Amelioration of cognition by hesperidin-conjugated cobalt oxide nanoparticles
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
Diabetes mellitus is one of the metabolic syndromes that is associated with cognitive deficit, dementia, and Alzheimer’s disease (AD) like pathology due to impaired insulin-signalling in the
brain, oxidative stress and mitochondrial dysfunction. Nanotechnology is one of the most promising techniques for targeting the brain. However, the toxicity of metal nanoparticles is one of
the biggest challenges to be studied. In this study, cobalt oxide nanoparticles are conjugated to a bioflavonoid, hesperidin, a natural antioxidant. The study is designed to assess the
efficacy and safety of the cobalt oxide conjugated hesperidin in the diabetes-induced cognitive deficit rat model. The neuropharmacological behaviour, in-vivo antioxidant status and level of
acetylcholinesterase, nitrite, amyloid β, and pro-inflammatory cytokines were determined for cobalt oxide conjugated hesperidin and compared with bare cobalt oxide nanoparticles and
hesperidin. The cobalt oxide conjugated hesperidin significantly improved learning and memory in the streptozotocin rat model. However, further studies are required to establish a cellular
and molecular mechanism involved in the neuroprotective activity of cobalt oxide-conjugated hesperidin.
Cognitive deficit is a disorder characterized by forgetfulness, amnesia, dementia, and progression to Alzheimer’s disease (AD)1. The prevalence of cognitive deficits is increasing
exponentially in India and globally2,3. Dementia is reported to be found in the population above 60 years of age and hardly before 60 years. Several pathological alterations like
mitochondrial dysfunction, impaired glucose metabolism, hyperglycemia, hyperinsulinemia, impaired insulin-signalling pathways, and oxidative stress play an important role in the development
of cognitive dysfunction or Alzheimer’s disease4. Some other neurological phenomena like neuroinflammation, neurodegeneration, increase in the levels of acetylcholinesterase and nitrite,
accumulation of amyloid beta peptides, hyperphosphorylation of tau proteins and deposition of neurofibrillary tangles also lead to loss of memory, dementia and AD5,6.
Diabetes mellitus is a metabolic disorder characterized by impaired glucose metabolism, insulin resistance or decreased insulin levels and associated with various secondary
complications7,8,9,10. The risk of cognitive deficit, dementia and AD is more profound in type II diabetes mellitus (T2DM) than in type I diabetes mellitus (T1DM)11. Abnormalities of insulin
and the insulin receptors in the brain cells lead to the development of cognitive impairment12,13,14. Other metabolic processes like oxidative stress, mitochondrial dysfunction, increased
levels of pro-inflammatory cytokines, and advanced glycation end-products have a great interlink between DM and cognitive dysfunction5,15. These also hamper the synaptic plasticity in the
brain16. The major antioxidant of the brain is glutathione (GSH), which is decreased in the diabetic brain of rats17 and the hippocampus and frontal cortex of the brain of patients with
memory impairment18,19. Hence, brain oxidative stress is an early biomarker for the identification of the risk of development of dementia, cognitive impairment and AD.
Nanotechnology has been explored in biomedical applications due to many advantages, such as increased potency, decreased dosing frequency, increased stability of drug nanoformulations,
bioavailability, targeted drug delivery, and reduced toxicity. Metal-based nanoformulations diagnose and treat diseases like cancer, diabetes, neurodegenerative disorders, and many more20.
However, neurotoxicity is one of the major limitations of delivering metal-based nanoformulations targeting the brain. The major drawback of metal nanoparticles is the elevation of reactive
oxygen species (ROS) and reactive nitrogen species (RNS)21. The elevated ROS and RNS cause mitochondrial damage, decrease ATP content, and regulate the expression of hypoxia-induced factor
alpha1, leading to morphological change and neuronal cell death22. Cobalt oxide nanoparticles (Co3O4 NPs) are reported to increase lipid peroxidation, leading to an increase in the levels of
malondialdehyde, causing neuroinflammation in the glial cells23, and elevating the inflammatory cytokine expressions like IL-1β and NLRP3, NADPH oxidase, and microglial marker Iba-1
expression24.
This research work is designed to synthesize the cobalt oxide nanoparticles conjugated with hesperidin and to assess its efficacy in a diabetes-induced cognitive deficit rat model. Though
hesperidin is reported to have excellent antioxidant and neuroprotective activity25,26, its low water solubility and less bioavailability to brain tissues limits its application for
blood-brain barrier permeability. The authors have studied the antioxidant and neuroprotective efficacy of gold and core-shell bimetallic Se@Au NPs of hesperidin10,27. In this study, the
conjugation of Co3O4 NPs to hesperidin is planned to study the neuroprotective activity through the antioxidant mechanism. The changes in acetylcholinesterase, nitrite and pro-inflammatory
cytokine levels were also evaluated.
Extra pure hesperidin was purchased from SRL, India, with a purity of 99%. Other reagents of high analytical grade required for our study were procured from local suppliers. The Cobalt
chloride, hexahydrate (Himedia, India) and HPLC grade water (Merck, India) needed for the synthesis of nanoparticles were procured from authentic suppliers.
The hesperidin conjugated cobalt oxide nanoparticle (HSP - Co3O4 NPs) was synthesized by the addition of 9 ml of 1 mM cobalt chloride hexahydrate with 1 ml of 0.1% w/v trisodium citrate as
reducing and stabilizing agent followed by addition of 1mL of HSP solution (500 µg/mL) at a temperature of 60–70 °C for a period of half an hour. Then, adding 1 drop of dilute sodium
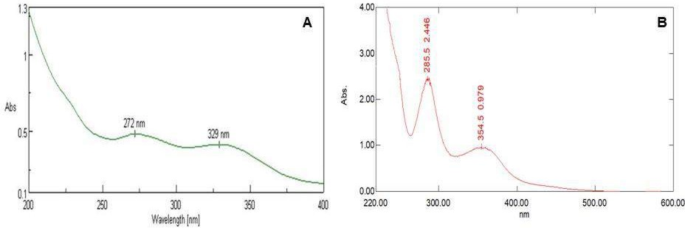
hydroxide solution led to a color change from white to light green, which signifies nanoparticle synthesis. The formation of HSP - Co3O4 NPs was confirmed by recording the localized surface
plasmon resonance (LSPR) in a UV-visible spectrophotometer.
The determination of surface plasmon resonance (SPR) confirmed the formation of magnetic nanoparticles. Characterization was done by Ultraviolet-visible spectroscopy (UV), Fourier Transform
Infrared Spectroscopy (FTIR), Zeta potential, and High-resolution Transmission Electron Microscopy (TEM) with Selected Area Electron Diffraction (SAED). All of these methods analyze the
shape and size of the particle as well as the surface area, dimension, and crystallinity. For the LSPR of HSP-Co3O4 NPs, the wavelength was chosen for scanning in the range of 250–800 nm by
the UV-visible spectrophotometer (JASCO, V-630). Zeta sizer Nano– ZS (Malvern Instrument Ltd., Malvern, UK) analyzed the hydrodynamic diameter, size distribution, and stability. Further, the
JOEL model JM2100 was employed for the TEM and SAED images of the synthesized HSP- Co3O4 NPs.
Thirty Wistar rats of either sex and weighing about 120-180 g were being issued from the central animal house of the School of Pharmaceutical Sciences, Siksha’ O’ Anusandhan (deemed to be)
University, Bhubaneswar. The rats were housed in polypropylene cages and provided sufficient water and food. The standard environmental condition was maintained at 25 ± 5 °C and relative
humidity of 25–35%. 12 h of light and the dark cycle was maintained per the principles and protocols of the Institutional Animal Ethical Committee, having approved proposal number
IAEC/SPS/SOA/117/2022.
Rats were once administered intraperitoneally 65 mg/kg streptozotocin (STZ). After five days, animals with fasting blood sugar > 250 mg/dl were divided into four groups (n = 6). Group I
animals were supposed to be a streptozotocin group and treated with vehicle (10 ml/kg i.p.) only. Group II animals were given cobalt nanoparticle solution (4 mg/kg, i.p.); Group III animals
received hesperidin solution (4 mg/kg, i.p.); Group IV animals were administered with hesperidin cobalt conjugated nanoparticle (4 mg/kg, i.p.) for 14 days. The neuropharmacological
behavioural studies such as Y maze, Elevated Plus Maze (EPM), and Radial Arm Maze (RAM) were conducted before and after the 14th day of treatment with the drug. After performing the
behavioural studies, the rats were kept under overnight fasting and decapitated under the anaesthetic condition via administration of ketamine (87.5 mg/kg i.p.) and xylazine solution (12.5
mg/kg i.p.). The brain, pancreas, and kidneys were isolated. The isolated brains (n = 3) were used to prepare homogenate to determine the antioxidant activity. The rest of the isolated
brains (n = 3) and the pancreas and kidney were stored in a 10% formalin solution for further histopathological studies. For histopathology studies, one more group of control rats (n = 3)
without any treatment were taken for comparison purposes.
Any alterations in the spontaneous behaviour of rats can be determined by evaluating spatial memory function in rats. The Y-Maze is used to analyze the altered behaviour. The recorded
observations were utilized to determine the percentage of spontaneous alternation behaviour (SAB) using the earlier formula5,28.
The approach by which rats’ memory and spatial learning functions are measured is called elevated plus maze (EPM). The time taken by each rat to move from the open arm end to the closed
walls with all four limbs was measured as transfer latency at the beginning of the trial. A cut-off time of 60 s was set for each trial. Similarly, after 24 h, the retention transfer latency
was recorded6,28.
The memory function of the rats is determined with the help of a radial arm maze (RAM) by analyzing the number of corrected responses shown by rats following the methods reported
earlier5,28.
To measure the activity of the enzyme superoxide dismutase (SOD), a blank solution containing 0.5 ml of 1 mM EDTA was prepared. 1 ml of 0.2 mM pyrogallol and 1.5 ml of tris buffer (0.05 mM)
were added to the blank solution. The test solution was prepared by adding the brain homogenate to the blank solution. The absorbance was measured at 420 nm in a UV-visible spectrophotometer
(JASCO, V-300). The following equation was used to compute the percentage of protection28,29
Where A control = absorbance of control A sample = absorbance of samples.
The concentration of malondialdehyde (MDA), an indicator of lipid peroxidation, was determined using UV-vis spectroscopy. The preparation of the blank was done by taking 2 ml (0.37% w/v) of
thiobarbituric acid (TBA), followed by mixing it with 2 ml (15% v/v) of trichloroacetic acid (TCA) and 2 ml (0.25 N) hydrochloric acid (HCl). 100 µl of brain homogenate was added to the
previously prepared blank to prepare the test solution. Then, the measurement of absorbance was recorded at 532 nm. The MDA concentration is then estimated from the standard calibration
curve. The results were then expressed in nmol/L29.
A UV-visible spectrophotometric method was used to measure the glutathione content. For the preparation of the assay mixture, 1 ml of 10 mM 5,5’-dithio-bis-(2-nitrobenzoic acid) (DTNB) was
mixed with 1 ml of 0.1 M phosphate buffer solution (PBS) at pH 7.4. The assay mixture was the control. Then, the test solution was prepared by adding 0.1 ml of brain homogenate to the
previously prepared assay mixture (control). At a wavelength of 412 nm, the absorbances of the control and the test solution were recorded. The percentage of protection was calculated using
Eq. (1)5,30.
Hydrogen peroxide’s (H2O2) ability to result in oxidation at 240 nm can be used to measure the catalase enzyme’s activity. Catalase activity was estimated by mixing 1.95 mL of phosphate
buffer (0.05 M, pH 7.4) with 1 mL of hydrogen peroxide (0.019 M). The test solution was prepared by combining 50 µL of brain homogenate with the resulting solution. The decomposition rate at
240 nm was then estimated by measuring the absorbance of the test solution and the blank. The catalase activity (CAT) unit is content/min/mg/protein31.
The Acetylcholinesterase (AChE) activity of the brain is measured by preparing an assay mixture of 3 ml phosphate buffer (0.01 M, pH = 8), 0.1 ml Acetylcholine iodide (0.075 M) and 0.1 ml
5,5′- dithio – bis − (2-nitrobenzoic acid) (DTNB) (10 mM) and taken as blank. Then, 50 µL of brain homogenate was added to the previously made assay mixture to prepare the test solution.
Then, with the help of UV-visible spectroscopy, the absorbance of the test solution and the blank were determined at 412 nm. The percentage of protection was computed following Eq. (1)32.
The Greiss reagent has been utilized to quantify the biological level of nitric oxide across the brain. At first, the assay mixture was prepared using the Greiss reagent of 500 µL containing
0.1% naphthylamine diamine dihydrochloric acid in water and 1% sulphadiazine in 5% phosphoric acid in a ratio of 1:1, and it was incubated at room temperature for a time of 5 min. Then, the
brain homogenate of 100 µL was added to prepare the test solution, and the absorbance was measured by UV-visible spectroscopy at 546 nm. The nitrite concentration was calculated and
expressed as µmol/mg/protein10.
The inflammatory mediators like interleukin-6 (IL-6) and tumour necrosis factor (TNF-α), the cytokines that induce neuronal inflammation, cause cognitive impairment by destroying the brain’s
hippocampal region. By the ELISA technique, the IL-6 production can be quantified. An ice-cold buffer solution (Tris-HCl, pH 7.4) of 20 mM was prepared, which contained EDTA (0.1 mM), PMSF
(0.5 mM) with benzamidine (0.5 mM), and DTT (0.1 mM) and to that solution, the brain homogenate was mixed. An ultrasonic disrupter was used to remove the total protein. Then, the solution
was kept for centrifugation for 30 min at 4 °C. The supernatant was collected and stored at 28 °C33,34.
The amyloid-β estimation was done by using a Sandwich ELISA kit. At first, the standard solution was prepared using the serial dilution method, and a standard calibration curve was prepared.
From this, a standard calibration curve was obtained. Then, the protein content was evaluated using the BCA (bicinchoninic acid) assay method. A microplate pre-coated with the matching
antibodies was incubated with approximately 100 µL containing 250 g of protein from the soluble fraction of Aβ1-42; a sample solution was added, and incubation was carried out for 90 min at
37 °C. Then, the microplate was washed using a wash buffer of 350 µL, and incubation was done for 60 min at 37 °C using a biotinylated detection antibody of 100 µL. After incubation, samples
were washed, and HRP-labelled conjugate was added to each well and kept in incubation for 30 min. Then, the reaction was stopped by adding 40 µL of stop solution after washing the samples.
The optical density was then evaluated using an ELISA reader at a wavelength of 450 nm after it had been preheated for a while at the same temperature34.
The tissues from different organs like the brain, kidney, and pancreas were piled up. A 10% formalin solution was used to preserve the collected tissues. Subsequently, the tissues were
subjected to fixation, followed by drying and cleansing using various solutions containing water, ethanol, and xylene. The resulting tissues are then fixed in the paraffin solution by
cutting around 5 to 6 μm thicknesses into pieces. Next, the tissues underwent staining with hematoxylin and eosin dye solutions. Subsequently, the stained tissues were placed on a trinocular
biological microscope to enhance visibility and facilitate observation at a magnification 10x35.
A one-way analysis of variance (ANOVA), followed by Tukey’s t-test, was used to analyze the data from the studies that were conducted. The value of p