Non-invasive sars-cov-2 rna detection and human transcriptome analysis using skin surface lipids
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT There have been several reports of skin manifestations in patients with coronavirus disease 2019 (COVID-19). However, it is unclear whether severe acute respiratory syndrome
coronavirus 2 (SARS-CoV-2) RNA can be detected on the skin surface, including the sebum, of these patients. In this study, SARS-CoV-2 RNA was detected using real-time reverse-transcription
polymerase chain reaction (RT-PCR) assay of skin surface lipids (SSLs) collected using an oil-blotting film from the faces of hospitalized patients with COVID-19. Human transcriptome
analysis was also performed using the same samples. In facial SSLs of patients with COVID-19, the RT-PCR positivity rate was 84.6% (11/13 samples) within 5 days and 30.4% (7/23 samples) by
6–10 days of symptom onset. In the transcriptome analysis, the most characteristic SSL-RNA profile was the upregulation of interferon-stimulated gene (ISG)-related genes, such as _ISG15_,
_IFITM1_, and _MX1_. This study presents an alternative technique using SSLs for non-invasive SARS-CoV-2 RNA detection and simultaneous analysis of human molecular pathogenesis in patients
with COVID-19. SIMILAR CONTENT BEING VIEWED BY OTHERS SKIN _STAPHYLOCOCCUS AUREUS_ DETECTION AND RELATIONSHIP TO ATOPIC DERMATITIS OUTCOMES USING CULTURE AND METAGENOMIC SEQUENCING Article
Open access 21 May 2025 PREVALENCE OF _MALASSEZIA_ SPECIES ON THE SKIN OF HIV-SEROPOSITIVE PATIENTS Article Open access 20 October 2020 AN RNA-SEQ ANALYSIS OF CORONAVIRUS IN THE SKIN OF THE
PANGOLIN Article Open access 09 January 2024 INTRODUCTION Since the beginning of the coronavirus disease 2019 (COVID-19) pandemic, real-time reverse-transcription polymerase chain reaction
(RT-PCR) of viral RNA isolated from nasopharyngeal swabs has been used for its clinical diagnosis. However, identification of the specimens carrying severe acute respiratory syndrome
coronavirus 2 (SARS-CoV-2) at detectable levels is still underway, and this can be critical in devising strategies to prevent SARS-CoV-2 transmission. It has been reported that SARS-CoV-2
can be detected in upper respiratory tract specimens, such as nasopharyngeal and oropharyngeal swabs, and in other specimens, such as saliva, sputum, bronchoalveolar fluid, feces, blood, and
urine1,2. The collection of nasopharyngeal swab fluid is invasive, uncomfortable for patients, and poses a risk of infection to healthcare workers; therefore, SARS-CoV-2 nucleic acid
detection using saliva specimens—which are as sensitive as nasopharyngeal swab fluid—is now widely used for diagnosis3. However, there are several limitations, such as collection of an
insufficient amount of saliva samples from which SARS-CoV-2 nucleic acid cannot be detected4 and difficulty in collecting saliva samples from infants and children. Cutaneous manifestations,
such as morbilliform rash, urticaria, vesicles, pseudo-chilblains, and vaso-occlusive lesions, are observed in some patients with COVID-195. Additionally, SARS-CoV-2 RNA has been detected in
skin biopsies of patients with COVID-196,7. Notably, the reports of SARS-CoV-2 RNA detection in sweat samples are controversial8,9,10. Moreover, it remains unclear whether SARS-CoV-2 can be
detected on the skin surface. We previously established a method using RNA isolated from skin surface lipids (SSLs) for biological analysis11. The method enables transcriptome analysis
using RNA extracted from SSLs collected using an oil-blotting film; it can be used to detect pathological molecular changes in patients with atopic dermatitis and Parkinson’s
disease12,13,14. It is a simple specimen collection method, and unlike blood or nasopharyngeal swabs, samples can be collected by nonmedical personnel, including the patients themselves. In
this study, we aimed to clarify whether SARS-CoV-2 RNA can be detected on the skin surface of patients with COVID-19. Facial SSLs of patients with COVID-19 were collected using an
oil-blotting film, and RT-PCR and patient transcriptome analyses were performed on these samples. Here, we established an alternative, non-invasive method for the detection of SARS-CoV-2 and
simultaneous analysis of the molecular pathogenesis of COVID-19 using an oil-blotting film. RESULTS PATIENT DEMOGRAPHIC AND CLINICAL FINDINGS Hospitalized patients with COVID-19 who were
enrolled between April 2020 and April 2021 (_n_ = 29) and healthy participants who underwent SSL sampling in 2019 (_n_ = 18) were included in this study (Supplementary Table 1). COVID-19
occurrence in patients was confirmed by RT-PCR assay for detection of SARS-CoV-2 RNA from nasopharyngeal swabs at hospitals. The median age of patients with COVID-19 was 38 years
(interquartile range: 28–53 years; range: 18–72 years), and 79.3% of the participants were male individuals. The first SSL sample was collected after a median of 6 days (interquartile range:
2.75–7 years; range: 1–10 days) after symptom onset, followed by a second SSL sample collection in some cases. Upon hospital admission, the severity of COVID-19 was classified as mild in 15
patients and moderate in 14 patients. SARS-COV-2 DETECTION IN SSLS OBTAINED FROM PATIENTS WITH COVID-19 SSLs were collected from the entire face using an oil-blotting film before washing
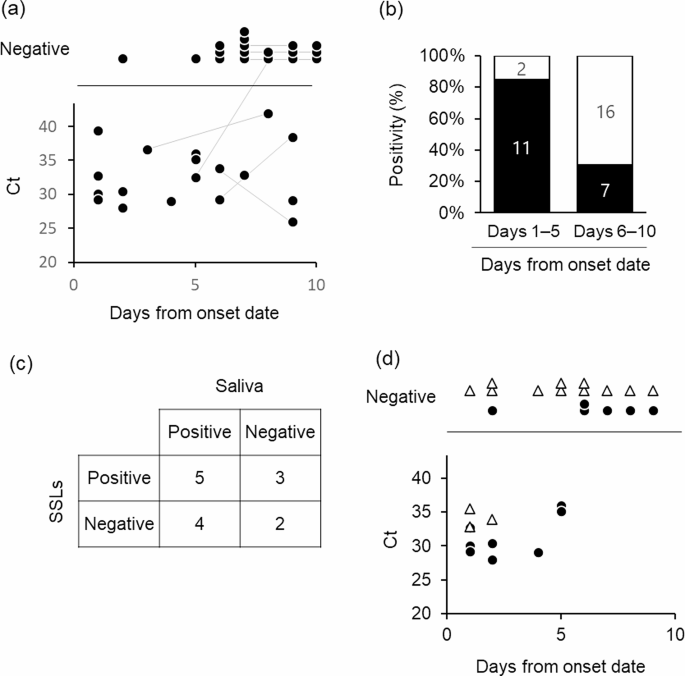
the face in the morning. SARS-CoV-2 was detected in 51.4% (19/37) SSL samples collected from the patients’ face within 10 days of symptom onset (one patient was asymptomatic) (Fig. 1a). The
RT-PCR positivity rates of SSL samples within 5 and 6–10 days of onset were 84.6% (11/13) and 30.4% (7/23), respectively (Fig. 1b). Saliva contamination of the SSL samples was expected as
they were obtained from the face; therefore, the results were compared with the RT-PCR results of saliva samples collected at the closest time point to the SSL samples. In 50% (7/14) of the
samples, the results of both tests matched (five positive and three negative), whereas they did not in the remaining 50% of the samples (Fig. 1c, Supplementary Fig. 1, Supplementary Table
2). Interestingly, in a few patients, the saliva samples showed negative results but the SSL samples showed positive results (3/14). The expression of the salivary marker histatin 3 (HTN3)15
was not observed in five SSL samples that were positive for SARS-CoV-2 RNA and had transcriptome data. Furthermore, we tested SSL samples collected from the body (armpits, chest, and
abdomen), where SARS-CoV-2 contamination from saliva via droplets was considered insignificant, and found that 3 out of 14 samples were positive. In particular, on the day after symptom
onset, two of three samples were positive, and on the second day after symptom onset, one of three samples of SSLs on the body was positive (Fig. 1d). Differences in the amount of SSLs
collected in each sample could affect the SARS-CoV-2-positivity rate; however, it was difficult to estimate the amount of SSLs in each sample because the quantity was low. Therefore, we
compared human internal standard gene expression levels between SARS-CoV-2-positive and -negative samples using RT-PCR and found no significant differences in _ACTB_ and _GAPDH_ levels in
the face and body (Fig. 2a, b, Supplementary Fig. 2a, b). The mean Ct value of human _ACTB_ in samples obtained from the face and body was 13.9 and 17.7, respectively, suggesting that the
amount of RNA collected was approximately 10 times lower in samples obtained from the body, which had fewer SSLs (Fig. 2c, Supplementary Fig. 2c). Finally, whole-genome sequencing of the
SARS-CoV-2-positive SSL samples resulted in strain identification in 12 of the 17 samples (Table 1): 2 samples with B.1.1 and 4 samples with B.1.1.284 among the samples collected between
April and July 2020; 3 samples with R.1 (EPI_ISL_12941049, EPI_ISL_12941050, EPI_ISL_18884079) and 3 samples with B.1.1.7 (EPI_ISL_12941051, EPI_ISL_12941052, EPI_ISL_18884080) among the
samples collected between March and April 2021. TRANSCRIPTOME ANALYSIS OF SSL SAMPLES FROM PATIENTS WITH COVID-19 Previous studies have shown that it is possible to analyze the human
transcriptome from SSL samples collected from the face using an oil-blotting film11. After preprocessing the sequence data, 45 samples (18 healthy participants and 27 patients with COVID-19)
and 3,835 genes were analyzed (Fig. 3a). The false discovery rate (FDR) was set to 0.05, and a comparison of the two groups, healthy participants and patients with COVID-19, resulted in the
identification of 302 differentially expressed genes (DEGs) (Fig. 3b, Supplementary Tables 3, 4). Analysis of 235 upregulated and 67 downregulated genes among the DEGs showed significant
results in Gene Ontology (GO) for biological process categories and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways. The top three terms for the upregulated genes were defense
response to the virus (GO:0051607), negative regulation of viral genome replication (GO:0045071), and response to the virus (GO:0009615) (Fig. 3c). These terms included a group of type I
IFN-related genes that are essential for antiviral immunity, and the expression of genes such as _ISG15_, _IRF7_, _IFITM1_, _IFITM3_, _IFI6_, and _MX1_ was significantly upregulated in
patients with COVID-19 (Fig. 3d). The expression of TNF pathway-related genes (_TNFSF10_, _TNFAIP6_, and _TNFSF13_) was significantly upregulated in patients with COVID-19 compared with that
in healthy controls (Fig. 3e). No significant differences were observed for the expression of the inflammation-related genes _IL1B_ and _NF-kB_ (Supplementary Fig. 3). The expression of
IL-6-induced genes, such as _IL6R_, _SOCS3_, and _STAT3_, was not included in the analysis because of their low expression levels. By contrast, analysis of downregulated genes revealed terms
related to cytoplasmic translation (GO:0002181), translation (GO:0006412), and ribosomes (hsa03010), suggesting that potential alterations in protein synthesis occurred in response to this
disease. DISCUSSION In this study, we attempted to detect SARS-CoV-2 and simultaneously analyze the human transcriptome using specimens obtained from a single oil-blotting film. To our
knowledge, this is the first report of detection of SARS-CoV-2 RNA from the skin surface. The patient or nurse followed the instructions in the manual to collect SSLs from the patient’s
face, and human mRNA—used as an indicator of proper sample collection—was detected in all 37 samples, suggesting that this oil-blotting film method is an easy and effective way for any
individual to collect specimens. A key advantage of this technique is its simplicity and non-invasive nature, making it especially useful for patients for whom conventional sampling methods
pose challenges, such as infants. A positive rate of 84.6% was obtained with facial SSLs isolated within 5 days of symptom onset, indicating that the sensitivity of this technique for
detecting SARS-CoV-2 RNA is relatively high in the early stages of symptom onset. Analysis of nasal swabs revealed that the SARS-CoV-2 RNA level peaked on day 4 of symptom onset16 and that
the viral load decreased as time progressed17. Although the date of peak viral loads was not clear in this study because of the small sample size, the overall trend in SSL samples of a high
positivity rate up to day 5 after symptom onset, followed by a decrease in the detection rate over time, was consistent with the results reported for nasal swabs. There are two possibilities
for the origin of SARS-CoV-2 nucleic acids detected in oil-blotting film samples: (i) persistence of droplets on the face and body and (ii) release of SARS-CoV-2 RNA on the skin via sebum
or sweat. SSL collection was performed with new nitrile gloves; therefore, contamination from the hands was eliminated as much as possible; however, the possibility of contamination from the
patient’s salivary droplets and nasal secretion on the face was not eliminated. Owing to the presence of RNase on the skin, it is unlikely that SARS-CoV-2 RNA in the droplets would remain
undegraded. It has been found that fatty acids in sebum inhibit RNase activity11, and it is possible that SARS-CoV-2 RNA remains undegraded in environments where droplets evaporate quickly
and sebum is abundant. Interestingly, in this study, there were some cases where SARS-CoV2 RNA was detected in SSL samples but not in saliva samples, and there were some cases where
SARS-CoV2 RNA was detected even on bodies where droplet contamination was considered low. To our knowledge, there are no reports of SARS-CoV-2 detection in sebum. On the contrary, there is a
report of SARS-CoV-2 RNA being detected in sweat10, as well as reports that SARS-CoV-2 RNA was not detected in sweat8,9. Considering the findings mentioned above, the possibility that
SARS-CoV-2 RNA was released on the skin via sebum or sweat cannot be denied. The origin of SARS-CoV-2 RNA in the specimens collected by oil-blotting film in this study was difficult to
determine, as there was insufficient evidence to reject the possibility of contamination from the patient’s salivary droplets and nasal secretion. However, this study provides the first
knowledge of SARS-CoV-2 detection on the skin surface, and we propose this as a novel methodology for non-invasive SARS-CoV-2 RNA detection in patients with COVID-19. In this study, human
transcriptome analysis was performed using RNA obtained from the same oil-blotting film used for SARS-CoV-2 detection. The most characteristic SSL-RNA profile of patients with COVID-19 was
the upregulation of interferon-stimulated (ISG)-related genes, such as _ISG15_, _IFITM1_, and _MX1_, which are typical genes involved in host immune response during SARS-CoV-2 infection18.
This gene expression profile is similar to that of the blood transcriptome of patients with COVID-1919. Regarding the skin, the levels of IFN-related genes and inflammation-related molecules
have been reported to be elevated in the biopsy gene expression profiles of skin lesions in patients with moderate-to-severe COVID-1920. In addition, the expression of BAFF (TNFSF13B) and
TRAIL (TNFSF10), members of the TNF superfamily, was upregulated in areas with severe lung tissue damage21. It is difficult to discuss the association between the SSL-RNA gene profile and
skin manifestations because the skin manifestations of patients with COVID-19 were not recorded in this study. The fact that the host response during acute respiratory infection was
detectable in SSL-RNA, as well as in the skin and lung tissues, is interesting. The expression profile of SSL-RNA is presumed to reflect its expression primarily in the epidermis, hair
follicles, and sebaceous glands11. ISG are also expressed in keratinocytes and cutaneous leukocytes, which are involved in immune responses in the skin22. It is important to note that it is
unclear whether changes in the SSL-RNA gene profile are a reflection of changes in keratinocytes, the main cells of the skin, or whether they also reflect the changes in immune cells.
Indeed, genes encoding macrophage receptor molecules (CD209 and CD163) and chemokines (CXCL2 and CCL2), which have been detected in skin punch biopsy20, were not detected in SSL-RNA profile
owing to low expression levels. However, this methodology, which can noninvasively detect the biological response to acute infection without biopsy, will help us understand the pathogenesis
of the disease. A limitation of the present study is the small number of patients tested. For the comparative study of the positivity rates of saliva and SSLs, sampling was not conducted on
the same day; therefore, sampling at the same time is necessary for a comprehensive discussion. It was not possible to determine the source of the SARS-CoV-2 RNA detected in SSLs.
Additionally, the detection of SARS-CoV-2 nucleic acids using RT-PCR alone does not indicate that the presence of infectious virus on the skin and, therefore, this should be discussed with
caution as a possible route of transmission. Finally, accurate negative controls need to be prepared and examined for possible future real-world diagnostic use of this method. If
false-negative results are caused by cross-contamination between infected and healthy individuals (for example, patients with COVID-19 sneezing on healthy individuals), it may be necessary
to reduce the false-negative rate, perhaps by waiting for a certain time after COVID-19 exposure before collecting sebum. In conclusion, this study presents a method for the simultaneous
detection of SARS-CoV-2 nucleic acids and host transcriptome analysis from SSLs collected using a single oil-blotting film. This method can be used as an alternative technique for convenient
pathogen detection and understanding the molecular pathogenesis of acute respiratory infections. METHODS STUDY DESIGN This study was approved by the Medical Research Ethics Committee of the
National Institute of Infectious Diseases (NIID; approval number: 1111) and the Ethical Committee of Kao Corporation (approval numbers: T293-200331 and T229-190615). This study was
conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all the participants or their legal representatives. The selection criteria were as
follows: (i) patients aged 20 years or older, (ii) patients with confirmed SARS-CoV-2 infection who visited a collaborating medical institution (patients with COVID-19) or hospitalized
patients with clinically suspected SARS-CoV-2 infection to be tested for infection, and (iii) patients who provided written informed consent themselves or through a legal representative. The
exclusion criteria were as follows: (i) patients who were judged by their physicians to be inappropriate for inclusion and (ii) patients whose health condition would worsen by having their
blood collected due to anemia or other reasons. Patients from whom consent was obtained were enrolled as study participants, and information on age, sex, date of symptom onset, clinical
symptoms related to COVID-19, and clinical examinations was obtained from their medical records. Severity was based on World Health Organization (WHO) categories. Specimens collected from
hospitalized patients in Japan between April 2020 and April 2021 were used for analysis. In the case of healthy controls, data from a study conducted in 2019, in which SSLs were collected
from healthy participants, were used. Our study examined both male and female participants, and similar findings have been reported for individuals of both sexes. SSL COLLECTION AND RNA
PREPARATION SSLs were collected from the entire face (forehead, temples, cheeks, nose, and chin) or body (armpits, chest, and abdomen) of the participants before washing the face in the
morning using an oil-blotting film (5.0 cm × 8.0 cm; 3 M Japan, Tokyo, Japan) by the patients or a nurse wearing a nitrile glove. The oil-blotting films with the SSLs were placed in
RNase-free 25-mL tubes containing molecular sieves 13 × 1/8 (FUJIFILM Wako Pure Chemical Corporation, Tokyo, Japan) and stored at − 80 °C. After cutting the oil-blotting film into small
pieces, QIAzol (1,425 µL; QIAGEN, Hilden, Germany) was added, and the samples were vortexed for 3 min; then, 1,300 µL of solution was collected in a tube. After adding 260 µL of chloroform,
the tube was vortexed for 10 s and centrifuged at 12,000 × _g_ for 15 min at 4 °C. The supernatant (aqueous layer; 750 µL) was collected and RNA was purified using the RNeasy Mini QIAcube
Kit (QIAGEN) with DNase treatment, according to custom protocol. Briefly, 85% ethanol (750 µL) was added to samples (upper aqueous phase; 750 µL), which were then mixed well and transferred
to a RNeasy mini column. After washing with RW1 buffer, DNase I treatment, re-washing with RW1 buffer, and washing with RPE buffer, the RNA was finally eluted by passing RNase-free water (50
µL) through the column twice to obtain RNA solution (100 µL). Subsequently, the purified RNA solution (100 µL) was ethanol precipitated with Ethachinmate (Nippon Gene, Tokyo, Japan) and
redissolved in RNase-free water (10 µL). SARS-COV-2 DETECTION Of the 10 µL RNA obtained from the oil-blotting film, 1 µL was diluted five-fold with water and used for virus detection. Cycle
threshold (Ct) values were measured using RT-PCR with the QuantiTect Probe RT-PCR kit (QIAGEN) and the NIID-N2 primer/probe set23 (Supplementary Table 5) targeting the SARS-CoV-2 nuclear
protein (N) region on an Applied Biosystems 7500 Fast Real-Time PCR System (Thermo Fisher Scientific, Waltham, MA, USA). The thermal cycling conditions were as follows: 50 °C for 30 min, 95
°C for 15 min, 45 cycles at 95 °C for 15 s, and 60 °C for 1 min. WHOLE-GENOME SEQUENCING The sample RNA was reverse transcribed using the SuperScript IV First-Strand Synthesis System (Thermo
Fisher Scientific) or LunaScript RT SuperMix Kit (New England BioLabs, Ipswich, MA, USA), and DNA amplicons for amplifying the SARS-CoV-2 genomic region were obtained using multiplex PCR
with ARTIC-N4 or N5 primers and Q5 Hot Start DNA polymerase (New England BioLabs)24. A library was prepared using the QIAseq FX DNA Library Kit (QIAGEN) and sequenced using the MiSeq System
(Illumina, San Diego, CA, USA). The obtained reads were mapped to the SARS-CoV-2 reference genome MN908947.3. Whole-genome sequencing was performed by Takara Bio, Inc. or the National
Institute of Infectious Diseases. AMPLISEQ TRANSCRIPTOME ANALYSIS Transcriptome analysis was performed as previously described, with a few modifications11. Briefly, RNA solution (1.5 µL) was
used for reverse transcription and sequencing library preparation using the Ion AmpliSeq Transcriptome Human Gene Expression Kit (Thermo Fisher Scientific, Waltham, MA, USA). In addition to
the previously described methods, 250 and 100 ng of T4 Gene 32 Protein (New England Biolabs) were added to the reverse transcription reaction mix and first-round PCR mix, respectively. The
library was then used for template preparation using the Ion 540 Chip Kit on an Ion Chef instrument (Thermo Fisher Scientific), and sequencing was performed on an Ion S5 XL System (Thermo
Fisher Scientific). The read count data were analyzed using the R software. Samples in which the percentage of detected genes was less than 20% of the target 20,802 genes were excluded from
the analysis as low-quality samples. Genes detected in more than 90% of the samples that met the criteria were normalized using the size factor in DESeq225 to extract genes with differential
expression (FDR < 0.05, |Log2FC| > 1). Biological process and KEGG pathway analyses were performed using the Database for Annotation, Visualization, and Integrated Discovery
(DAVID)26,27,28. INTERNAL STANDARD GENE EXPRESSION ANALYSIS Owing to the limited amount of SSL-RNA, the remaining samples from the AmpliSeq analysis were used for the evaluation of human
internal standard genes using RT-PCR. In other words, pre-amplified amplicons were used as cDNA templates using an Ion AmpliSeq™ Transcriptome Human Gene Expression kit (Thermo Fisher
Scientific). In accordance with the sequences included in the Ion AmpliSeq™ Transcriptome Human Gene Expression Core Panel provided by Thermo Fischer Scientific, the primers for RT-PCR were
designed using Primer-BLAST (NCBI) and are shown in Supplementary Table 6. They were designed as nested primers, which were specified to amplify the region in the first PCR using the Ion
AmpliSeq™ Transcriptome Human Gene Expression kit. Human _ACTB_ and _GAPDH_ were quantified as stably expressed genes in the SSL-RNA. RT-PCR was performed with reaction mixtures comprising
0.75 µL of each forward and reverse primers of each gene (250 nM), 1.0 µL of template (1:10 dilution), and 2.5 µL of THUNDERBIRD Next SYBR qPCR Mix (TOYOBO, Osaka, Japan) using the
QuantStudio™ 12 K Flex Real-time PCR System (Thermo Fisher Scientific). The thermal cycling conditions were as follows: 50℃ for 2 min, 95℃ for 10 min, 40 cycles at 95℃ for 15 s and at 60℃
for 1 min; specificity of the PCR products was evaluated using melting curve analysis. STATISTICS To assess the significance of the differences between the two groups, Welch’s _t_-test was
used. We extracted DEGs between healthy controls and patients with COVID-19 using the likelihood ratio test with Benjamini–Hochberg’s FDR < 0.05 and |Log2FC| > 1 as threshold values.
DATA AVAILABILITY Source data are available as Supplementary Data Values file. REFERENCES * Wang, W. et al. Detection of SARS-CoV-2 in different types of clinical specimens. _JAMA_. 323,
1843–1844 (2020). CAS PubMed Google Scholar * Peng, L. et al. SARS-CoV-2 can be detected in urine, blood, anal swabs, and oropharyngeal swabs specimens. _J. Med. Virol._ 92, 1676–1680
(2020). Article CAS PubMed PubMed Central Google Scholar * Wyllie, A. L. et al. Saliva or nasopharyngeal swab specimens for detection of SARS-CoV-2. _N Engl. J. Med._ 383, 1283–1286
(2020). Article PubMed Google Scholar * Matic, N. et al. Practical challenges to the clinical implementation of saliva for SARS-CoV-2 detection. _Eur. J. Clin. Microbiol. Infect. Dis._
40, 447–450 (2021). Article CAS PubMed Google Scholar * Polly, S. & Fernandez, A. P. Common skin signs of COVID-19 in adults: An update. _Cleve Clin. J. Med._ 89, 161–167 (2022).
Article PubMed Google Scholar * Majumdar, R. et al. SARS-CoV-2 RNA detection in formalin-fixed paraffin-embedded (FFPE) tissue by droplet digital PCR (ddPCR). _Clin. Chim. Acta_ 532,
181–187 (2022). Article CAS PubMed Google Scholar * Marzano, A. V. et al. SARS-CoV-2 detection by digital polymerase chain reaction and immunohistochemistry in skin biopsies from 52
patients with different COVID-19-associated cutaneous phenotypes. _Dermatology_. 239, 584–591 (2023). Article CAS PubMed Google Scholar * Fathizadeh, H. et al. Study presence of COVID-19
(SARS-CoV-2) in the sweat of patients infected with COVID-19. _Microb. Pathog_ 149, 104556 (2020). Article CAS PubMed PubMed Central Google Scholar * Arslan, B., Bercin, S., Aydogan,
S., Islamoglu, Y. & Dinc, B. SARS-CoV-2 is not found in the sweat of COVID-19 positive patients. _Ir. J. Med. Sci._ 191, 27–29 (2022). Article CAS PubMed Google Scholar * Recalcati,
S., Tonolo, S., Meroni, E. & Fantini, F. SARS-CoV-2 in the sweat of COVID-19-positive patients: A possible route of transmission? _J. Eur. Acad. Dermatol. Venereol._ 35, e865–e866
(2021). Article CAS PubMed PubMed Central Google Scholar * Inoue, T. et al. Non-invasive human skin transcriptome analysis using mRNA in skin surface lipids. _Commun. Biol._ 5, 215
(2022). Article CAS PubMed PubMed Central Google Scholar * Shima, K. et al. Non-invasive transcriptomic analysis using mRNAs in skin surface lipids obtained from children with
mild-to-moderate atopic dermatitis. _J. Eur. Acad. Dermatol. Venereol._ 36, 1477–1485 (2022). Article CAS PubMed PubMed Central Google Scholar * Yamamoto-Hanada, K. et al. mRNAs in skin
surface lipids unveiled atopic dermatitis at 1 month. _J. Eur. Acad. Dermatol. Venereol._ 37, 1385–1395 (2023). Article CAS PubMed Google Scholar * Uehara, Y. et al. Non-invasive
diagnostic tool for Parkinson’s disease by sebum RNA profile with machine learning. _Sci. Rep._ 11, 18550 (2021). Article ADS CAS PubMed PubMed Central Google Scholar * Song, M., Bai,
H., Zhang, P., Zhou, X. & Ying, B. Promising applications of human-derived saliva biomarker testing in clinical diagnostics. _Int. J. Oral Sci._ 15, 2 (2023). Article CAS PubMed
PubMed Central Google Scholar * Frediani, J. K. et al. The new normal: Delayed peak SARS-CoV-2 viral loads relative to symptom onset and implications for COVID-19 testing programs. _Clin.
Infect. Dis._ 78, 301–307 (2024). Article PubMed Google Scholar * Puhach, O., Meyer, B. & Eckerle, I. SARS-CoV-2 viral load and shedding kinetics. _Nat. Rev. Microbiol._ 21, 147–161
(2023). CAS PubMed Google Scholar * Sarkar, L., Liu, G. & Gack, M. U. ISG15: Its roles in SARS-CoV-2 and other viral infections. _Trends Microbiol._ 31, 1262–1275 (2023). Article CAS
PubMed Google Scholar * Hadjadj, J. et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. _Science_ 369, 718–724 (2020). Article ADS CAS
PubMed PubMed Central Google Scholar * Domizio, J. D. et al. The cGAS-STING pathway drives type I IFN immunopathology in COVID-19. _Nature_ 603, 145–151 (2022). Article ADS CAS PubMed
PubMed Central Google Scholar * Ohue-Kitano, R. et al. Medium-chain fatty acids suppress lipotoxicity-induced hepatic fibrosis via the immunomodulating receptor GPR84. _JCI Insight_. 8,
e165469 (2023). Article PubMed PubMed Central Google Scholar * Greenberg, E. et al. (ed, N.) Circadian control of interferon-sensitive gene expression in murine skin. _Proc. Natl. Acad.
Sci. U S A_ 117 5761–5771 (2020). Article ADS CAS PubMed PubMed Central Google Scholar * Shirato, K. et al. Development of genetic diagnostic methods for detection for novel
coronavirus 2019(nCoV-2019) in Japan. _Jpn J. Infect. Dis._ 73, 304–307 (2020). Article CAS PubMed Google Scholar * Itokawa, K., Sekizuka, T., Hashino, M., Tanaka, R. & Kuroda, M.
Disentangling primer interactions improves SARS-CoV-2 genome sequencing by multiplex tiling PCR. _PLoS One_ 15, e0239403 (2020). Article CAS PubMed PubMed Central Google Scholar * Love,
M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. _Genome Biol._ 15, 550 (2014). Article PubMed PubMed Central Google
Scholar * Kanehisa, M. & Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. _Nucleic Acids Res._ 28, 27–30 (2000). Article CAS PubMed PubMed Central Google Scholar * Huang da,
W., Sherman, B. T. & Lempicki, R. A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. _Nat. Protoc._ 4, 44–57 (2009). Article PubMed
Google Scholar * Sherman, B. T. et al. A web server for functional enrichment analysis and functional annotation of gene lists (2021 update). _Nucleic Acids Res._ 50, W216–W221 (2022).
Article ADS CAS PubMed PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS We thank the clinical staff at the Hospital of the Institute of Medical Science, University of
Tokyo, National Center for Global Health and Medicine, and Tokyo Metropolitan Cancer and Infectious Diseases Center, Komagome Hospital. We also thank GISAID for sharing and comparing our
data with data submitted globally.This work was supported by the Japan Agency for Medical Research and Development (AMED) [grant numbers: JP21fk0108104 and JP23fk0108637 (to T.S.)]. AUTHOR
INFORMATION Author notes * Satoshi Kutsuna Present address: Department of Infection Control and Prevention, Graduate School of Medicine, Faculty of Medicine, Osaka University, Osaka, Japan *
Yusuke Miyazato Present address: Department of Internal Medicine, Hashimoto Municipal Hospital, Wakayama, Japan AUTHORS AND AFFILIATIONS * Biological Science Research, Kao Corporation, 2606
Akabane, Ichikai-machi, Haga-gun, 321- 3497, Tochigi, Japan Tetsuya Kuwano, Yui Ueda, Maeko Iwamura, Naoto Takada & Takayoshi Inoue * Department of Pathology, National Institute of
Infectious Diseases, 1-23-1 Toyama, Shinjuku, 162-8640, Tokyo, Japan Takayuki Kanno, Minoru Tobiume, Yuichiro Hirata, Harutaka Katano & Tadaki Suzuki * Division of Infectious Diseases,
Advanced Clinical Research Center, University of Tokyo, Tokyo, Japan Michiko Koga, Takeya Tsutsumi & Hiroshi Yotsuyanagi * Department of Infectious Diseases and Applied Immunology,
Hospital of Institute of Medical Science, University of Tokyo, Tokyo, Japan Michiko Koga, Hiroyuki Nagai, Takeya Tsutsumi & Hiroshi Yotsuyanagi * Department of Allergy and Rheumatology,
Institute of Medical Science, University of Tokyo, Tokyo, Japan Noritada Yoshikawa * Disease Control and Prevention Center, National Center for Global Health and Medicine, Tokyo, Japan
Satoshi Kutsuna, Yusuke Miyazato, Noriko Kinoshita-Iwamoto & Norio Ohmagari * Department of Infectious Diseases, Tokyo Metropolitan Cancer and Infectious Diseases Center Komagome
Hospital, Tokyo, Japan Taiichiro Kobayashi, Kazuaki Fukushima, Masaru Tanaka & Akifumi Imamura * AIDS Research Center, National Institute of Infectious Diseases, Tokyo, Japan Tetsuro
Matano & Ai Kawana-Tachikawa * Department of AIDS Vaccine Development, Institute of Medical Science, University of Tokyo, Tokyo, Japan Tetsuro Matano & Ai Kawana-Tachikawa Authors *
Tetsuya Kuwano View author publications You can also search for this author inPubMed Google Scholar * Takayuki Kanno View author publications You can also search for this author inPubMed
Google Scholar * Minoru Tobiume View author publications You can also search for this author inPubMed Google Scholar * Yuichiro Hirata View author publications You can also search for this
author inPubMed Google Scholar * Harutaka Katano View author publications You can also search for this author inPubMed Google Scholar * Michiko Koga View author publications You can also
search for this author inPubMed Google Scholar * Hiroyuki Nagai View author publications You can also search for this author inPubMed Google Scholar * Takeya Tsutsumi View author
publications You can also search for this author inPubMed Google Scholar * Noritada Yoshikawa View author publications You can also search for this author inPubMed Google Scholar * Hiroshi
Yotsuyanagi View author publications You can also search for this author inPubMed Google Scholar * Satoshi Kutsuna View author publications You can also search for this author inPubMed
Google Scholar * Yusuke Miyazato View author publications You can also search for this author inPubMed Google Scholar * Noriko Kinoshita-Iwamoto View author publications You can also search
for this author inPubMed Google Scholar * Norio Ohmagari View author publications You can also search for this author inPubMed Google Scholar * Taiichiro Kobayashi View author publications
You can also search for this author inPubMed Google Scholar * Kazuaki Fukushima View author publications You can also search for this author inPubMed Google Scholar * Masaru Tanaka View
author publications You can also search for this author inPubMed Google Scholar * Akifumi Imamura View author publications You can also search for this author inPubMed Google Scholar * Yui
Ueda View author publications You can also search for this author inPubMed Google Scholar * Maeko Iwamura View author publications You can also search for this author inPubMed Google Scholar
* Naoto Takada View author publications You can also search for this author inPubMed Google Scholar * Takayoshi Inoue View author publications You can also search for this author inPubMed
Google Scholar * Tetsuro Matano View author publications You can also search for this author inPubMed Google Scholar * Ai Kawana-Tachikawa View author publications You can also search for
this author inPubMed Google Scholar * Tadaki Suzuki View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS Conceptualization, T.S.; Methodology,
T. Kuwano (T.K.1) and T.I.; Investigation, T.K.1, T. Kanno (T.K.2), M.T., Y.H., H.K., Y.U., M.I., and N.T.; Resources, M.K., H.N., T.T., N.Y., H.Y., S.K., Y.M., N.K.I., N.O., T. Kobayashi
(T.K.3), K.F., M.T., and A.I.; Project Administration, A.K.T. and T.S.; Funding Acquisition, T.S.; Supervision, T.M., A.K.T., and T.S.; Writing – Original Draft, T.K.1; Writing –Review &
Editing, T.K.1, T.I., T.M., A.K.T., and T.S. CORRESPONDING AUTHORS Correspondence to Tetsuya Kuwano or Tadaki Suzuki. ETHICS DECLARATIONS COMPETING INTERESTS T. Kuwano (T.K.1), T. Kanno
(T.K.2), M.T., N.T., T.I., T.M., A.K.T., and T.S. are the inventors of the patented method of SARS-CoV-2 detection in SSLs. The authors declare no competing interests. ADDITIONAL INFORMATION
PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. ELECTRONIC SUPPLEMENTARY MATERIAL Below is the link
to the electronic supplementary material. SUPPLEMENTARY MATERIAL 1 SUPPLEMENTARY MATERIAL 2 RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons
Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give
appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission
under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons
licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by
statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit
http://creativecommons.org/licenses/by-nc-nd/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Kuwano, T., Kanno, T., Tobiume, M. _et al._ Non-invasive SARS-CoV-2 RNA
detection and human transcriptome analysis using skin surface lipids. _Sci Rep_ 14, 26057 (2024). https://doi.org/10.1038/s41598-024-77862-0 Download citation * Received: 14 June 2024 *
Accepted: 25 October 2024 * Published: 30 October 2024 * DOI: https://doi.org/10.1038/s41598-024-77862-0 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this
content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
KEYWORDS * SARS-CoV-2 * COVID-19 * Skin * Sebum * Transcriptome * Skin surface lipid