A novel translational model of atherosclerosis, the ex vivo pump-perfused amputated human limb model
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT The preclinical study of atherosclerosis has traditionally centred around the use of small animal models, translating to large animal models, prior to first-in-man studies. We
propose to disrupt this paradigm by designing an ex vivo pump perfused human limb model. The novel model consists of taking a freshly amputated limb and incorporating it into an ex situ
pump-perfused bypass system (akin to extracorporeal membrane oxygenation), circulating warmed, oxygenated blood. The circuit incorporates an introducer sheath and guiding catheter for
intravascular imaging and X-ray angiography. Regular monitoring is performed using blood gas analysis, aiming for physiological parameters. The model maintains oxygen saturations > 99%
for the length of perfusion (up to 6-h). Clinical grade X-ray angiography, intravascular ultrasound and optical coherence tomography have been successfully performed. Indocyanine green, a
near-infrared fluorescent dye that localises to atherosclerotic plaque, has been injected into the system and left to circulate for 90-min. Fluorescence reflectance imaging of the dissected
arterial bed confirmed uptake in areas of calcific atherosclerotic plaque on intravascular imaging. This is the first demonstration of an ex vivo pump-perfused “living” limb experimental
model of atherosclerosis, which shows promise for future studies in translational interventional imaging and molecular targeting. SIMILAR CONTENT BEING VIEWED BY OTHERS ELECTRONIC VASCULAR
CONDUIT FOR IN SITU IDENTIFICATION OF HEMADOSTENOSIS AND THROMBOSIS IN SMALL ANIMALS AND NONHUMAN PRIMATES Article Open access 18 March 2025 MODELING EARLY STAGE ATHEROSCLEROSIS IN A PRIMARY
HUMAN VASCULAR MICROPHYSIOLOGICAL SYSTEM Article Open access 27 October 2020 IN VITRO INVESTIGATION OF THE IMPACT OF PULSATILE BLOOD FLOW ON THE VASCULAR ARCHITECTURE OF DECELLULARIZED
PORCINE KIDNEYS Article Open access 20 August 2021 INTRODUCTION The traditional developmental pipeline of a novel molecular targeting agent, using atherosclerosis as an example, consists of
the identification of an unmet clinical need, target discovery through interrogation and modulation of molecular cellular pathways in in vitro models, to in vivo translation with small
animal models (e.g., mice and rats), prior to larger animal models, which are closer to human size and biology (e.g., rabbits and pigs), to demonstrate continued efficacy and absence of
toxicity. Finally, following this, clinical studies must be conducted, ranging from small-scale first-in-man to large phase III clinical trials, testing against the current clinical
standard. This translational pipeline is fraught with difficulties, with significant dropout of potential agents even after extensive and very promising in vivo testing, when a large amount
of time, money and research effort will already have been invested. The validity of animal models of atherosclerosis to patients with atherosclerotic cardiovascular disease in clinical
practice has been questioned in this regard, with all models suffering from human translation applicability, cost, and ethical considerations. For example, transgenic mouse models, which are
the most commonly utilised animal model, lack atherosclerotic lesion complexity, spontaneous plaque rupture or other atherothrombotic complications, rendering them models of atherogenesis
rather than true cardiovascular disease1. Also, their small size means that intravascular experimental techniques cannot be performed. Larger animals with closer physiology to humans, where
more clinically-orientated intravascular techniques can be employed, are more expensive to maintain, often more difficult to handle and have greater ethical considerations2. As such, in this
study we have designed, developed, and tested a novel translational model of atherosclerosis, centred around perfusion of an amputated human limb that would otherwise be clinical waste.
This model will aim to provide a novel means of evaluating molecular targeting agents utilising human tissue, that should serve to both streamline translational pipelines and reduce our
reliance on animals in preclinical atherosclerotic research. Lower limbs amputated on clinical grounds will be used for the study, which de facto will have a significant burden of
atherosclerosis, with chronic limb ischaemia representing the most common indication for amputation3. The limb will be incorporated into a bespoke extra-corporeal perfusion circuit that aims
to maintain physiological parameters and facilitate sophisticated clinical and preclinical intravascular imaging techniques coupled with fluoroscopy. The limb will be perfused with warmed
and oxygenated packed red cells mixed with balanced crystalloid via a modified oxygenator and a pulsatile pump. The pump will be set to mimic the cardiac output the limb would have received
in situ. Measurement of pH, electrolytes, haemoglobin, lactate and oxygenation will be performed, with appropriate optimisation to ensure physiological parameters are maintained, as
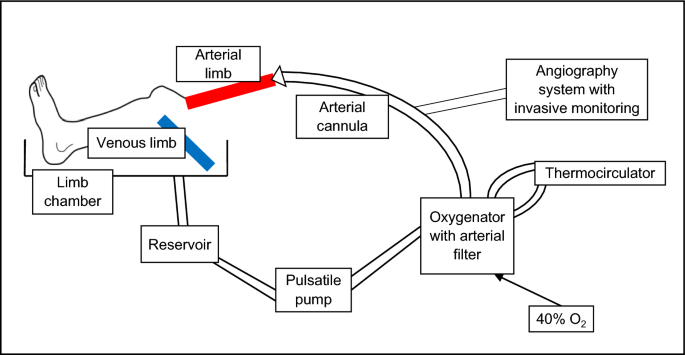
feasible. RESULTS The limb model has been successfully developed, antegradely perfusing amputated human lower limbs with warmed and oxygenated blood. Figure 1 provides a schematic
representation of the limb circuit. After limb perfusion via cannulation of the femoral or popliteal artery (depending on length of resection), blood returns via incision of proximal veins
and is collected passively into a reservoir. Blood is then pumped in a pulsatile fashion through an oxygenator and arterial filter prior to returning the limb via the arterial cannula. In
parallel, a thermocirculator provides a warming circuit to heat the perfusate to 37 °C prior to re-circulation. This model allows the passage of an introducer sheath and guiding catheter for
intravascular imaging and X-ray angiography, and has been incorporated into an experimental catheter laboratory. Figure 2 provides images of the limb model during use. LIMB PERFUSION The
perfusate comprised either expired packed red cells and balanced crystalloid in a 1:1 ratio. Sodium heparin was added to the perfusate to limit haemostasis. The pump was set at 60 systole
%/40 diastole % (to equate to an approximated clinical blood pressure) and at a pump output of 15 mL per stroke (to represent approximately 10% of clinical stroke volume), with an average
pump rate of 70 per minute (equating to a heart rate of 70 beats per minute). Thus, total pump output, or ‘cardiac output’ to the limb was estimated at 1050 mL/min. Circuit flow (mL/min) was
approximated to 6–10% of donor estimated cardiac output (pulse pressure/[(systolic blood pressure + diastolic blood pressure) × heart rate]), which is an approximation of the blood flow a
leg would receive in vivo. Monitoring for satisfactory limb perfusion was performed using continuous pulse oximetry, hourly peripheral temperature and post-limb perfusate testing every hour
for pH, oxygen saturation, lactate, glucose, bicarbonate, sodium, potassium, and haemoglobin concentrations. The perfusate was adjusted according to these parameters with sodium chloride
containing 40 mmol potassium, 1.26% sodium bicarbonate and 10% dextrose in solution as required, aiming for physiological electrolyte normal ranges. Haemoglobin concentration was targeted at
40–60 g/L, with further packed red cells/crystalloid added as required. This haemoglobin target range was based on prior studies with upper limb perfusion, where this lower than physiologic
level resulted in less vascular congestion4. Oxygen saturations were targeted at > 99%. In addition, continuous haemodynamic monitoring was performed using beat-to-beat invasive blood
pressure, heart rate and oxygen saturations. Figure 3 provides an overview of the measured parameters during example limb perfusion. Gas exchange is excellent, with the partial pressure of
oxygen maintaining > 15 kPa and oxygen saturations > 99% throughout. In addition, carbon dioxide levels were very low at < 1.5 kPa. Haemoglobin stayed around the target range of
40–60 g/L throughout. The skin temperature of the limb did take a significant length of time to rise towards normothermia, likely due to the cold ischaemic time on ice packs during transit
prior to perfusion. Unfortunately, despite exchanging blood volume with fresh crystalloid and packed red cells, it was not possible during this experiment to generate a non-acidotic pH or
suppress the lactataemia. ANGIOGRAPHY AND INTRAVASCULAR IMAGING Following the establishment of limb perfusion, angiography is performed in each limb, confirming arterial perfusion, and
identifying areas of atherosclerosis. Figure 4 and corresponding Movies 1–5 provide example fluoroscopic acquisitions using iodinated contrast obtained in one limb model obtained from an
individual who was a prior smoker with diabetes mellitus, hypertension and hypercholesterolaemia; demonstrating successful intubation of the popliteal artery at knee-level (A); just distal
to the popliteal trifurcation below the knee, with anterior tibial, posterior tibial and peroneal branches shown (B); at foot-level with plantar arterial branches of the posterior tibial
artery demonstrated (C). Following successful wiring of the vessel of interest (posterior tibial artery) (D) using a 0.014″ coronary guidewire, intravascular imaging runs were performed,
with intravascular ultrasound (IVUS) (E) and optical coherence tomography (OCT) (F) catheters passed over-the-wire. Intravascular imaging plays an important role in clinical interventional
cardiology, to both assess atherosclerotic lesion morphology prior to possible percutaneous coronary intervention (PCI) and evaluate the post-PCI result. Accordingly, intravascular imaging
has an important role in translational research, permitting the detailed analysis of effective molecular targeting to varying atherosclerotic plaque characteristics. IVUS is a commonplace
tool in modern clinical cardiac catheter laboratories, that utilises an ultrasonic transducer at the distal catheter tip to visualise the arterial wall with a good resolution of 100 µm5. OCT
is a near-infrared light-based imaging modality that generates high-resolution cross-sectional images of the vessel wall, with a resolution of 10–15 µm6. In the experimental limb model,
IVUS and OCT imaging was performed of the vascular bed, from popliteal artery to distal posterior tibial artery of the same limb shown in Fig. 4. Example images and movies acquired using
these tools are displayed in Figs. 5 and 6, as well as corresponding Movies 6 and 7. Figs. 5 and 6 and Movies 6/7 demonstrate evidence of atherosclerotic plaques that could not be readily
appreciated angiographically. The obtained intravascular imaging is of clinical calibre and is easily interpretable by a trained operator. EVALUATION OF A NEAR-INFRARED ATHEROSCLEROTIC
MOLECULAR TARGETING AGENT As a proof-of-principle of utilising the limb model for the assessment of a molecular targeting atherosclerosis agent, indocyanine green was tested. Indocyanine
green is a near-infrared agent (excitation 780/emission 830 nm), which targets lipid and macrophages in areas of increased endothelial permeability (atherosclerotic plaques). Moreover,
indocyanine green is readily available as it is used clinically with laser ophthalmoscopy for the evaluation of the retinochoroidal circulation. Indocyanine green at a dose of 2 mg/kg (limb
weight) was injected post-limb (akin to intravenous injection) and left to circulate within the perfusion system. Indocyanine green usually has a human plasma half-life of 3–5 min, with
hepatic excretion. Of course, in the limb model there is no liver metabolism; thus, the agent will remain in circulation until the perfusate is changed. In an experimental in vivo human
carotid study utilising indocyanine green to visualise carotid plaques, the average time from intravenous injection to ex vivo imaging was 99 min7. Accordingly, 90-min after injection, limb
perfusion was ceased, and the system drained of all perfusate. The limb was then perfused with fresh crystalloid to flush any residue or unbound indocyanine green and therefore reduce
background fluorescent signal. Next, the perfused arterial tissue was dissected and opened en face, prior to performing fluorescence reflectance imaging (FRI), shown in Fig. 7. This displays
the dissected tibioperoneal trunk bifurcating into posterior tibial and peroneal arteries, imaged under white light in (A) and using FRI in (B). There is increased fluorescence signal in
the tibioperoneal artery proximal to the bifurcation, which is a predilection site for atherosclerosis, as well as in the posterior tibial artery. The area of high intensity fluorescence
towards the distal end of the posterior tibial artery section corresponds to regions of circumferential calcification seen on both IVUS and OCT in Figs. 5 and 6. DISCUSSION This study
details the design, development and validation of a novel experimental model of atherosclerosis. The benefits of this novel amputated human limb model of atherosclerosis lie in that it could
reduce our dependency on animal models of atherosclerosis in preclinical research, whilst at the same time also improving applicability of the preclinical models to humans, thus optimising
clinical relevance, and hopefully increasing the efficiency of clinical translation. Although establishing the limb perfusion circuit was initially expensive, once set up, there is a
possibility that this model may be cost saving, versus breeding/purchasing, feeding and housing animals (especially large species), as well as any necessary procedures, such as balloon iliac
injury in the case of the rabbit model of atherosclerosis. Rabbit models of experimental atherosclerosis are common, owing to their relatively inexpensive maintenance and easy handling.
Experimental use is declining however, likely related to the ready commercial availability of cheaper and more acceptable transgenic mouse models. Rabbit models include Watanabe heritable
hyperlipidaemic rabbits, which develop significant hypercholesterolaemia and hypertriglyceridaemia accompanied by widespread atherosclerotic lesions with a normal diet8. Other approaches
include New Zealand White rabbits fed a high-fat diet and more recently, Apolipoprotein E-deficient rabbits have been generated using genome editing9. Rabbit models develop largely foam cell
atherosclerotic lesions, rather than the fibrous lesions in mice and more complex mixed lesions in humans. Rabbits are obviously larger in size than mice, and therefore iliac vessel
cannulation is possible, permitting catheter-based intravascular imaging of the aorta, but not the coronary arteries. This has been harnessed to generate a novel model of atherosclerosis,
whereby catheter-directed balloon injury is performed to disrupt the endothelium of the iliac vessels, resulting in rapid neointimal thickening, lipid accumulation, smooth muscle cell
infiltration and foam cell generation. This creates atherosclerotic lesions in an accelerated fashion (within 2-weeks), much faster than the four to eight months that would be required
otherwise10. Rabbit lipoprotein metabolism is closer to human than mouse lipoprotein metabolism, however there are important differences. For example, rabbits lack Apolipoprotein A-II (an
important component of High-Density Lipoprotein in humans) and have low levels of hepatic lipase11. Again, different to humans, rabbits develop significant atherosclerotic lesions in the
aortic arch and descending thoracic aorta2. Larger animal models of experimental atherosclerosis are principally pigs and non-human primates. The advantages of using these larger species
over smaller species, are that more complex intravascular catheter-based techniques can be employed, owing to their increased size and more human-like cardiovascular anatomy. Pigs are the
most used larger species for experimental atherosclerosis modelling, owing to their human-like anatomy, closer genetic resemblance to humans and similar atherosclerosis predilection sites,
in the coronary, abdominal aorta and iliofemoral arteries. However, atherosclerotic plaques in porcine models often do not advance to become more complex lesions, at least in a reasonable
experimental timescale. Furthermore, spontaneous plaque rupture and thrombosis is rare. Pig models consist of lines with natural mutations in the Apolipoprotein B and Low-Density
Lipoprotein-Receptor genes, diabetic hypercholesterolaemic pigs, as well as genetically engineered strains, for example, Low-Density Lipoprotein-Receptor knock-out. In contrast to mice and
rabbits, pigs are more expensive to maintain and more difficult to handle2. Porcine models however have been successfully utilised to study atherosclerotic plaque development in response to
disturbed coronary flow using clinical calibre in vivo intravascular imaging12, model ischaemia–reperfusion injury and restenosis following angioplasty, as well as being used in stent
development13. Non-human primates, for example, cynomolgous and rhesus monkeys, develop atherosclerosis on high-fat diets, and can be useful experimental models for human atherosclerosis.
However, they are used very infrequently, owing to the necessary level of regulation, expense, ethical considerations, as well as high level of specialised training required. Nonetheless,
non-human primate studies have been utilised to study psychosocial and behavioural aspects of atherosclerosis13. Maintenance of a physiological environment is desirable to allow the
experimental set-up to continue running for a prolonged period without limb decomposition, to assess probe binding and localisation at a timepoint after initial administration, simulating
intravenous construct injection. As an example, ex situ perfusion of an amputated human upper limb from brain-dead organ donors has been successfully performed for 24-h (n = 5), in an
attempt to prolong allograft storage times for transportation and potential transplantation. Using a similar experimental circuit design, neuromuscular electrical stimulation resulted in
appropriate contractile responses throughout perfusion and histological examination post-perfusion demonstrated no evidence of myocyte injury4. Unfortunately, despite exchanging blood volume
with fresh crystalloid and packed red cells, it has not been possible to generate a non-acidotic pH or suppress the lactataemia in this model. This is likely a result of a degree of muscle
breakdown and anaerobic respiration of the heavily diseased amputated limb tissue, as well as the lower pH and raised lactate concentration of stored packed red cells14. However, this raises
the unique possibility of utilising the limb model to study the effects of various physiological stressors that may promote plaque rupture within human vessels. Achieving physiological
levels of sodium, potassium and glucose was problematic, possibly due to the large swings in electrolyte/ glucose levels induced by the addition of large volumes of crystalloids within a
smaller circulating volume (the circuit volume is approximately 2 L, compared to 5 L in an adult male). Using glucose as an example, a very large jump in glucose concentration is seen at 5-h
in Fig. 3, due to blood sampling occurring soon after the administration of 10% dextrose solution. Transfusion-related hyperkalaemia is a well identified phenomenon, especially with blood
that has been stored for longer periods, due to reduced cellular function and potassium leakage into the extracellular space15. The red cells used in this experiment will have been stored
for a prolonged period prior to use, and thus will carry a significant potassium load. Moreover, as above, given the smaller circulating volume of the limb circuit and packed red cells
forming half the circulating volume, any changes in electrolyte levels are magnified. Indeed, transfusion-related hyperkalaemia has been noted to be more problematic in neonatal and
paediatric populations16. Important limitations include the heavily atherosclerotic nature of the amputated limbs, which will restrict the ability to achieve optimal physiological conditions
for limb perfusion. Despite the significant disease burden, successful perfusion has always been achieved, likely due to the preservation of viable tissue within resection margins during
the amputation. In addition, although animal models are often used to demonstrate targeting probe function, data on harm or toxicity is also generated. This will not be possible with the
limb model. Likewise, evaluating pharmacokinetics will not be possible with the limb model, nor the assessment of activatable or pro-drug compounds. Moreover, animal models are routinely
used to assess therapeutic efficacy of an investigational agent: this will be difficult to achieve with the limb model, without the ability to run the model for sufficient time to allow a
therapeutic agent time to demonstrate efficacy. Additionally, although this is human-based and great efforts have gone to optimise the model within physiological parameters, it remains an
artificial system, where clinical applicability and relevance will still be questioned. A further consideration is the relevance of peripheral atherosclerotic plaques to coronary or
cerebrovascular atherosclerotic disease. Peripheral atherosclerosis tends to have greater burden of calcification, including vascular medial calcification. As a result, plaque rupture of
peripheral plaques is less common, and occlusive clinical disease is more often the result of embolic phenomena or in situ thrombosis17. There are further considerations regarding tissue
stability for the use of this model in other research avenues. For example, we have not utilised prolonged perfusion times and demonstrated continuing limb viability. However, as discussed
above, high levels of oxygenation in the venous perfusate were achieved across all experimental time points, as well as successful tissue warming following perfusion and a declining lactate
concentration, all suggesting potential model stability. On a practical level, once perfusion has been established, the model requires little input, aside from perfusate sampling and
adjusting conditions accordingly. One option to experimentally evaluate model viability would be to obtain both proximal and distal limb skin tissue biopsies and carry out a cell viability
or metabolic activity assay at varying timepoints, with the aim of demonstrating continuing model stability of both tissue samples. Another potential utility of this model would be in
evaluating vascular physiological functions in response to various stimuli, potentially with invasive physiological assessments, as performed routinely in clinical interventional cardiology
practice. However, this model may not be ideal to evaluate vascular physiology, given the artificial nature of the perfusion circuit, with potential confounding cellular effects of altered
flow dynamics as well as the acidotic cellular environment. Moving forwards, challenges with this novel experimental model will include further optimisation of perfusion conditions, aiming
to normalise the cellular environment for both pH and electrolyte balance. Performing prolonged perfusion experiments (> 24 h) with demonstration of continued tissue viability will also
be an important next step. Once these have been established, testing novel molecular targeting agents on human tissue, with detection via catheter based near-infrared fluoroscopic techniques
as well as other non-invasive imaging modalities, will be an exciting prospect. Successful ex situ molecular targeting of human atherosclerotic plaque has been demonstrated for the first
time, through this novel translational model of atherosclerosis, using clinical calibre intravascular imaging with IVUS and OCT to permit plaque signal colocalisation. Potential future uses
include the preclinical testing of developmental molecular probes, as well as trialling new intravascular imaging catheters, without the requirement for animal models and with the hope of
more streamlined bench to bedside translation. METHODS ETHICAL PERMISSIONS Ethical permission for the collection of, and research on, human clinical tissue for this study was granted by the
Imperial College Healthcare Tissue Bank (REC Wales 17/WA/0161), as a formal subcollection (CAR_RK_17_070). No additional clinical samples were required or obtained for this study. All
methods were performed in accordance with necessary institutional guidelines and regulations. Patients were approached to donate tissue to the study following screening by the referring
vascular surgical team and routine clinical pre-amputation counselling, arranged from pre-operative clinic. All participants received the appropriate patient information leaflet for use of
leftover tissue for research purposes, as per the Imperial College Healthcare Tissue Bank, prior to the consenting process. All patients provided written informed consent for tissue
collection at the time of consenting for the operation itself. EX SITU PERFUSION SYSTEM A custom-made radiolucent operating table was designed that incorporated a decline and funnel to
facilitate venous blood return back into the bypass circuit. The funnel was connected via ¼’’ tubing (Tygon Tubing, Harvard Apparatus, Cambridge, UK) to the venous inlet of a modified
oxygenator with integrated arterial filter (CAPIOX Baby FX05RW, Terumo, Surrey, UK), allowing the venous reservoir to fill passively under gravity. The arterial filter served to remove any
clots or debris generated within the perfusion system. The reservoir outlet was connected to the inlet of a pulsatile blood pump, with ball valves and smooth flow to minimise haemolysis
(55-3321 pulsatile blood pump for dogs/ monkeys, Harvard Apparatus, Cambridge, UK), through a ¼″–½″ adapter (Harvard Apparatus, Cambridge, UK) and ½″ tubing. The pump outlet was connected
through a ½″–¼″ adapter to ¼″ tubing back to the arterial inlet of the oxygenator. The oxygenator was supplied with a sweep gas of 40% oxygen: air mixture via ¼″ tubing. A parallel circuit
supplying warmed water to the oxygenator at 37 °C was set up using a thermocirculator (TC120, Grant Instruments, Cambridge, UK), connected via ½″ tubing. The arterial outlet port of the
oxygenator was connected via ¼″ tubing to one arm of a Y-connector (Harvard Apparatus, Cambridge, UK). Further ¼″ tubing incorporating a femoral sheath (9French Input Introducer Sheath,
Medtronic, Dublin, Ireland) was connected to the other arm. The Y-connecter was then joined via further ¼″ tubing to a femoral arterial cannula (Medtronic EOPA Elongated One-Piece Arterial
cannula, Dublin, Ireland), supplying the amputated limb with warmed and oxygenated perfusate. LIMB PROCUREMENT All patients undergoing elective planned limb amputations were eligible to
donate tissue to the study. Limbs were collected immediately from vascular operating theatre and flushed with heparinised saline to remove clots and prevent in situ thrombosis. Ligatures
were tied around the main proximal artery that would be used for antegrade perfusion, as well as the large proximal veins for outflow, locking the heparinised saline within the vascular bed.
LIMB PERFUSION Firstly, the arterial cannula was sutured into the femoral/popliteal artery (depending on length of resection), and corresponding veins incised to facilitate venous return.
Following establishment of the perfusion circuit, the limb was thoroughly flushed via the pulsatile pump with heparinised saline to remove any in situ clot. The system was then drained to
remove the saline wash and exchanged for perfusate. The perfusate comprised either expired clinical-grade or research-grade packed red cells (Non-Clinical Issue National Health Service Blood
and Transplant Service, Colindale, UK) and balanced crystalloid (Plasma-Lyte 148 [pH 7.4], Baxter, Berkshire, UK) in a 1:1 ratio. Packed red cells were used for their oxygen carrying
capacity, rather than an acellular perfusate. Sodium heparin was added to the perfusate, at a concentration of 3,000 units/kg in 0.9% sodium chloride, to limit haemostasis. Monitoring for
satisfactory limb perfusion was performed using continuous pulse oximetry (AVAX, Contec Medical Systems, China); hourly peripheral temperature of the skin on the sole (TempIR, USA); and
post-limb perfusate testing every hour for pH, oxygen saturation, lactate, glucose, bicarbonate, sodium, potassium, and haemoglobin concentrations (GEM 4000, Werfen UK, Cheshire, UK). In
addition, continuous haemodynamic monitoring was performed using a S/5 Compact Anaesthesia Monitor with beat-to-beat invasive blood pressure, heart rate and oxygen saturation modules (M-ESTR
module) (Datex-Ohmeda, Wisconsin, USA). IMAGING Interventional cardiac guide catheters (for example, a 6French multipurpose MB1 Launcher guiding catheter [Medtronic, Dublin, Ireland]) were
introduced over a J-tipped 0.035″ wire to the artery of study and connected to a 3-port standard angiography manifold, that permits injection of iodinated contrast, invasive monitoring and a
port for administration of other agents. Omnipaque 300 (GE Healthcare, Illinois, USA) was used as the contrast agent for angiographic studies. X-ray fluoroscopy was performed using a
portable C-arm (Fluoroscan Insite FD Mini C-arm Extremities Imaging System, Hologic, Manchester, UK). Following this, 0.014″ coronary guidewires, such as a Runthrough NS Extra Floppy
(Terumo, Tokyo, Japan), could be manipulated to the distal arterial bed using conventional interventional techniques. Over this wire, intravascular imaging catheters could be passed distal
to the area of interest and manual/automated pullback performed. Readily available imaging systems included IVUS (Volcano Eagle Eye Platinum, Philips, Amsterdam, Netherlands) and OCT
(Dragonfly Optis, Abbott Cardiovascular, Illinois, USA). IVUS was performed with manual pullback at a rate of 1 mm/s, whilst OCT was performed with an automated pullback of 20 mm/s. DATA
AVAILABILITY The data that support the findings of this study are available from the corresponding author, RK, upon reasonable request. REFERENCES * Getz, G. S. & Reardon, C. A. Animal
models of atherosclerosis. _Arterioscler. Thromb. Vasc. Biol._ 32, 1104–1115. https://doi.org/10.1161/atvbaha.111.237693 (2012). Article CAS PubMed PubMed Central Google Scholar * Emini
Veseli, B. _et al._ Animal models of atherosclerosis. _Eur. J. Pharmacol._ 816, 3–13. https://doi.org/10.1016/j.ejphar.2017.05.010 (2017). Article CAS PubMed Google Scholar * Kalbaugh,
C. A. _et al._ Trends in surgical indications for major lower limb amputation in the USA from 2000 to 2016. _Eur. J. Vasc. Endovasc. Surg._ 60, 88–96.
https://doi.org/10.1016/j.ejvs.2020.03.018 (2020). Article PubMed Google Scholar * Werner, N. L. _et al._ Ex situ perfusion of human limb allografts for 24 hours. _Transplantation_ 101,
e68–e74. https://doi.org/10.1097/tp.0000000000001500 (2017). Article PubMed Google Scholar * Ono, M. _et al._ Advances in IVUS/OCT and future clinical perspective of novel hybrid catheter
system in coronary imaging. _Front. Cardiovasc. Med._ 7, 119 (2020). Article PubMed PubMed Central Google Scholar * Araki, M. _et al._ Optical coherence tomography in coronary
atherosclerosis assessment and intervention. _Nat. Rev. Cardiol._ 19, 684–703 (2022). Article PubMed PubMed Central Google Scholar * Verjans, J. W. _et al._ Targeted near-infrared
fluorescence imaging of atherosclerosis: Clinical and intracoronary evaluation of indocyanine green. _JACC Cardiovasc. Imaging_ 9, 1087–1095. https://doi.org/10.1016/j.jcmg.2016.01.034
(2016). Article PubMed PubMed Central Google Scholar * Watanabe, Y. Serial inbreeding of rabbits with hereditary hyperlipidemia (WHHL-rabbit). _Atherosclerosis_ 36, 261–268.
https://doi.org/10.1016/0021-9150(80)90234-8 (1980). Article CAS PubMed Google Scholar * Niimi, M. _et al._ ApoE knockout rabbits: A novel model for the study of human hyperlipidemia.
_Atherosclerosis_ 245, 187–193 (2016). Article CAS PubMed Google Scholar * Jain, M., Frobert, A., Valentin, J., Cook, S. & Giraud, M. N. The rabbit model of accelerated
atherosclerosis: A methodological perspective of the iliac artery balloon injury. _J. Vis. Exp._ https://doi.org/10.3791/55295 (2017). Article PubMed PubMed Central Google Scholar * Fan,
J. _et al._ Rabbit models for the study of human atherosclerosis: From pathophysiological mechanisms to translational medicine. _Pharmacol. Ther._ 146, 104–119.
https://doi.org/10.1016/j.pharmthera.2014.09.009 (2015). Article CAS PubMed Google Scholar * Pedrigi, R. M. _et al._ Inducing persistent flow disturbances accelerates atherogenesis and
promotes thin cap fibroatheroma development in D374Y-PCSK9 hypercholesterolemic minipigs. _Circulation_ 132, 1003–1012. https://doi.org/10.1161/circulationaha.115.016270 (2015). Article
PubMed Google Scholar * Shim, J., Al-Mashhadi, R. H., Sørensen, C. B. & Bentzon, J. F. Large animal models of atherosclerosis—New tools for persistent problems in cardiovascular
medicine. _J. Pathol._ 238, 257–266. https://doi.org/10.1002/path.4646 (2016). Article CAS PubMed Google Scholar * Oyet, C., Okongo, B., Onyuthi, R. A. & Muwanguzi, E. Biochemical
changes in stored donor units: Implications on the efficacy of blood transfusion. _J. Blood Med._ 9, 111–115. https://doi.org/10.2147/jbm.S163651 (2018). Article CAS PubMed PubMed Central
Google Scholar * Raza, S. _et al._ A prospective study on red blood cell transfusion related hyperkalemia in critically ill patients. _J. Clin. Med. Res._ 7, 417–421.
https://doi.org/10.14740/jocmr2123w (2015). Article PubMed PubMed Central Google Scholar * Altun, D. _et al._ Measuring potassium level in packed red blood cells before using: Word of
caution for congenital cardiac surgery. _J. Card. Surg._ 37, 535–541. https://doi.org/10.1111/jocs.16158 (2022). Article PubMed Google Scholar * Narula, N., Olin, J. W. & Narula, N.
Pathologic disparities between peripheral artery disease and coronary artery disease. _Arterioscler. Thromb. Vasc. Biol._ 40, 1982–1989. https://doi.org/10.1161/atvbaha.119.312864 (2020).
Article CAS PubMed Google Scholar Download references FUNDING Ramzi Khamis is funded by a British Heart Foundation Clinical Research Fellowship (FS/17/16/32560). Adam Hartley is funded
by a Wellcome Trust Clinical Research Fellowship (220572/Z/20/Z). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Vascular Sciences Section, National Heart and Lung Institute, Imperial
College, Hammersmith Hospital, London, UK Adam Hartley, Jonathan Afoke, Guiqing Liu, Samuel Owen, Reza Hajhosseiny, Prakash Punjabi, Dorian Haskard & Ramzi Khamis * Hammersmith Hospital,
Imperial College Healthcare NHS Trust, London, UK Adam Hartley, Reza Hajhosseiny, Kimberly Hassen, Prakash Punjabi & Ramzi Khamis * Imperial College London and Imperial Vascular Unit,
Imperial College Healthcare NHS Trust, Waller Unit, Mary Stanford Wing, St Mary’s Hospital, Praed Street, London, W2 1NY, UK Joseph Shalhoub Authors * Adam Hartley View author publications
You can also search for this author inPubMed Google Scholar * Jonathan Afoke View author publications You can also search for this author inPubMed Google Scholar * Guiqing Liu View author
publications You can also search for this author inPubMed Google Scholar * Samuel Owen View author publications You can also search for this author inPubMed Google Scholar * Reza Hajhosseiny
View author publications You can also search for this author inPubMed Google Scholar * Kimberly Hassen View author publications You can also search for this author inPubMed Google Scholar *
Prakash Punjabi View author publications You can also search for this author inPubMed Google Scholar * Dorian Haskard View author publications You can also search for this author inPubMed
Google Scholar * Joseph Shalhoub View author publications You can also search for this author inPubMed Google Scholar * Ramzi Khamis View author publications You can also search for this
author inPubMed Google Scholar CONTRIBUTIONS Study conception and design: A.H., R.K.; data collection: A.H., S.O., R.H., J.A., G.L., K.H., J.S.; analysis and interpretation of results: A.H.,
R.K.; draft manuscript preparation: A.H., R.K.; project supervision: P.P., D.H., R.K. All authors reviewed the results and approved the final version of the manuscript. CORRESPONDING AUTHOR
Correspondence to Ramzi Khamis. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE Springer Nature remains
neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION Supplementary Video 1. Supplementary Video 2. Supplementary Video 3.
Supplementary Video 4. Supplementary Video 5. Supplementary Video 6. Supplementary Video 7. SUPPLEMENTARY INFORMATION. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a
Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit
to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are
included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons
licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of
this licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Hartley, A., Afoke, J., Liu, G. _et al._ A novel translational
model of atherosclerosis, the ex vivo pump-perfused amputated human limb model. _Sci Rep_ 14, 17244 (2024). https://doi.org/10.1038/s41598-024-67635-0 Download citation * Received: 13
February 2024 * Accepted: 15 July 2024 * Published: 27 July 2024 * DOI: https://doi.org/10.1038/s41598-024-67635-0 SHARE THIS ARTICLE Anyone you share the following link with will be able to
read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing
initiative KEYWORDS * Atherosclerosis * Molecular targeting * Translational research