A rapid and low-cost method to fabricate well of the well (wow) dishes with arbitrary 3d microwell shapes for improved embryo culture
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Despite its high cost, the success rate for in vitro fertilization (IVF) remains < 33% in humans, driving the need for new techniques to improve embryo culture outcomes. The
well-of-the-well (WOW) culture system is a platform for in-vitro mammalian embryo culture that has been shown to enhance the developmental competence of embryos and clinical pregnancy rates
in humans. However, discovery and testing of the best design for optimal embryo culture quality is hindered by the lack of a method to flexibly produce WOW dishes of various designs. Here,
we present a low-cost and simple method to fabricate WOW dishes with microwells of arbitrary shapes and dimensions. We use a low-cost 3D printing service to fabricate a
poly(dimethylsiloxane) (PDMS)-based WOW insert that can be paired with a standard in vitro fertilization (IVF) dish to create WOW dishes with new microwell shapes, including pyramidal and
hemispherical designs. We validate the fabrication quality of the WOW inserts and demonstrate the utility of the assembled WOW dishes for observation and grading of mouse embryo quality.
Moreover, our results indicate that WOW dishes with hemispherical microwells result in better culture outcomes than traditional flat-bottomed IVF dishes and those with other microwell
shapes, including the semi-elliptical microwells used in commercial WOW dishes. The proposed fabrication strategy thus provides a way to rapidly fabricate and test new WOW dishes that may
bolster IVF success rates. SIMILAR CONTENT BEING VIEWED BY OTHERS GENERATING HUMAN BLASTOIDS MODELING BLASTOCYST-STAGE EMBRYOS AND IMPLANTATION Article 15 February 2023 A MICROFLUIDICS-BASED
STEM CELL MODEL OF EARLY POST-IMPLANTATION HUMAN DEVELOPMENT Article 11 December 2020 MICROTECHNOLOGY-BASED METHODS FOR ORGANOID MODELS Article Open access 05 October 2020 INTRODUCTION The
well-of-the-well (WOW) culture system1,2 is a platform for _in-vitro_ mammalian embryo culture that has been shown to enhance the developmental competence of embryos and clinical pregnancy
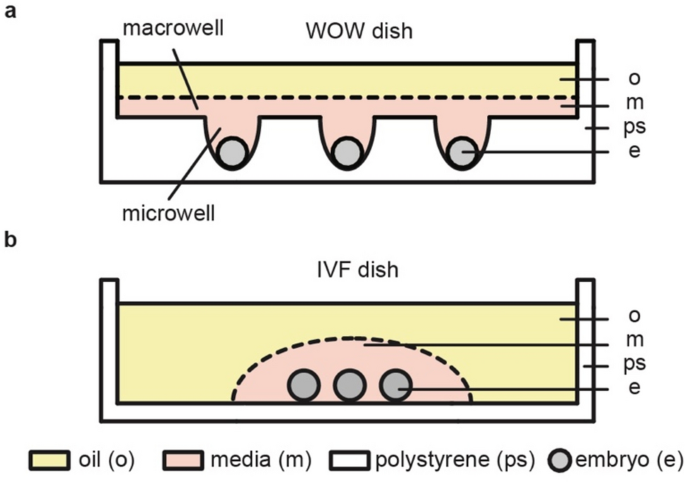
rates in humans compared to traditional embryo culture dishes3,4,5. In a WOW dish, each embryo is cultured in a confined space termed a “microwell” but has access to a larger “macrowell”
holding a small volume of culture media that is shared among all embryos with overlay oil (Fig. 1a). In contrast, embryos cultured in a traditional in vitro fertilization (IVF) dish are not
individually confined but share a droplet of culture media with overlay oil (Fig. 1b). Research shows that the shapes and dimensions of microwells in WOWs affect embryo culture outcomes; 6,7
however, rigorous, systematic research is lacking about which microwell shape provides the best culture quality. A major limitation in the ability to conduct this research has been the lack
of cost-effective tools to rapidly iterate the fabrication of WOW dishes with arbitrary shapes and dimensions. Given the low success of current human IVF procedures, there is a clear need
for new tools, such as WOW dishes of optimal design, that may improve clinical outcomes. When first introduced in 2000, WOW dishes were handmade by using heated darning needles to melt the
base of a standard IVF dish1,3, resulting in microwells of semi-ellipsoid shape. The manual nature of the hand-melting process, however, can introduce heterogeneities in the dimensions of
the fabricated microwells, leading to variability in culture outcomes and hindering systematic assessment. Moreover, the melting process can lead to the release of toxic materials that are
potentially harmful to human embryos. Today, commercial WOW dishes are typically manufactured by polystyrene (PS) mold injection2 and are available with microwells having truncated cone,
semi-ellipsoid and conical shapes. This fabrication process is optimal for high-volume manufacturing, but it is not compatible with the need to fine-tune design parameters to test various
microwell shapes and dimensions for research studies. Commercial WOW dishes are also 10 times more costly than traditional IVF dishes, which lowers their desirability and accessibility.
Photolithography has been used to create poly(dimethylsiloxane) (PDMS)-based WOW dishes templated from SU-8 molds8,9; however, the SU-8 mold development process is complex and laborious.
Moreover, since multi-layer (i.e., 3D) structures are hard to achieve in conventional photolithography, the range of possible microwell shapes is limited with this strategy. To date, only
single-layer, cylindrical microwells have been reported8. Conversely, the broader cell culture community has developed several methods to fabricate cell culture dishes having microwells with
a variety of 3D shapes, including conical microwell arrays10, omega-shaped microwell arrays11, and pyramidal microwell arrays12. These methods often use commercially available structures
(e.g., computer chips, acrylic sheets) as the mold or leverage advanced manufacturing processes to create custom molds. Unfortunately, the use of commercially available materials for molding
limits the options for design of the microwell shape, and the custom mold-development processes used to date are time-consuming and costly, which restrict rapid prototyping and production.
For example, using a projection micro stereolithography (PmSL) 3D printing service, a petri dish-sized mold (30 × 30 × 6 mm) can cost $1000 to $10,000 and take ten hours to seven days to
print, respectively, depending on the printing quality. Here we present a rapid and low-cost method for WOW fabrication to facilitate systematic investigation of the optimal WOW design for
human embryo culture. Our strategy involves use of digital light projection (DLP) 3D printing to fabricate a low-cost PDMS-based WOW insert with controllable microwell shapes and dimensions.
When paired with a standard IVF dish, one can quickly and easily create a novel WOW dish to explore optimal culture conditions. In this work, we validate the physical quality of the WOW
inserts fabricated with this strategy, demonstrate the utility of the PDMS WOW dishes for observation and grading of mouse embryo quality, and report the first use of novel-shaped microwells
(hemispherical and pyramidal) in WOW dishes for embryo culture. METHODS WOW DISH FABRICATION Our WOW dish comprises two components: a PDMS WOW insert (containing both microwell and
macrowell structures) and an IVF dish. The overall fabrication process (Fig. 2) involves creation of the WOW insert from a 3D-printed negative mold and attachment of the WOW insert to the
IVF dish. The negative mold can be reused to fabricate numerous WOW inserts at low cost: the initial mold printing cost was roughly $60, and the materials cost for each molded insert was
just $0.77. Details of the fabrication process and design we tested are described below. NEGATIVE MOLD DESIGN AND FABRICATION We designed a 28-mm-diameter negative mold (Fig. 2a) in
SolidWorks (Dassault Systèmes) to template WOW inserts comprising four cylindrical macrowells (diameter: 6 mm, depth: 3 mm), each capable of holding up to 81 µl of culture media. Each
macrowell contains nine identical microwells in one of four different designs, each 200-µm deep: large truncated cones (top diameter: 400 µm, base diameter: 200 µm); small truncated cones
(top diameter: 300 µm, base diameter: 100 µm); hemispheres (diameter: 400 µm) and square pyramids (side length: 400 µm). The microwell shapes and dimensions included in the design were
selected to demonstrate the versatility of the fabrication technique: the specific shapes we chose correspond to a combination of existing (i.e., truncated conical) and novel (i.e.,
pyramidal and hemispherical) shapes for WOW culture. Note that pyramidal designs have been shown to enhance cell culture in other contexts12. The negative mold was 3D-printed (Core 530,
B9Creations) using high-detail resin (B9R-8-HD-SLATE, B9Creations) with an axial resolution of 20 µm and a lateral resolution of 15 µm. A complete CAD drawing of the negative mold is
included in Fig. S1. To prevent adhesion of PDMS to the mold in subsequent steps, the mold was cleaned by air plasma treatment in a plasma cleaner (PDC-32G, Harrick Plasma) for 30 s. It was
then placed inside a desiccator along with a dish (08–732-105, Fisher Scientific) of 100 µl of trichloro(1H, 1H, 2H, 2H-perfluorooctyl)silane (PFOCTS, Sigma Aldrich) and subjected to high
vacuum overnight (at least 12 h). Finally, the mold was transported to an oven and baked at 75 °C for an hour. WOW INSERT FABRICATION Figure 2b shows the workflow of WOW insert fabrication.
PDMS (Sylgard 184, DOW) was prepared using a standard recipe of 10:1, base:curing agent, degassed for 30 min, and then poured directly on the negative mold until the mold was full. A second,
20-min degassing step was subsequently performed to remove any trapped air bubbles. Then, we covered the PDMS-filled mold with a glass slide (71860-01, Electron Microscopy Sciences) to form
a flat bottom for the WOW insert and cured it at 75 °C in an oven for at least three hours. After that, we removed the glass slide and retrieved the cured WOW insert from the mold. WOW DISH
ASSEMBLY Figure 2c shows the workflow of WOW dish assembly. The WOW insert was glued to an IVF dish (150,255, NUNC, Thermo Fisher) by casting a layer of uncured PDMS to the flat base of the
dish and curing the assembly for at least 48 h at room temperature. The use of the IVF dish was necessary to provide a stable bottom support for the WOW dish and to leverage the vented lid
that comes with the IVF dish to facilitate air exchange during culturing. The assembled WOW dishes were placed in an ultrasonic cleaner with distilled water for 20 min, dried with a nitrogen
blow gun and sterilized under UV light for an hour before use. MOLD AND WOW INSERT CHARACTERIZATION To verify the printing and fabrication quality of the negative mold and resulting WOW
inserts, we used micro-computed tomography (µCT) to visualize the large-scale structures (i.e., macrowells) and optical coherence tomography (OCT) to visualize small-scale structures (i.e.,
microwells). ΜCT IMAGING The macrowell templates (i.e., cylinders) in the negative mold and the macrowells of the WOW insert were imaged by µCT (µCT 40, Scanco Medical AG) at an energy of 70
kVp and resolution of 10 µm. The resulting volumetric data were loaded in ImageJ for visualization and measurement. Standard deviation (STD) projections along the z-axis were generated to
characterize the diameters of the extensive wells. The heights of the macrowell templates and corresponding depths of the macrowells were measured along the two orthogonal planes. Each
measurement was repeated three times to enable averaging. OCT IMAGING Despite the high resolution of µCT, the poor contrast of resin and PDMS in air limits the visualization of
microstructures. Thus, we used optical coherence tomography (OCT, TEL221PSC1, Thorlabs) to characterize the dimensions of the microstructures. OCT images covering a 2 × 2-mm scanning area
(1000 × 1000 points) were acquired for each of the four macrowell regions on the negative mold and WOW insert. Both the negative mold and the WOW insert were carefully adjusted to be flat to
the OCT scanner head prior to imaging. The volumetric data were loaded in ImageJ for visualization and measurement. STD projections along the z-axis were generated to characterize the
dimensions of lateral (i.e., x–y plane) structures. Specifically, we measured the following structure dimensions of all nine microwells in each macrowell: the average top and base diameters
of truncated conical shapes, the average diameters of hemispherical shapes, and the average side lengths of pyramidal shapes. Three cross-sectional images spanning the centers of the
microwell structures were extracted to measure their profiles. MOLD REUSABILITY TESTING To test the reusability of the negative mold, we fabricated 30 WOW inserts from the same mold. The
15th and 30th WOW inserts were collected for examination and imaged with OCT as described above. STD projections along the z-axis were generated to compare the structural consistency. The
corresponding projection images of the same microwell structures in the two inserts were registered in MATLAB (MathWorks) for direct comparison. EMBRYO CULTURE IN PDMS WOW DISHES To
demonstrate the utility of the PDMS WOW dishes for embryo culture, we cultured single-cell mouse embryos and graded the blastocysts that resulted after four days. In this study, only the
pyramidal, hemispherical, and large truncated conical microwells were used: the small truncated conical microwells were not used so that their smaller opening dimension would not be a
confounding factor in comparing the culture outcomes, as has been noted previously13. Table 1 summarizes the number of embryos included in the study. Each row indicates the number of embryos
that were cultured over the same experimental period. The variability in the row totals is due to exclusion of embryos lost during handling and transport prior to culture. During each
experiment, the control group of embryos was grown in a standard IVF dish, without the use of a WOW insert. The other experimental groups of embryos were all placed in the same WOW dish,
with no more than one embryo in a given microwell. CULTURE DISH PREPARATION On Day 0, we filled the microwells of a WOW dish with culture media using micropipettes (7–72-2170, Cooper
Surgical) under a stereomicroscope (SM-2BYY, AmScope) with an attached, 37 °C warm plate (TCS-100, AmScope). We then pipetted 80 µl culture media into each of the macrowells and carefully
inspected the microwells for the appearance of bubbles. In general, we found that heating the microwells and using warm media eliminates the presence of bubbles. Thus, WOW dishes were kept
in a 37 °C incubator before use. During media loading, WOW dishes were kept on the warm plate, and the media was maintained at 37 °C by a dry bath (14955218, Fisher Scientific). If observed,
trapped bubbles appearing in microwells were removed by pushing culture media through a micropipette to flush them away with gentle pressure. About 3 ml culture oil was then pipetted into
the WOW dishes to cover the media. Preparation of the IVF culture dishes was as follows: a droplet of 80 µl of culture media was loaded and covered by 3 ml of culture oil. Both WOW dishes
and IVF culture dishes were placed in the incubator to equilibrate overnight. EMBRYO THAWING AND CULTURE Cryopreserved one-cell murine embryos (B6C3F1 × B6D2F1, Embryotech Laboratories) were
used in the study. On Day 1, a straw of 20 embryos was exposed to room temperature for two minutes and immersed in a 37 °C water bath for one minute. Then the straw was moved from the water
bath and wiped dry with a tissue. We cut the straw at the plug and seal and pushed all of its contents into a droplet of 250 µl of embryo culture media (MR-106-D, EmbryoMax, Sigma) that had
been pre-warmed in an IVF dish. After that, we immediately rinsed the embryos twice in two droplets of 250 µl of pre-warmed culture media in IVF dishes set aside for rinsing. The embryos
were left in the second rinsing droplet and incubated for 10 min at 37 °C under 6% CO2 to rehydrate. Warmed embryos were then seeded in the Day 0, pre-equilibrated WOW dishes and IVF dishes
and cultured for 96 h. EMBRYO CULTURE IN PDMS DISHES Properties of a cell culture substrate, such as stiffness and hydrophobicity, are known to affect cell proliferation. To isolate the
impacts of culture quality due to the PMDS substrate (i.e., material effects), we cultured single-cell mouse embryos on a layer of PDMS (i.e., PDMS dishes) or polystyrene (i.e., IVF dishes)
and graded the blastocysts that resulted after 96 h for quality comparison. The protocols of PDMS dish fabrication, culture dish preparation, and embryo thawing and culture are described in
the supplemental S1. The number of embryos used in the experiment is summarised in Table 2. Each row represents number of embryos uses in each replication. The variability of embryo numbers
in different replication is due to the variations in number of embryos per straw and exclusion of embryos lost during handling and transport prior to culture. EMBRYO CULTURE IN COMMERCIAL
WOW DISHES To assess the performance of our PDMS WOW dishes to commercial WOW dishes (VitaVitro) made of polystyrene, we compared the embryo culture quality in the hemispherical microwells
of PDMS WOW dishes (i.e., microwells of the best culture quality as described in results and discussion) and the semi-elliptical microwells of commercial WOW dishes. Each commercial WOW dish
has 16 microwells in a macrowell. The microwell diameter measured 0.317 ± 0.001 mm (± 1 standard error of the mean, SEM) and the microwell depth measured 0.260 ± 0.001 mm (± 1 SEM) using
OCT. The OCT images of the microwell are shown in supplemental Fig. S2. Comparing with the hemispherical microwells in our WOW dish, the semi-elliptical microwells in commercial WOW dish
have a smaller diameter and larger depth. We cultured one-cell mouse embryos in the semi-elliptical microwells of commercial WOW dishes and graded the blastocysts that resulted after four
days. During each experiment, a control group of embryos was grown in a standard IVF dish to ensure quality of the straw from the source. The protocols for commercial WOW dish preparation
and embryo thawing and culture are described in the supplemental S2. The number of embryos used in the experiment is summarised in Table 3. Each row represents number of embryos uses in each
replication. The variability of embryo numbers across replications is due to variations in the number of embryos per straw and exclusion of embryos lost during handling and transport prior
to culture. Note that the macrowell size in the commercial WOW dishes is smaller than that in the PDMS WOW dishes. The commercial dishes used a of 50 µl of culture media as instructed by the
manufacture, compared to 80 µl for which the PDMS WOW dishes were designed. Previous study has shown that embryo density in WOW dishes does not impact the blastocyst rate and
quality14,15,16; therefore, the volume difference is unlikely impactful to culture quality in the study. The culture media in control groups was kept 80 µl to be consistent with the control
group of PDMS WOW dishes. CULTURE QUALITY COMPARISON EMBRYO IMAGING After 96 h of culture (Day 4), embryos were imaged with an inverted microscope for quality assessment. To prevent optical
aberrations introduced by the base structure of the microwells from affecting the quality assessment, imaging of embryos cultured in both fabricated and commercial WOW dishes was performed
in glass-bottom dishes (P35G-1.0-20-C, MatTek) that had been pre-equilibrated in the incubator the night before. For embryos in PDMS the WOW dish, each glass-bottom dish comprised three
droplets of 30 µl of culture media covered with 3 ml of culture oil. Embryos from the same macrowell (i.e., cultured in same microwell shapes) were grouped together into the same droplet.
For embryos in the commercial WOW dish, each glass-bottom dish comprised one droplet of 30 ul media covered with 3 ml culture oil, wherein embryos were grouped from commercial WOW dish. Then
the dish was loaded into a stage-top incubator at 37 °C under 6% CO2 (ECU- HOC-IV, In Vivo Scientific) and imaged by integrated modulation contrast (IMC) microscopy (DMI8, Leica) using a 20
× objective (378–824-5, Mitutoyo). The PDMS dishes and IVF dishes were directly loaded to the stage-top incubator and imaged by the same microscope. Digital IMC images of the full field of
view were saved. To avoid grading bias, any embryos seen in a collapsed state (due to spontaneous collapse or trauma from pipetting) were incubated for an additional 1.5 h to allow time for
re-expansion prior to saving the final image. EMBRYO GRADING Each image was labelled with a random code known only to the experimenter. Randomly-labelled images were then sent to three
embryologists for independent grading following the Gardner blastocyst grading system17, which involves assessing the degree of expansion, the quality of the inner cell mass (ICM) and the
quality of the trophectoderm (TE) with a letter grade. The three-letter blastocyst morphology gradings were converted to a single-number score, the blastocyst quality score (BQS)18, which is
the product of the degree of expansion (numbered one to six), and grades give for the ICM and TE (A = three, B = two and C = one). Embryos that did not become blastocysts were graded as
zeros. The mean BQS across three embryologists was calculated for each embryo to represent its quality. BLASTOCYST RATE We calculated the blastocyte rate of each replication for IVF dishes
and for each microwell shape in PDMS WOW dishes. The mean blastocyst rate was then calculated across all replications for a given experimental condition. The mean blastocyst rate of the PDMS
WOW dishes was obtained by averaging the blastocyst rates of all microwell groups. We confirmed the normal distribution of the blastocyst rate in all experimental groups by
Kolmogorov–Smirnov normality test. Next, a paired Tukey one-way analysis of variance (ANOVA) was used to compare the blastocyst rates of each microwell shape, the PDMS WOW dish as a whole
and the IVF dish. An embryo was considered not to have reached the blastocyst stage if at least two of the three embryologists graded it as a non-blastocyst. Such embryos were excluded from
the subsequent BQS comparison. MEAN BQS ANALYSIS We first confirmed the normal distribution of the mean BQS in all experimental groups by Kolmogorov–Smirnov normality test. With the
confirmation of normal distribution, a paired Tukey one-way analysis of variance (ANOVA) was used to perform statistical analysis to compare culture outcomes in the study comparing PDMS WOW,
flat PDMS, and commercial WOW dishes. ETHICAL STATEMENTS This study is performed in accordance with relevant guidelines and regulations. All methods are reported in accordance with ARRIVE
guidelines. RESULTS AND DISCUSSION CHARACTERIZATION OF LARGE-SCALE STRUCTURES Figure 3 shows µCT images of the 3D-printed mold and corresponding WOW insert. The values for the designed and
measured dimensions are summarized in Table S1. The reported values represent the average of three repeated measurements at each location. Qualitatively, it is evident that the large-scale
structures in the mold match the expected design. The large-scale lateral structures in the PDMS WOW inserts also nearly match the expected designs, although they are uniformly smaller than
the corresponding mold dimensions. PDMS shrinkage, a known phenomenon during PDMS curing19, was observed in the axial structures (i.e., WOW insert thickness, H’, and macrowell depths,
h1’–h4’). Notably, the shrinkage was limited (< 5.35%) and did not affect the use of WOW dishes. CHARACTERIZATION OF SMALL-SCALE STRUCTURES Figure 4 shows OCT images of the microwell
templates of the mold (a–h) and corresponding microwell structures of the insert (i–p). The measured dimensions are summarized in Table S2, where each measurement represents the average of
single measurements taken at each of the nine microwells. As expected, the small-scale structures in the 3D-printed mold nearly matched the designs, as do the small-scale lateral structures
in the PDMS WOW inserts. Similar to the macrowells, PDMS shrinkage was observed for the axial structures of microwells (i.e., microwell depths); however, the shrinkage was limited (<
7.84%) and did not affect the use of WOW dishes. Qualitatively, the images in Fig. 4 also show the strong similarity across microwell replicates, which suggests the repeatability and
reliability of the embryo growth conditions across microwells. TEST OF MOLD REUSABILITY Figure 5 shows the consistency of inserts replicated with the same mold. STD z-projections of the OCT
images of the 15th and 30th WOW insert replicates are shown as an interlaced pattern. The strong continuity of the image indicates that structures are well preserved even after many
replicates, suggesting high reusability of the mold, which can lower fabrication and production costs. COMPARISON OF CULTURE QUALITY IN PDMS WOW DISHES Of the 83 embryos cultured, 70 reached
blastocysts, which is one measure of embryo quality. The average Day-5 blastocyst rate of embryos cultured in our WOW dish was 88.7%. In general, the blastocyst rate for each microwell
shape was higher than that of control embryos grown in IVF dishes (78.0%) by nearly 10%; however, no significant differences were found in any paired groups, when evaluated using Tukey
one-way ANOVA. The high blastocyst rate confirms the WOW dish culture conditions are suitable for embryo growth. Figure 6 presents a visual summary of these results. The hatched pattern of
bars corresponds to dishes with flat bottoms, such as the truncated cone microwells and the standard IVF dish. Overall, embryos grown in PDMS WOW dishes also show better blastocyst quality
than those grown in IVF dishes when evaluated using the BQS score. The mean scores ± 1 SEM across all experiments for the different groups were as follows: 13.21 ± 1.45 (IVF dish), 28.63 ±
2.78 (hemispherical WOW), 17.35 ± 2.60 (pyramidal WOW) and 18.88 ± 3.01 (truncated conical WOW). Embryos cultured in hemispherical WOWs show the best blastocyst quality, and the trend was
consistent with grades from all three embryologists despite general differences in the average BQS assigned by each embryologist (Fig. 7a). A Tukey one-way ANOVA was applied to analyse the
BQS of each grader. In the BQS of the grader 1 (G1), statistically significant differences were found between the hemispherical and pyramidal dish groups, the hemispherical and truncated
cone dish groups, and the hemispherical and IVF dish groups. In the BQS of the grader 2 (G2), statistically significant differences were found between the hemispherical and pyramidal dish
groups, and the hemispherical and IVF dish groups. No significant differences were found in other paired groups. In the mean BQS comparison (Fig. 7b), statistically significant differences
were found between the hemispherical and pyramidal dish groups, the hemispherical and truncated cone dish groups, and the hemispherical and IVF dish groups, while no significant differences
were found in other paired groups, when evaluated using Tukey one-way ANOVA. Across all graders, hemispherical microwells yielded better BQS. As this work is the first to use hemispherical
microwells to culture embryos, one outcome of this fabrication process is that we have demonstrated a dish shape leading to better quality embryos. Previous efforts to demonstrate WOW
culture of human embryos in truncated cone microwells5 yielded a live birth rate of 41.5%, an improvement over IVF dishes by 8.6%. Given our results showing that hemispherical wells
outperform truncated cones IVF dishes in both blastocyst rate and BQS, we anticipate that this shape will also improve the live birth rate. In our experiment, all WOW-dish embryos were
individually confined, while IVF-dish embryos were grown as a group. Notably, the WOW-dish embryos within a single microwell type were all connected by the same ~ 80-µL droplet formed in the
macrowell, which is the definition of group culture2,5,20. Hence, this experiment is not a comparison of the effects of individual versus group culture. Indeed, group culture has already
been shown to be superior to individual culture21, likely because it enables sharing of growth factors. We thus believe that the superior BQS results of the hemispherical microwell group are
due to the shape alone, as the general size of the microwell was controlled by maintaining a constant opening and depth across all tested shapes. The superiority of the hemispherical shape
is not surprising, as the 3D culture environment offered by the hemispherical microwell likely better mimics the in-vivo environment. COMPARISON OF CULTURE QUALITY IN PDMS DISHES Of the 40
embryos cultured, 39 embryo reached blastocyst. The average Day-5 blastocyst rate of embryos cultured in PDMS dishes was 100% and in IVF dishes was 95%. The mean BQS scores ± 1 SEM for PDMS
and IVF dishes are 15.33 ± 1.72 and 19.29 ± 0.94, respectively. As tested by Tukey one-way ANOVA, no statistically significant difference was found between the culture quality of PDMS and
IVF dishes (Fig. 8), indicating that the PDMS substrate alone does not improve the culture quality. Therefore, the enhancement of culture quality in PDMS WOW dishes is not a material effect
of using PDMS. COMPARISON OF CULTURE QUALITY IN COMMERCIAL WOW DISHES Of the 37 embryos cultured, 30 embryo reached blastocyst. The average Day-5 blastocyst rate of embryos cultured in
commercial WOW dishes was 79.0% and in IVF dishes (i.e., control) was 83.3%. The mean BQS scores ± 1 SEM for the IVF and commercial WOW dishes are 14.82 ± 1.01 and 18.42 ± 2.24 respectively.
Tukey one-way ANOVA was performed on the mean BQS of the control groups of hemispherical PDMS WOW dishes and commercial WOW dishes to confirm that there is no quality difference of embryos
used in these two experiments. The same analysis was performed between the embryos cultured in hemispherical PDMS WOW dishes and commercial WOW dishes (Fig. 9). A statistically significant
difference was found between the mean BQS of hemispherical PDMS WOW dishes and commercial WOW dishes, indicating that our WOW dishes provide better culture quality than commercial WOW dishes
with semi-elliptical shapes. CONCLUSIONS In this study, we proposed a new method for low-cost and rapid fabrication of WOW dishes with arbitrary microwell shapes and dimensions. The
flexibility of the fabrication method enabled us to produce WOW dishes with multiple microwell designs in a single dish, including pyramidal and hemispherical designs that have never been
used for embryo culture before. The dishes we present here used four different designs, but the method is versatile to produce any combination of microwell designs, which can facilitate
highly parallelized experiments and testing to identify the optimal microwell shape and dimensions. In theory, one could conceive of having a larger variety of designs in a single dish or of
varying the combination of designs that share a single macrowell. Although our WOW dishes show enhanced culture quality, the optimal WOW design is unknown. This experiment is to demonstrate
that better culture quality can be achieved by varying WOW design. To that end, we proposed the fabrication method to offer a simple method for researchers to investigate and research the
better WOW design. The one-step PDMS molding method we introduce to fabricate WOW inserts is simple to perform and can be used to convert any standard IVF dish to a WOW dish. It is also
convenient to make multiple WOW inserts, as may be necessary to perform large-scale studies that validate the statistical significance of the results. The low cost of each replicate
contributes to lowering the cost to fabricate multiple dishes, as may be needed to enable large-scale studies. We found that pre-warming the WOW dishes and culture media to 37 °C before
filling the wells is useful to reduce bubbles during handling. The PFOCTS coating applied to the mold to prevent adhesion of the PDMS can cause the PDMS not to cure. However, during the WOW
dish assembly, we left the dish in room temperature for at least 48 h to let all PDMS cure. Therefore, this issue did not affect the integrity of WOW insert structures and embryo culture in
this study. In the future, alternative coating materials, such as parylene-c, can be applied to eliminate the curing concern22. Although this method is very versatile and can enable almost
any arbitrary design, the limited axial resolution of the DLP 3D printer affects the accuracy of shapes that have round structures. This defect can be observed in Fig. 4a, where the
hemisphere template appears slightly flattened on the top. The limited axial resolution also makes the roughness of the microwell edges hard to control. Such roughness can be observed in
Fig. 4e as ridges on the pyramidal templates. These ridges are also seen in the microscope images of the mold and WOW insert shown in Figs. S3 and S4, respectively. The effects of microwell
base curvature and edge roughness on embryo culture quality are unknown, but analysis of the embryo culture results reveals that the PDMS WOW dishes are robust for culture and that
hemispherical microwells produce better quality blastocysts than IVF dishes and other microwell designs. In conclusion, the proposed method for fabricating novel WOW dishes will make it
possible to rigorously investigate the effects of microwell shapes and dimensions on embryo growth. Our results indicate that hemispherical microwells yield better culture outcomes than
traditional IVF dishes, pyramidal microwells and truncated cone microwells. We also verify that the observed improvements in embryo quality are not due to the use of PDMS (i.e., material
effects) alone. Our WOW dishes with hemispherical microwells also outperform commercial WOW dishes of semi-elliptical microwells. Altogether, these results suggest that translation of our
technology to in vitro fertilization clinics may provide new tools to bolster IVF success rates. DATA AVAILABILITY The data that support the findings of this study are available from the
corresponding author upon reasonable request. REFERENCES * Vajta, G. _et al._ New method for culture of zona-included or zona-free embryos: The Well of the Well (WOW) system. _Mol. Reprod.
Dev._ 55, 256–264 (2000). Article PubMed CAS Google Scholar * Vajta, G., Parmegiani, L., Machaty, Z., Chen, W. B. & Yakovenko, S. Back to the future: Optimised microwell culture of
individual human preimplantation stage embryos. _J. Assist. Reprod. Genet._ 38, 2563–2574 (2021). Article PubMed PubMed Central Google Scholar * Vajta, G. _et al._ The Well-of-the-Well
system: An efficient approach to improve embryo development. _Reprod. Biomed. Online_ 17, 73–81 (2008). Article PubMed Google Scholar * Iwayama, H. _et al._ Clinical application of a
microwell system to in vitro culture of human preimplantation embryos. _J. Mamm. Oval Res._ 25, 167–171 (2008). Article Google Scholar * Fancsovits, P. _et al._ Prospective-randomized
study comparing clinical outcomes of IVF treatments where embryos were cultured individually or in a microwell group culture dish. _Biol. Future_ 73, 229–236 (2022). Article Google Scholar
* Feltrin, C. _et al._ Effectiveness of microwell-based in vitro culture systems for zona-free cloned bovine embryos. _Acta Sci. Vet._ 43, (2015). * Hoelker, M. _et al._ Effect of embryo
density on in vitro developmental characteristics of bovine preimplantative embryos with respect to micro and macroenvironments. _Reprod. Domest. Anim._ 45, 138–145 (2010). Google Scholar *
Chung, Y. H. _et al._ Microwells support high-resolution time-lapse imaging and development of preimplanted mouse embryos. _Biomicrofluidics_ 9, 1–10 (2015). Article Google Scholar *
Watanabe, H. _et al._ An evaluation of continuous human embryo culture using the wow dish. _Fertil. Steril._ 104, e317–e318 (2015). Article Google Scholar * Thomsen, A. R. _et al._ A deep
conical agarose microwell array for adhesion independent three-dimensional cell culture and dynamic volume measurement. _Lab Chip_ 18, 179–189 (2018). Article CAS Google Scholar * Kim,
K., Kim, S. H., Lee, G. H. & Park, J. Y. Fabrication of omega-shaped microwell arrays for a spheroid culture platform using pins of a commercial CPU to minimize cell loss and crosstalk.
_Biofabrication_ 10, 045003 (2018). Article ADS PubMed Google Scholar * Futrega, K. _et al._ The microwell-mesh: A novel device and protocol for the high throughput manufacturing of
cartilage microtissues. _Biomaterials_ 62, 1–12 (2015). Article PubMed CAS Google Scholar * Pereira, D. C., Dode, M. A. N. & Rumpf, R. Evaluation of different culture systems on the
in vitro production of bovine embryos. _Theriogenology_ 63, 1131–1141 (2005). Article PubMed Google Scholar * Kang, S.-S. _et al._ The efficacy of the well of the well (WOW) culture
system on development of bovine embryos in a small group and the effect of number of adjacent embryos on their development. _Zygote_ 23, 412–415 (2014). Article PubMed Google Scholar *
Sugimura, S. _et al._ Effect of embryo density on in vitro development and gene expression in bovine in vitro-fertilized embryos cultured in a microwell system. _J. Reprod. Dev._ 59, 115
(2013). Article PubMed CAS Google Scholar * Vajta, G., Parmegiani, L., Machaty, Z., Chen, W. B. & Yakovenko, S. Back to the future: Optimised microwell culture of individual human
preimplantation stage embryos. _J. Assist. Reprod. Genet._ https://doi.org/10.1007/s10815-021-02167-4/Published (2021). Article PubMed PubMed Central Google Scholar * Hardarson, T., Van
Landuyt, L. & Jones, G. The blastocyst. _Hum. Reprod._ 27(Suppl 1), 72–91 (2012). Article Google Scholar * Rehman, K. S. _et al._ Late stages of embryo progression are a much better
predictor of clinical pregnancy than early cleavage in intracytoplasmic sperm injection and in vitro fertilization cycles with blastocyst-stage transfer. _Fertil. Steril._ 87, 1041–1052
(2007). Article PubMed Google Scholar * Madsen, M. H., Feidenhans’l, N. A., Hansen, P. E., Garnæs, J. & Dirscherl, K. Accounting for PDMS shrinkage when replicating structures. _J.
Micromech. Microeng._ 24, 127002 (2014). Article CAS Google Scholar * Wydooghe, E. _et al._ Individual commitment to a group effect: Strengths and weaknesses of Bovine embryo group
culture. _Reproduction_ 148, 519–529 (2014). Article PubMed Google Scholar * Ebner, T. _et al._ Group culture of human zygotes is superior to individual culture in terms of blastulation,
implantation and life birth. _Reprod. Biomed. Online_ 21, 762–768 (2010). Article PubMed CAS Google Scholar * O’grady, B. J. _et al._ Lab on a Chip Rapid prototyping of cell culture
microdevices using parylene-coated 3D prints †. _Lab Chip_ 21, 4814 (2021). Article PubMed PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS We thank Kelly Morgan and
Amelia Bass for assistance with embryo grading. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Vanderbilt Biophotonics Center and Department of Biomedical Engineering, Vanderbilt University,
Nashville, TN, 37232, USA Yunqin Zhao, Taehyung Yoon & Audrey K. Bowden * Tennessee Fertility Institute, Franklin, TN, 37067, USA Jennifer Miller & Christopher P. Montville Authors *
Yunqin Zhao View author publications You can also search for this author inPubMed Google Scholar * Taehyung Yoon View author publications You can also search for this author inPubMed Google
Scholar * Jennifer Miller View author publications You can also search for this author inPubMed Google Scholar * Christopher P. Montville View author publications You can also search for
this author inPubMed Google Scholar * Audrey K. Bowden View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS Y.Z. developed the mold, fabricated
the microwell dishes, conducted the embryo culture and imaging, and performed data analysis. T.Y. created the computer-aided design of the mold. J.M. conducted and arranged the embryo
grading. C.P.M. provided access to the clinical resources and editorial review of the manuscript. A.K.B. advised on methods implementation, study design, data analysis and provided
resources. All authors contributed to the manuscript creation. CORRESPONDING AUTHOR Correspondence to Audrey K. Bowden. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no
competing interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0
International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s)
and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material
derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a
credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted
use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/. Reprints and permissions
ABOUT THIS ARTICLE CITE THIS ARTICLE Zhao, Y., Yoon, T., Miller, J. _et al._ A rapid and low-cost method to fabricate well of the well (WOW) dishes with arbitrary 3D microwell shapes for
improved embryo culture. _Sci Rep_ 14, 19757 (2024). https://doi.org/10.1038/s41598-024-70517-0 Download citation * Received: 24 May 2024 * Accepted: 19 August 2024 * Published: 26 August
2024 * DOI: https://doi.org/10.1038/s41598-024-70517-0 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable
link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative