Untargeted metabolomics reveal the corrective effects of scorpion on epileptic mice
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Scorpion is a commonly used drug in traditional Chinese medicine for treating epilepsy, although the exact mechanisms are not yet fully understood. This study aimed to compare the
treatment effects of Scorpion water extract (SWE) and Scorpion ethanol extract (SEE) on mice with pentetrazole-induced epilepsy and investigate the possible mechanisms through metabolomics
methods. A pentetrazole-induced epileptic mice model was used to assess the corrective effects of SWE and SEE. Untargeted metabolomics, utilizing UPLC-Q-TOF/MS, was employed to analyze the
metabolic profiles of mice and identify metabolic changes following scorpion treatment. The results revealed that only SWE showed therapeutic effects in epileptic mice. Metabolomics analysis
demonstrated significant alterations in metabolic signatures between the pentetrazole-induced epileptic mice and SWE groups. By utilizing orthogonal partial least squares discrimination
analysis, 44 and 108 potential biomarkers in mouse serum were identified in positive and negative ion modes, respectively. Differential metabolites related to epilepsy were then used to
pinpoint relevant pathways in epileptic mice, such as linoleic acid metabolism, biosynthesis of unsaturated fatty acids, glycerophospholipid metabolism, and ether lipid metabolism. In
conclusion, this study highlights the corrective effects of Scorpion on epileptic mice and provides insight into the underlying metabolic pathways involved in its efficacy. SIMILAR CONTENT
BEING VIEWED BY OTHERS ALTERATIONS IN SERUM METABOLOMICS DURING THE FIRST SEIZURE AND AFTER EFFECTIVE CONTROL OF EPILEPSY Article Open access 19 August 2024 A COMPREHENSIVE INVESTIGATION OF
_CLERODENDRUM INFORTUNATUM_ LINN. USING LC-QTOF-MS/MS METABOLOMICS AS A PROMISING ANTI-ALZHEIMER CANDIDATE Article Open access 05 January 2025 TOWARDS A BETTER UNDERSTANDING OF IDIOPATHIC
EPILEPSY THROUGH METABOLIC FINGERPRINTING OF CEREBROSPINAL FLUID IN DOGS Article Open access 26 June 2024 INTRODUCTION Epilepsy is a chronic neurological disorder characterized by a
persistent tendency to experience seizures1. According to the World Health Organization (WHO), epilepsy contributes to about 1% of the global disease burden, ranking fourth among
neuropsychiatric disorders after depression, alcoholism, and cerebrovascular disease, with a similar impact to breast and lung cancer2. It affects over 65 million individuals worldwide, with
causes ranging from acute brain injuries and genetic mutations to metabolic disorders and autoimmune conditions3,4. The social, cognitive, and economic burdens of epilepsy are substantial,
making the development of effective treatments a key focus in epilepsy research. Traditional Chinese medicine (TCM) has shown promising efficacy in managing epilepsy with minimal side
effects5,6,7. The dried body of _Buthus martensii_ Karsch, known as Scorpion, is frequently used in China for epilepsy treatment8,9,10. Nevertheless, the precise mechanisms through which
Scorpion alleviates epilepsy symptoms remain unclear. Metabolomics has emerged as a powerful tool for identifying disease-related biomarkers and potential drug targets across various stages
of illnesses11,12. Metabolomics plays a crucial role in uncovering the corrective mechanisms of Chinese medicine, particularly in recent TCM research13. A widely adopted technique,
UPLC-Q-TOF/MS14, known for its high resolution and sensitivity, was employed in our study to investigate these mechanisms. Specifically, we aimed to analyze how Scorpion treats epilepsy. We
induced an epileptic mice model using pentetrazole. Using UPLC-Q-TOF/MS for untargeted metabolomics, we identified potential biomarkers and assessed the overall metabolic changes induced by
Scorpion in epileptic mice. This research contributes to a deeper understanding of Scorpion’s corrective mechanisms in epilepsy treatment. MATERIALS AND METHODS CHEMICAL REAGENTS AND
MATERIALS HPLC-grade acetonitrile, methanol, and formic acid were purchased from TEDIA (Fairfield, OH, USA). Ultra-pure water was processed by the Milli-Q water purification system
(Millipore, Bedford, USA). Ethanol was obtained from Beijing Shiji (Beijing, China). Pentetrazole (PTZ) was purchased from Aladdin (Shanghai, China). Scorpion was purchased from a local
market in 2023. PREPARATION OF SCORPION EXTRACTS Ten g of Scorpion was crushed and sieved through a No. 20 mesh. It was then added to 500 ml of water and boiled for 40 min. After filtering
by vacuum suction filtration, the residue was subjected to the same process: adding another 500 ml of water and boiling it for another 40 min for extraction. The filtrate was combined and
concentrated using rotary evaporator at 50 ℃ before being freeze-dried to obtain the scorpion water extract (SWE). Ten g of Scorpion was crushed and sieved through a No.20 mesh. It was then
added to 500 ml of 50% ethanol solution. Extraction was performed using a magnetic mixer under 50 ℃ and 1000 rpm for 12 h. After filtering by vacuum suction filtration, the residue was
subjected to the same conditions for another extraction. The filtrates were combined and concentrated using rotary evaporator at 50 ℃ before being freeze-dried to obtain the scorpion ethanol
extracts (SEE). ANIMALS AND DRUG ADMINISTRATION Twenty-seven male Kunming mice weighing 20–22 g were supplied by the Experimental Animal Center of Jilin University. All animal experiments
and husbandry have been carried out under the guidelines of the Animal Ethics Committee of Jilin Medical University (Approval Code: 20230126, Approval Data: Match 10th, 2023). All
experimental methods were performed in accordance with the ARRIVE guidelines (https://arriveguidelines.org), guidelines of the Chinese Institution of Laboratory Animal Sciences
(https://cnilas.org/en/). Twenty-seven mice were divided into the model group (n = 10), SWE group (n = 8), and SEE group (n = 9). Every morning, mice in the SWE and SEE groups were orally
administered the corresponding Scorpion extracts once at a dosage of 1g/kg15,16. The control group was given an equal amount of physiological saline. Administration was carried out
continuously for 7 days. Thirty min after final administration on day 7, pentetrazole (80 mg/kg) was injected to induce acute epileptic seizures in mice. After injecting pentetrazole, the
mice were immediately placed into transparent compartments marked with designated groups, and the camera was turned on to record the seizure activity of all mice within the compartments for
30 min. According to the Racine scale17,18 (Grade 0, no convulsion observed,Grade I, head nodding, eye blinking, neck movement, salivation, or wet dog-like tremor,Grade II, rhythmic head
nodding,Grade III, head clonus with forelimb tremor; Grade IV, Grade III with hindlimb tonic–clonic movements; Grade V, generalized tonic–clonic seizure with falling), we recorded the Grade
V seizures of each mouse, calculated the seizure frequency, and analyzed the effect of the scorpion venom extract on mouse seizures. The seizure frequency data were further processed by
Student’s _t_-test (SPSS19.0, Chicago, IL, USA) to get p value. And _p_ < 0.05 indicates significant differences between groups. SAMPLE COLLECTION AND PREPARATION Whole blood samples were
collected after recording seizures in mice. Blood was collected in an Eppendorf tube and then centrifuged at 2000×_g_ for 10 min at 4 °C to obtain the serum. Methanol (400 µL) was added to
the serum sample (100 µL) for protein precipitation. After vortex-mixing for 1 min and centrifuging at 12,000×_g_ for 10 min, the supernatant was separated and lyophilized. Residues were
re-dissolved in acetonitrile–water mixture (1:1, v/v) (100 µL) and centrifuged at 12,000×_g_ for 15 min at 4 °C. The supernatant was subjected to UPLC-QTOF/MS analysis. A pooled “quality
control” (QC) sample was prepared by mixing equal aliquots (10 μL) from all prepared serum samples. All prepared samples were stored at 4 °C. UPLC-Q-TOF/MS ANALYSIS Chromatographic
separation was performed using a Shimadzu LC20AD ProminenceTM UPLC system equipped with a Thermo Fisher Golden C18 column (2.1 × 50 mm, 1.9 µm) maintained at a temperature of 35°C. Mobile
phase A consisted of 0.1% formic acid in water, and mobile phase B consisted of 0.1% formic acid in ACN. The gradient for mobile phase B was programmed as follows: 5% (0 to 1 min), 5–35% (1
to 14 min), 35–95% (14 to 20 min), 95% (20 to 22 min), and 95–5% (22 to 22.1 min). The system was then equilibrated at 5% for 2.9 min. The injection volume was set to 5.0 µL, and a 0.3
mL/min flow rate was employed. Q-TOF/MS analysis was conducted on a Triple-TOF 5600 + MS (SCIEX, Concord, Canada) with an ESI source. The MS parameters were as follows: positive or negative
ion mode, source temperature: 500 ℃, ion spray voltage: + 5500 or − 4500 V, nebulizer gas (N2): 55 psi, heater gas (N2): 60 psi; curtain gas (N2) of 35 psi, and declustering potential: 100
V. Full-scan MS data were acquired from m/z 100 to 1000 in TOF/MS mode with collision energy 5 eV. The MS/MS data were acquired in IDA mode using the following settings: collision energy of
35 eV, rolling collision energy with a spread of 15 eV, the mass range of precursors were from m/z 100 to 1000, and the mass range of MS/MS spectra were from m/z 100 to 1000. DATA ANALYSIS
AND IDENTIFICATION OF POTENTIAL BIOMARKERS After UPLC-Q-TOF/MS analysis, the raw data obtained from the model group, SWE group, and QC samples were imported into MS-DIAL software 4.10
(http://prime.psc.riken.jp/) to perform data preprocessing, including peak identification, peak matching, and peak alignment of retention time and mass. The peak alignment was performed
using a reference file (QC data). The retention time tolerance was set at 0.1 min, and the MS1 tolerance was set at 0.01 Da. The data collection parameters were as follows: an MS1 tolerance
of 0.01 Da and an MS2 tolerance of 0.02 Da. The peak detection parameters were a minimum peak height of 3000 amplitude and a mass slice width of 0.1 Da. The resultant dataset, containing m/z
value, retention time, the normalized intensity, and the sample code, was used to perform the multivariate statistical analysis. Then, the dataset was saved as .csv files and imported into
SIMCA software 13.0 (Umetrics, Umea, Sweden) to conduct the orthogonal partial least squares-discriminant analysis (OPLS-DA). In the OPLS-DA model, ions with variable importance in
projection (VIP) 1 values larger than 1 were highlighted and were further filtered by Student’s t-test (SPSS19.0, Chicago, IL, USA). The metabolites with _p_ < 0.05 were considered
significant and were selected as potential biomarkers. After screening for the accurate molecular weight and fragment information of the candidate variable fragments from MS-DIAL software
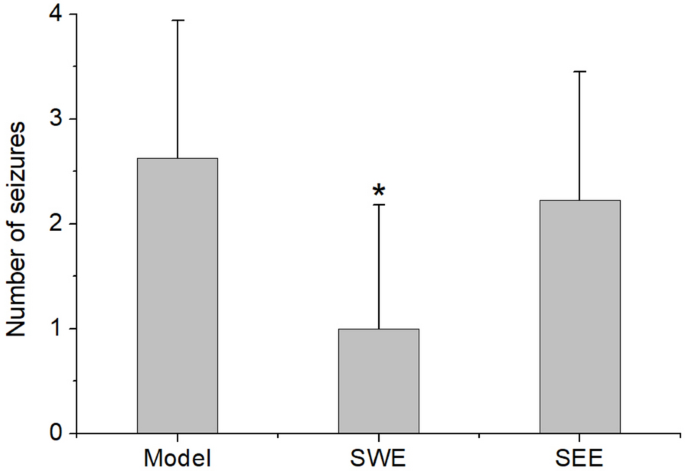
4.10, biomarkers were identified. MetaboAnalyst 5.0 (https://www.metaboanalyst.ca/) was used to identify metabolic pathways. RESULTS EFFECTS OF SCORPION ON EPILEPTIC MICE As shown in Fig. 1,
the number of seizures in the model, SWE, and SEE groups were 2.63 ± 1.32 (n = 8), 1.00 ± 1.19 (n = 10), and 2.22 ± 1.23 (n = 9), respectively. Compared with the model group, the number of
seizures in the SWE group was significantly lower. Moreover, there was no significant difference in the number of seizures between the model and SEE groups. The results indicated that only
SWE showed good effects on epileptic mice, and the extraction method played an important role in the pharmacological function of Scorpion. Therefore, the serum samples from the SWE group
were used in the following metabolomics study. METABOLOMIC ANALYSIS To systematically elucidate the mechanisms by which SWE ameliorate epilepsy, serum samples were analyzed using
UPLC-Q-TOF/MS in both positive and negative ion modes. Representative total ion chromatograms (TIC) of serum samples from both the model and SWE groups were obtained under optimal conditions
(Fig. 2). After data preprocessing, 880 and 655 peaks were detected in positive and negative ion modes, respectively. Before statistical analysis, method validation was performed to
evaluate the repeatability and stability of this established method. A QC sample was analyzed three times at the beginning of an analytical run and then once every five samples to provide
robust quality assurance for each peak. The repeatability of the established methods was evaluated with the relative standard deviation (RSD) of the peaks in 7 QC samples. More than 80% of
ions were detected in a QC sample with an RSD of less than 20% for positive and negative ion modes, indicating the excellent repeatability and stability of the present methods (Fig. S1).
There were 1 positive ion peak and 8 negative ion peaks which has high RSD (more than 30%) between QC samples. And the peaks with high RSD had been excluded before OPLS-DA. To evaluate the
systemic changes of the metabolome in epileptic mice treated with SWEs, OPLS-DA was used to explore the tendency of metabolic profiles between the model and SWE groups. A positive ion
OPLS-DA model was established with R2 (Y)= 0.9581 and Q2 (cum)= 0.8670, suggesting that the established model had prominent fitness and predictability. Similarly, the negative ion OPLS-DA
model had prominent fitness and predictability (R2 = 0.9354 and Q2 = 0.9127). Permutation test (n = 200) was further performed to validate the model. No overfitting was found because the
permutated R2 and Q2 values on the left are lower than the original point on the right (Fig. S2). As shown in Fig. 3A,B, all samples were within the Hotelling T2 (0.95) ellipse, and the
OPLS-DA sore plot suggested that clear separations from the model and SWE groups were observed in both positive and negative ion modes. The excellent separation between the SWE group and
model groups in the OPLS-DA score plots indicated that the SWE group had completely different metabolic profiles compared with the model group. IDENTIFICATION OF POTENTIAL ENDOGENOUS
BIOMARKERS To identify key metabolites distinguishing between the model and SWE groups, OPLS-DA was employed to generate S-plots (Fig. 3C,D). These plots visualize covariance and correlation
relationships from the model, along with Variable Importance in Projection (VIP) values. This approach reduces false positives in metabolite selection. Initially, features with VIP greater
than 1 were selected as significant variables, further screened using Student’s t-test to identify metabolites significantly differing between groups. Finally, total 154 ions (46 from
positive and 108 from negative) with p < 0.05 were retained as potential biomarkers, highlighted in red on the S-plot (Fig. 3C,D). Next, the selected biomarkers were identified based on
the MS and MS/MS information recorded in the MS-DIAL metabolomics database (https://systemsomicslab.github.io/compms/msdial/main.html#MSP). The ion features with statistical significance
(_p_ < 0.05 and VIP > 1.0) were identified and are summarized in Table 1. Specifically, compared to the model group, the SWE-treated mice exhibited altered levels in a total of 19
metabolites, all of which were found to be decreased (Fig. 4). ANALYSIS OF METABOLIC PATHWAY OF POTENTIAL BIOMARKERS Topology and pathway enrichment analyses were conducted to assess the
role of metabolites in biological reactions based on their positions within related pathways and to identify key metabolomic pathways. All identified potential biomarkers were analyzed using
MetaboAnalyst for metabolic pathway analysis, presented through an interactive visualization system (Fig. 5A,B). The results of metabolic pathway enrichment and topological analysis
indicated that treatment of PTZ-induced epileptic mice primarily affected metabolic pathways such as linoleic acid metabolism, biosynthesis of unsaturated fatty acids, glycerophospholipid
metabolism, and ether lipid metabolism. These pathways are interconnected, as illustrated in Fig. 6 and Table S1. The corrective effects of SWE on epileptic mice were exerted by reducing the
levels of ten metabolites, including stearic acid, palmitic acid, docosahexaenoic acid, eicosapentaenoic acid, gamma-linolenic acid, arachidonic acid, linolenic acid,
lysophosphatidylcholine 18:1, 1-stearoyl-sn-glycero-3-phosphocholine, and phosphatidylcholine lyso 17:0. DISCUSSION Scorpion is a type of animal-derived traditional Chinese medicine,
commonly used in clinical practice as a decoction and sometimes prepared as a tincture. According to the results of this study, SWE have a certain corrective effect on epileptic mice, while
SEE show no significant corrective effect. When clinically using Scorpion to treat epilepsy, more consideration may be given to its use as a decoction. Epilepsy is a chronic condition
characterized by recurrent seizures resulting from abnormal neuronal activity in the brain. This abnormal activity can be caused by neuronal overexcitation or dysfunctional coordination of
neuronal firing. The etiology of epilepsy involves various factors, including genetic predisposition, brain injury, metabolic abnormalities, and others19,20,21. Recent studies reveal that
epilepsy might be induced by a metabolic etiology and epileptic seizures also contribute to changes of small endogenous metabolites22, Ingrid E Scheffer et al., 2017). A report about
children with drug-refractory epilepsy indicated that the top two enriched metabolic pathways involved in the drug-refractory epilepsy condition were the biosynthesis of unsaturated fatty
acids and linoleic acid metabolism, and a decrease in fatty acids and an increase in triglycerides were associated with the response to anti-seizure medications therapy23. Similarly, our
study showed that the Scorpion’s ability to treat epileptic mice was also related to these two metabolic pathways by decreasing the levels of stearic acid, palmitic acid, docosahexaenoic
acid, eicosapentaenoic acid, gamma-linolenic acid, arachidonic acid, linolenic acid. The biomarkers stearic acid, palmitic acid, docosahexaenoic acid, and eicosapentaenoic acid have been
identified in cerebrospinal fluid, as previously reported24,25. The observed fluctuation in the levels of these four metabolites within both serum and cerebrospinal fluid post-SWE treatment
in epileptic mice indicates potential correlations. However, a deeper understanding of the true connections between these serum and cerebrospinal fluid variations and the specific changes in
these four metabolites within the cerebrospinal fluid remains a subject for further investigation. Additionally, the study failed to pinpoint the distinct ways SWE and SEE regulate the
metabolism of epileptic mice, nor did it identify the differing components of the two extracts. The differences in the chemical composition of SWE and SEE, and how their related active
compounds impact the metabolism of epileptic mice, will be explored in our future research. CONCLUSION The study focused on the effects of Scorpion on epileptic mice, with results
demonstrating significant corrective effects specifically from SWE treatment. An untargeted metabolomic analysis using UPLC-Q-TOF/MS was then implemented to investigate the serum metabolome
profiles in epileptic and SWE-treated mice systematically. This approach aimed to uncover the corrective effects of SWE on metabolic imbalances associated with epilepsy. The application of
OPLS-DA further elucidated the action mechanisms of SWE, leading to the identification of 19 potential biomarkers in serum samples linked to metabolic pathways such as linoleic acid
metabolism. In conclusion, our research offers a comprehensive analysis of SWE’s corrective effects in epilepsy, providing insights into metabolic abnormalities and revealing the corrective
mechanisms of animal-derived Traditional Chinese Medicine at a holistic level. DATA AVAILABILITY The data supporting the findings of this study are available within the article. Additional
datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request. REFERENCES * Robert, S. F. et al. ILAE official report: A
practical clinical definition of epilepsy. _Epilepsia_ 55, 475–482 (2014). Article MATH Google Scholar * Ali, A. Global health: Epilepsy. _Semin. Neurol._ 38, 191–199 (2018). Article
PubMed MATH Google Scholar * Ingrid, E. S. et al. ILAE classification of the epilepsies: Position paper of the ILAE commission for classification and terminology. _Epilepsia._ 58, 512–521
(2017). Article MATH Google Scholar * Vezzani, A., Balosso, S. & Ravizza, T. Neuroinflammatory pathways as treatment targets and biomarkers in epilepsy. _Nat. Rev. Neurol._ 15,
459–472 (2019). Article PubMed MATH CAS Google Scholar * Sun, T. et al. Efficacy and safety of Chinese herbal medicine in post-stroke epilepsy: A systematic review and meta-analysis.
_Front. Pharmacol._ 14, 1286093 (2023). Article PubMed PubMed Central CAS Google Scholar * Wu, J. et al. Research progress on the treatment of epilepsy with traditional Chinese
medicine. _Phytomedicine._ 120, 155022 (2023). Article PubMed MATH CAS Google Scholar * Zhang, R. & Wang, X. Research progress in the treatment of epilepsy by traditional Chinese
medicine. _Acad. J. Med. Health Sci._ 4, 72–76 (2023). MATH Google Scholar * Chen, Q. et al. Effects of scorpion venom heat-resistant peptide on the hippocampal neurons of Kainic
acid-induced epileptic rats. _Braz. J. Med. Biol. Res._ 54, e10717 (2021). Article PubMed PubMed Central CAS Google Scholar * Rong, P. et al. Chinese herbal compounds containing
scorpion in the treatment of epilepsy: A protocol for systematic review and meta-analysis. _Medicine._ 100, e25134 (2021). Article PubMed PubMed Central Google Scholar * Sun, Y. et al.
Effects of scorpion venom heat-resistant protein on seizure behavior and expression of proenkephalin in rats with kainate-induced epilepsy. _Neurophysiology._ 45, 319–322 (2013). Article
MATH CAS Google Scholar * Claudio, T. et al. Exploring the potential role of metabolomics in COPD: A concise review. _Cells._ 13, 475 (2024). Article MATH Google Scholar * Leila, A. et
al. Metabolomic biomarkers of endometriosis: A systematic review. _J. Endometr. Uter. Diso._ 7, 100077 (2024). Article MATH Google Scholar * Ding, J., Jiang, T., Qian, J., Wang, G. &
Liu, S. Metabolomic profiles delineate the effect of Sanmiao wan on hyperuricemia in rats. _Biomed. Chromatogr._ 31, e3792 (2017). Article Google Scholar * Susan, T. O. et al.
Applications of chromatographic methods in metabolomics: A review. _J. Chromatogr. B._ 1239, 124124 (2024). Article MATH Google Scholar * Liang, Y. et al. Effects of ethanol extract of
scorpion on the mRNA expression of hippocampus GFAP in rats with chronic Epilepsia. _China Pharm._ 23, 4033–4035 (2012). Google Scholar * Wu, M. & Zhang, X. Experimental study on acute
toxicity of “Qufeng Zhidong Decoction” and its scorpion in mice. _SH. J. TCM Dec._ 42, 77–79 (2008). MATH Google Scholar * Ronald, J. R. Modification of seizure activity by electrical
stimulation: Cortical areas. _Electr. Clin. Neur._ 38, 1–12 (1975). Article MATH Google Scholar * Wu, L., Qin, Y., Yuan, H., Zhu, Y. & Hu, A. Anti-inflammatory and neuroprotective
effects of insulin-like growth factor-1 overexpression in pentylenetetrazole (PTZ)-induced mouse model of chronic epilepsy. _Brain Res._ 1785, 147881 (2022). Article PubMed CAS Google
Scholar * David, J. T. et al. Standards for epidemiologic studies and surveillance of epilepsy. _Epilepsia._ 52, 2–26 (2011). Article MATH Google Scholar * Tracy, G. et al. Updated ILAE
evidence review of antiepileptic drug efficacy and effectiveness as initial monotherapy for epileptic seizures and syndromes. _Epilepsia._ 54, 551–563 (2013). Article Google Scholar *
Wolfgang, L., Heidrun, P., Sanjay, M. S. & Annamaria, V. Drug resistance in epilepsy: Clinical impact, potential mechanisms, and new innovative treatment options. _Pharmacol. rev._ 72,
606–638 (2020). Article MATH Google Scholar * Wang, D., Wang, X., Kong, J., Wu, J. & Lai, M. GC–MS–Based metabolomics discovers a shared serum metabolic characteristic among three
types of epileptic seizures. _Epilepsy Res._ 126, 83–89 (2016). Article PubMed MATH CAS Google Scholar * Guo, H. et al. Integrating metabolomics and lipidomics revealed a decrease in
plasma fatty acids but an increase in triglycerides in children with drug-refractory epilepsy. _Epilepsia Open._ 8, 466–478 (2023). Article PubMed PubMed Central Google Scholar * Alfred,
N. F., Matthew, C., Abby, J. C., Sarah, P. E. & Michael, G. H. Polyunsaturated fatty acid composition of cerebrospinal fluid fractions shows their contribution to cognitive resilience
of a pre-symptomatic Alzheimer’s disease cohort. _Front. Physiol._ 11, 83 (2020). Article Google Scholar * Židó, M., Kačer, D., Valeš, K., Zimová, D. & Štětkářová, I. Metabolomics of
cerebrospinal fluid amino and fatty acids in early stages of multiple sclerosis. _Int. J. Mol. Sci._ 24, 16271 (2023). Article PubMed PubMed Central Google Scholar Download references
FUNDING This research was funded by the Science and Technology Project from the Department of Science and Technology of Jilin Province (Grant No. YDZJ202201ZYTS268). AUTHOR INFORMATION
AUTHORS AND AFFILIATIONS * School of Pharmacy, Jilin Medical University, Jilin, 132013, Jilin, China Lele Li, Shengyu Ge, Heyun Zhu & Bo Feng * Jilin Ginseng Academy, Changchun
University of Chinese Medicine, Changchun, 130117, Jilin, China Yang Wang Authors * Lele Li View author publications You can also search for this author inPubMed Google Scholar * Shengyu Ge
View author publications You can also search for this author inPubMed Google Scholar * Yang Wang View author publications You can also search for this author inPubMed Google Scholar * Heyun
Zhu View author publications You can also search for this author inPubMed Google Scholar * Bo Feng View author publications You can also search for this author inPubMed Google Scholar
CONTRIBUTIONS L.Z. data collection and analysis; G.W. conception and design; W.S. material preparation; X.M. review the manuscript. CORRESPONDING AUTHORS Correspondence to Yang Wang, Heyun
Zhu or Bo Feng. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ETHICAL APPROVAL The Animal Ethics Committee of Jilin Medical University (Approval Code:
20230126, Approval Data: Match 10th, 2023) reviewed and approved this animal study. The study is reported in accordance with ARRIVE guidelines (https://arriveguidelines.org) and guidelines
of the Chinese Institution of Laboratory Animal Sciences (https://cnilas.org/en/). ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional
claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION 1. SUPPLEMENTARY INFORMATION 2. SUPPLEMENTARY INFORMATION 3. SUPPLEMENTARY
INFORMATION 4. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any
non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the
Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of
it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material
is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission
directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Li,
L., Ge, S., Wang, Y. _et al._ Untargeted metabolomics reveal the corrective effects of scorpion on epileptic mice. _Sci Rep_ 15, 937 (2025). https://doi.org/10.1038/s41598-024-84028-5
Download citation * Received: 17 August 2024 * Accepted: 19 December 2024 * Published: 06 January 2025 * DOI: https://doi.org/10.1038/s41598-024-84028-5 SHARE THIS ARTICLE Anyone you share
the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer
Nature SharedIt content-sharing initiative KEYWORDS * Scorpion * Untargeted metabolomics * UPLC-Q-TOF/MS * Epilepsy * Metabolic pathways * Metabolites