinations using biopolymer-based systems: advances in physicochemical properties, stability and bioavailability. _Crit. Rev. Food Sci. Nutr._ 60, 2083–2097 (2020).
Article CAS PubMed Google Scholar * César, G. F. et al. The effects of polyphenols and other bioactives on human health. _Food Funct._ 10, 514–528 (2019). Article Google Scholar *
Talmaciu, A. I. et al. A comparative analysis of the green techniques applied for polyphenols extraction from bioresources. _Chem. Biodivers._ 12, 1635–1651 (2015). Article CAS PubMed
Google Scholar * Hu, Y. et al. Recent technologies for the extraction and separation of polyphenols in different plants: A review. _J. Renew. Mater._ 10 (6), 1471–1490 (2022). Article CAS
Google Scholar * Kumar, M. N. et al. Extraction of polyphenols from hybrid Eucalyptus leaves using ultrasound: parametric and antioxidant studies. _Environ. Qual. Manage._ 58 (7),
3935–3945 (2023). ADS Google Scholar * Wagare, D. S. et al. Sustainable solvents in chemical synthesis: a review. _Environ. Chem. Lett._ 19, 3263–3282 (2021). Article CAS Google Scholar
* Sara, C. et al. Extraction techniques with deep eutectic solvents. _Trends Anal. Chem._ 105, 225–239 (2018). Article ADS Google Scholar * Mónica, A. et al. Deep eutectic solvents
(DES) based on sulfur as alternative lubricants for silicon surfaces - ScienceDirect. _J. Mol. Liq._ 295 (C), 111728–111728 (2019). Google Scholar * Dai, Y. et al. Application of natural
deep eutectic solvents in the extraction of Quercetin from vegetables. _Molecules_ 24(12) (2019). * Wang, W. et al. Development and optimization of green extraction of polyphenols in
michelia Alba using natural deep eutectic solvents (nades) and evaluation of bioactivity. _Sustainable Chem. Pharm._ 37, 101425 (2024). Article CAS Google Scholar * .Alfaleh, A. A. et al.
Systematic study on date palm seeds (Phoenix dactylifera L.) extraction optimisation using natural deep eutectic solvents and ultrasound technique. _Sci. Rep._ 14, 16622 (2024). Article
PubMed PubMed Central Google Scholar * Amine, D. et al. Deep eutectic solvent-based sonication assisted dispersive liquid-liquid Microextraction using Box-Behnken optimization for the
determination of patent blue V in food and drug samples. _J. Food Compos. Anal._ 135, 106634 (2024). Article Google Scholar * Wang, T. et al. Enhanced and green extraction polyphenols and
furanocoumanns from Fig Ficus carica L) leaves using deep eutectic solvents. _J. Pharm. Biomed. Anal._ 145, 339–345 (2017). Article CAS PubMed Google Scholar * Wei, Z. F. et al. Appli
cation of natural deep eutectic solvents for extraction and determin ation of phenolics in Cajanus Cay an leaves by ultra performance liquid chromatography. _Sep. Purif. Technol._
149:237–244 . * Ruan, Y. H. et al. Optimization of extraction process and component analysis of Catechin from eutectic solution by response surface Methodology[J]. _Food Ind._ 41 (3),
121–125 (2020). Google Scholar * Jun, C. et al. Efficient extraction of pro antho Cyanidin from Ginkgo biloba leaves employingrationally designed deep eutectic solvent-water mixture and
evaluation of the anti oxidant activity. _Pharmaceut Biomed._ 158, 317–326 (2018). Article Google Scholar * Li, Z. et al. Characterization of glucosinolates in 80 broccoli genotypes and
different organs using UHPLC-Triple-TOF-MS method. _Food Chem._ 334, 127519 (2021). Article CAS PubMed Google Scholar * Liu, Z. et al. Pulsed electric field as an alternative
Pre-treatment for drying to enhance polyphenol extraction from fresh tea leaves. _Food Bioprocess Technol._ 12 (01), 183–192 (2019). Article CAS Google Scholar * Mondal, M. et al.
Purification of polyphenols from green tea leaves and performance prediction using the blend Hollow Fiber ultrafiltration membrane. _Food Bioprocess Technol._ 12 (06), 438 (2019). Article
Google Scholar * Duan, Q. et al. Extraction of Moringa oleifera seed oil by SCF-CO2 and analysis of its constitution. _China Oils Fats_. 35, 76–79 (2010). Google Scholar * Meng, L. S. et
al. Composition and antioxidant activity of anthocyanins from Aronia melanocarpa cultivated in Haicheng Liaoning, China. _Food Bioscience_. 30 (C), 106102–106102 (2019). Google Scholar *
Xing, C. et al. Ultrasound-assisted deep eutectic solvents extraction of glabridin and Isoliquiritigenin from Glycyrrhiza glabra: optimization, extraction mechanism and in vitro
bioactivities. _UltrasonicsSonochemistry_ 83, 105946 (2022). CAS Google Scholar * Cui, Q. et al. Deep eutectic solvent-based microwave-assisted extraction of Genistin, genistein and
apigeninfrom pigeon pea roots. _Sep. Purif. Technol._ 150, 63–72 (2015). Article CAS Google Scholar * Liu, C. H. et al. Preparation of nanofluids based on Urea/choline chloride eutectic
solvent system and its thermal properties. _Acta Chem. Eng._ 72 (03), 1333–1341 (2021). CAS Google Scholar * Dai, Y. et al. Natural deep eutectic solvents as a new extraction media for
phenolic metabolites in Carthamus tinctorius L. _Anal. Chem._ 85, 6272–6278 (2013). Article CAS PubMed Google Scholar * Zhang, Q. et al. Deep eutectic solvents:syntheses, properties and
applications. _Chem. Soc. Rev._ 41, 7108–7146 (2012). Article ADS CAS PubMed Google Scholar * Tian, Y. T. et al. Process optimization and mechanism analysis of polyphenols for
extraction of apple peels by ultrasonic assisted natural eutectic solvents. _Mod. Food Technol._ 196, 1–14 (2024). Google Scholar Download references ACKNOWLEDGEMENTS This work was
supported by Ningbo Public Welfare Fund Project(Grant 2021S069). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * College of Biological and Environmental Science, Zhejiang Wanli University,
No.8 South Qianhu Road, Yinzhou District, Ningbo, 315000, Zhejiang, China Bingqing Wang, Peiyun Chen, Huien Zhang, Yanlei Chen & Liqing Chen Authors * Bingqing Wang View author
publications You can also search for this author inPubMed Google Scholar * Peiyun Chen View author publications You can also search for this author inPubMed Google Scholar * Huien Zhang View
author publications You can also search for this author inPubMed Google Scholar * Yanlei Chen View author publications You can also search for this author inPubMed Google Scholar * Liqing
Chen View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS Bingqing Wang, Liqing Chen, and Huien Zhang, Peiyun Chen wrote the main manuscript
text and Yanlei Chen, Peiyun Chen prepared all figures. All authors reviewed the manuscript. CORRESPONDING AUTHOR Correspondence to Peiyun Chen. ETHICS DECLARATIONS COMPETING INTERESTS I
declare that the authors have no competing interests as defined by Springer, or other interests that might be perceived to influence the results and/or discussion reported in this paper.
CONSENT FOR PUBLICATION All authors have approved the final manuscript and agree with submission to Scientific reports. This manuscript has not been published, accepted for publication or
submitted elsewhere. The authors declare no conflicting financial interests or personal relationships that may interfere with data collection, analysis or reporting for this research. ETHICS
APPROVAL Not applicable. CONSENT TO PARTICIPATE Not required for this study. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in
published maps and institutional affiliations. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International
License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the
source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived
from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line
to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will
need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/. Reprints and permissions ABOUT THIS
ARTICLE CITE THIS ARTICLE Wang, B., Chen, P., Zhang, H. _et al._ Optimization of polyphenols extraction by deep eutectic solvent from broccoli stem and characterization of their composition
and antioxidative effects. _Sci Rep_ 15, 16066 (2025). https://doi.org/10.1038/s41598-025-00632-z Download citation * Received: 30 November 2024 * Accepted: 29 April 2025 * Published: 08 May
2025 * DOI: https://doi.org/10.1038/s41598-025-00632-z SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable
link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * Polyphenol extraction * Deep eutectic
solvent * Composition * Broccoli stem * Antioxidant activity