Turning glucosinolate into allelopathic fate: investigating allyl isothiocyanate variability and nitrile formation in eco-friendly brassica juncea from south korea
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Leaf mustard (_Brassica juncea_ L.) is explored for its biofumigant properties, derived from its secondary metabolites, particularly allyl isothiocyanate (AITC), produced during the
enzymatic breakdown of glucosinolates like sinigrin. The research examines eight leaf mustard cultivars developed in Yeosu city, South Korea, focusing on their genetic characteristics, AITC
concentration and nitriles formation rates from glucosinolates. Results indicate that the allelopathic effects, largely dependent on AITC concentration and enzymatic activity, vary across
cultivar. Sinigrin and AITC constitute 79% and 36%, respectively, of glucosinolate and its hydrolysis products. The cultivar 'Nuttongii' demonstrates significant potential for
inhibiting weeds, exhibiting the highest AITC concentration at 27.47 ± 6.46 µmole g−1 These outcomes highlight the importance of selecting mustard cultivars for biofumigation based on their
glucosinolate profiles and hydrolysis product yields. The study also identifies a significant genetic influence on AITC and nitrile formation, suggesting that epithiospecifier protein
modulation could enhance both allelopathic and other beneficial effects. Collectively, the research underscores the promise of mustard as a sustainable, environmentally friendly alternative
to traditional herbicides. SIMILAR CONTENT BEING VIEWED BY OTHERS SYNERGISTIC EFFECTS OF HERBICIDES AND GIBBERELLIC ACID ON WHEAT YIELD AND QUALITY Article Open access 03 March 2025
ASSESSMENT OF INDUCED ALLELOPATHY IN CROP-WEED CO-CULTURE WITH RYE-PIGWEED MODEL Article Open access 07 May 2024 COMPREHENSIVE QUANTITATIVE EVALUATION AND MECHANISM ANALYSIS OF INFLUENCING
FACTORS ON YIELD AND QUALITY OF CULTIVATED _GASTRODIA ELATA_ BLUME Article Open access 27 May 2025 INTRODUCTION Biofumigation refers to the biochemical processes within plants that result in
the production of secondary metabolites, thereby contributing to their allelopathic effects on surrounding organisms1,2. As the challenge of herbicide-resistant weeds continues to expend,
biofumigation stands out as an eco-friendly alternative in agriculture, harnessing the power of cover crops3,4. Biofumigation not only addresses the issue of herbicide resistance but also
brings about notable impacts, including changes in soil pH attributed to the persistent use of herbicide5,6. The Brassica family, particularly Brown mustard (_Brassica juncea_), is well
known for its production of chemicals known as isothiocyanates by previous studies7. The brown mustard, commonly known as leaf mustard, has been utilized for pest and bacteria suppression8.
The unique ability of leaf mustard to generate isothiocyanates underscores its significance not only in culinary, where it contributes to the distinctive taste and spiciness of the plant,
but also as a valuable resource for pest and bacterial control in various agricultural systems. Nevertheless, further research is required to effectively implement this concept in weed
control, particularly in understanding the interactions between allelochemicals and weeds. Leaf mustard, for instance, is rich in glucosinolates, especially sinigrin, which is recognized as
the origin of its unique taste and spiciness9. Physical damage to the tissues of leaf mustard serves as a catalyst, initiating an enzymatic reaction involving myrosinase. This enzyme is
responsible for the conversion of sinigrin into allyl isothiocyanate (AITC)10,11. AITC, a compound found in leaf mustard, has been observed to exhibit a range of biological effects,
including anti-cancer, anti-obesity, antiviral, and antibacterial properties. These effects arise primarily due to its capacity to inhibit the cell growth cycle12,13,14. Consequently, the
cell cycle arrest attribute of leaf mustard enhances its value as a cover crop15. Previous studies have shown that AITC can cause damage to the root hairs of lettuce seeds, leading to
drought stress and significantly inhibiting plant growth at higher concentrations16. The presence and concentration of epithiospecifier protein (ESP) in plant tissues are pivotal in
determining whether the hydrolysis of glucosinolates results in the production of AITC or epithionitriles, thus affecting the profile of allelopathic compounds produced17. Despite various
studies on mustard, the knowledge about the enzymatic reactions and hydrolysis products in relation to ESP is still not fully understood. Moreover, although there have been studies, such as
those by Frazie, et al.18, on glucosinolate and its hydrolysis products in leaf mustard, research specifically focusing on their allelopathic effects is comparatively limited. Hence, the
goal of this study is to clarify the allelopathic effects and potential applications of various mustard cultivars. The Ministry of Agriculture, Food, and Rural Affairs of South Korea has
been developed a genetic resource collection project aimed at developing new leaf mustard cultivars, including ‘Nuttongii’, ‘Sundongii’, ‘Sindongii’, ‘Zzangdolii’, ‘Ssamdolii’, ‘Kkottolii’,
‘Alsami’, and ‘Mekomi’ since 2001. The allelopathic potential of leaf mustard varieties bred in Yeosu city has not been extensively studied. There is a necessity for comprehensive
laboratory-scale research to establish the suitable concentration for utilizing these cultivars as cover crops. Previous research has revealed the biofumigation effects of leaf mustard, but
there remains a scarcity of information regarding the allelopathic potential of diverse leaf mustard cultivars19. This study delves into the genetic diversity of mustard compounds with a
focus on their allelopathic characteristics. This inquiry is crucial in identifying the most suitable mustard cultivar for use as a cover crop. It also involves a comparison between the
widely utilized ‘Dolsan’ mustard variety, which is the most popular cultivar, and eight locally bred cultivars from Yeosu City. The objective is to underscore the potential of Korean mustard
varieties as biofumigation agents, presenting an innovative approach to weed management. This comparative analysis is vital for understanding the variations in effectiveness and possible
applications in agricultural methodologies. MATERIALS AND METHODS PLANT CULTIVATION AND HARVEST Eight leaf mustard cultivars (‘Nuttongii’, ‘Sundongii’, ‘Sindongii’, ‘Zzangdolii’,
‘Ssamdolii’, ‘Kkottolii’, ‘Alsami’, and ‘Mekomi’) and the common ‘Dolsan’ cultivar mustard (_Brassica juncea_ Coss, Worldseed Agricultural Corporation, Gyeonggi-do, Republic of Korea), were
sown into 50-cell seedling trays and incubated in green house at average of 24 °C. After two weeks, at 3 March 2021, the seedlings were transplanted in a randomized block design with three
replications per cultivar. The cultivars were conventionally cultivated at the Chonnam National University farm in Naju, Jeollanam Province, Republic of Korea (34°58′28.4″N, 126°45′59.3″E)
until the time of harvest. Leaf mustards were harvested at the bolting stage in accordance with prior research findings, which have indicated that the highest sinigrin concentration is
typically observed at this bolting stage20. The harvest dates for leaf mustard cultivars were categorized based on their bolting times, with harvests occurring on April 30, 2021, or May 21,
2021. Given that the average cultivation period for leaf mustard is 60 days, the cultivars were classified according to whether bolting occurred within the 60-day cultivation period from the
transplanting date or after this period. Cultivars that bolted within 60 days from transplanting to harvest were classified as the early-harvested group (‘Dolsan (control)’, ‘Alsami’,
‘Sundongii’, ‘Kkottolii’), while those that bolted after 60 days were classified as the late-harvested group (‘Nuttongii’, ‘Mekomi’, ‘Sindongii’, ‘Zzangdolii’ ‘Ssamdolii’). Morphological
traits including days to flowering, bolting rate, leaf length, number of leaves, fresh weight, and days to maturity were determined at day of harvest. PLANT MATERIAL PREPARATION On the day
of harvest, six undamaged plants were selected from each replication. The longest leaf length, number of leaves, and the fresh weight were measured. For sample collection, a total of four
fresh mustard plants per each replication (_n_ = 3) were removed the dirt, chopped, and pooled together. The samples were immediately frozen using liquid nitrogen and then freeze-dried at −
80 °C under 5 mTorr pressure (MCFD8508, IlshineBioBase Co. Ltd., Dongducheon, Korea). The freeze-dried leaf mustard samples were ground using a coffee grinder (BCG-620SP, Bean Cruise, Seoul,
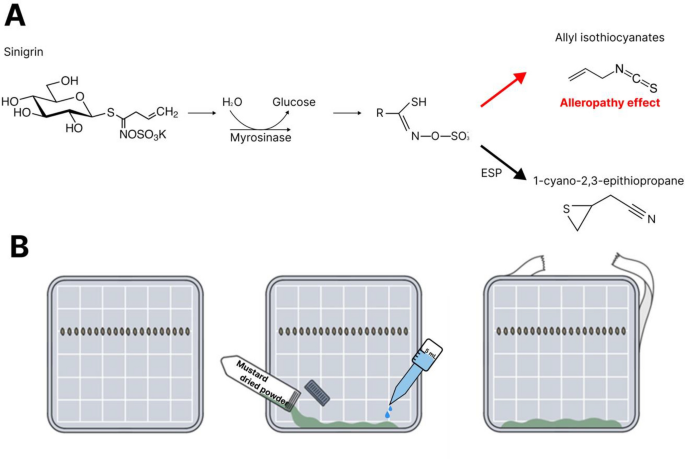
Korea) and stored in a freezer at − 20 °C until further analysis. ALLELOPATHIC BIOASSAY IN AGAR PETRI DISH Experiments was modified as using plant leaf to plant powder on agar gel according
to published method16. 50 mL of 2% agar gel was poured into square petri dishes (126.4 mm diameter) and cooled to solidify. The lettuce seeds (_Lactuca sativa_ L.) cultivar, ‘Summer Andong’
(Kyoungshinseed, Uiseong, Korea), were used for the allelopathy test. Twenty seeds were placed horizontally on the agar surface, and the test species were then added to the agar gel (Fig.
1A). 50 mg of mustard powder and 0.5 mL of water were added to each petri dish (Fig. 1B). In case of non-treated sample, 0.5 mL of water was added. The petri dishes were sealed with parafilm
to prevent gas exchanges of inside air16. Finally, the petri dishes were incubated at 26 °C, tilted at a 120° angle to prevent leakage of the liquid. After 24 h, the germination rates were
evaluated by counting the number of germinated seeds. After three days, the shoot and root lengths were measured using ImageJ (Version 1.52; National Institutes of Health). One day after,
the number of germinated individuals in each petri dish was counted and exhibited as germination rate (% of non-treatment). Subsequently, root and shoot length were measured on 3rd day after
germination. GLUCOSINOLATE ANALYSIS The extraction and analysis of glucosinolate in the mustard samples followed a previously described method with slight modifications of ISO 9167-1 and
previous publication21. Briefly, 200 mg of sample powder was mixed with 2 mL of 70% methanol and 500 µL of 1 mM internal standard (glucosinalbin, isolated from _Sinapis alba_ seeds) and
vortexed for 10 s. The mixture was heated on a heating block at 95 °C for 10 min, then cooled on ice for 5 min. The extracts were centrifuged at 3,000 × g for 10 min. The supernatant was
moved into a glass tube, and the residue was re-extracted twice with 2 mL of 70% methanol. The extracted supernatants were combined, and 1 mL was transferred to a 2 mL tube. Then, 150 µL of
lead/barium acetate solution (0.5 M) was added, vortexed for 10 s, and centrifuged at 15,000 × g for 1 min. The centrifuged mixture was drained into a polyprep column filled with DEAE
Sephadex A-25 (GE Healthcare, Piscataway, NJ, USA). The column was washed sequentially with 3 mL of 0.02 M pyridine acetate and 3 mL of deionized water. After the water had passed through,
500 µL of sulfatase solution (20 U mL−1, _Helix pomatia_ Type-1, Sigma-Aldrich, St. Louis, MO, USA) was added to the column, and it was incubated overnight at room temperature. The eluent
was collected in a 12 × 75 mm glass tube, vortexed for 10 s, and filtered through a 0.22 µm syringe filter into an HPLC vial. The sample was injected into an HPLC (Agilent 1100, Agilent
Technologies, Palo Alto, PA, USA) equipped with a Kromail RP-C18 column (250 mm × 4.6 mm; 5 µm; 100 Å) (AkzoNobel, Bohus, Sweden) at a flow rate of 1.5 mL min−1. The mobile phase A (HPLC
grade water) and mobile phase B (acetonitrile) were used in a gradient system: 0 min, 1.5% B; 4 min, 4% B; 20 min, 20% B; 21 min 80% B; 22 min 80% B; 24 min, 1.5% B; 26 min, 1.5% B. The UV
response factors were adjusted to each glucosinolate for quantification. GLUCOSINOLATE HYDROLYSIS PRODUCTS ANALYSIS To extract hydrophilic glucosinolates and myrosinase from 75 mg of
freeze-dried leaf mustard powder, 1.5 mL of deionized water was used. The sample was left to incubate at room temperature for 1 h, after which the mixture was centrifuged at 12,000 × g for 5
min. 500 µL of the supernatant was then transferred to a 1.5 mL polytetrafluoroethylene (PTFE) tube22. To perform the liquid–liquid extraction, a solvent mixture of 490 µL dichloromethane
(DCM) and 5 µL phenyl isothiocyanate (dissolved in DCM at a concentration of 1 mg mL−1) was used. The mixture was incubated at 37 °C for 1 h, then vortexed briefly, and subsequently
centrifuged at 12,000 × g for 1 min. 200 µL of the DCM layer was transferred to a vial with an insert, and 1 µL of the sample was injected into a gas chromatograph (Nexis GC-2030, Shimadzu,
Kyoto, Japan) coupled to a gas chromatograph–mass spectrometer (GC/MS-QP 2020 NX, Shimadzu, Kyoto, Japan) and an autosampler with an Injector (AOC-20i PLUS, Shimadzu, Kyoto, Japan). The
chromatographic separation was performed using a capillary column (DB-5MS, Agilent Technologies, Santa Clara, CA, USA; 30 m × 0.25 mm coated with 0.25 µm film). The sample was held at 40 °C
for 1 min, and then the oven temperature was increased to 200 °C at 15 °C min, and further increased to 300 °C at 25 °C∙min−1. The injector and detector temperatures were set at 260 °C and
300 °C∙min−1, respectively, and the flow rate of helium carrier gas was 1.2 mL min−1. The AITC standard curve (0.03 µmole–0.20 µmole) was used for hydrolysis products quantification.
MYROSINASE ACTIVITY AND ESP NITRILE FORMATION Nitrile formation (%) was measured to estimate the ESP activity, as ESP enhances the formation of nitriles over isothiocyanates23. The methods
were followed previous study by Ku, et al.24. Nitrile formation of a leaf mustard was determined by incubating concentrated horseradish root extract with crude protein extract of the sample,
and it was analyzed using GC/MS. 10 g of horseradish powder were mixed with 100 mL of 70% methanol. After vortexing the supernatant, it was centrifuged at 4000 × g for 5 min. Then, the
mixture was boiled in the heating block until all the methanol solvent had evaporated. 1 mL of concentrated horseradish root extract was transferred to a 2 mL microcentrifuge tube and mixed
with 25 mg of leaf mustard powder and at 12,000 × g for 3 min. The 200 µL of supernatant was transferred to a 1.5 mL PTFE tube and mixed with 1 mL of DCM. Samples were incubated at room
temperature for 1 h. The mixture was then vortexed for 10 s and centrifuged at 12,000 × g for 3 min. The bottom organic layer was transferred into a vial with an inserter, and 1 µL of the
sample was injected into a GC/MS. The sample was held at 40 °C for 1 min. The oven temperature was increased to 180 °C at 15 °C∙min−1 and then increased to 200 °C at 20 °C∙min−1. The
injector and detector temperatures were set at 260 °C and 300 °C, respectively. The flow rate of helium carrier gas was 1.2 mL∙min−1 and the split ratio was 1:9. AITC standard curve (0.03
µmole–0.20 µmole) was used to quantify the hydrolysis rate of nitriles. DATA ANALYSIS Statistical analyses were conducted using JMP (JMP Pro software ver. 16.0, SAS Institute, Cary, NC,
USA), where Tukey’s HSD test (_p_ ≤ 0.05) and Pearson’s correlation analysis among the nine cultivars were performed. The correlation matrix was computed in RStudio (RStudio Inc., Boston,
USA), utilizing packages such as ggplot2 and corrplot, to analyze eleven variables including sinigrin, total GSL, CETP, AITC, total GSHP, shoot length, root length, total length, germination
rate, nitrile formation, and myrosinase activity. One-way ANOVA was carried out using Excel (version 2021, Microsoft Corporation, Redmond, Washington, USA). Variance components were
estimated from the mean squares obtained in the analysis of variance25. These components, comprising error variance (VE) and genotypic variance (VG), were calculated according to a formula
detailed in reference26. In this study, genotypic variance represented the sum of squares for the cultivars. Narrow sense heritability (_h_2) was estimated following the methodology
described by Abu-Ellail, et al.27. The coefficient of variation for each trait was calculated based on the mean of the genotypes. $${h}^{2} = VG/VE$$ GUIDELINES OF EXPERIMENTAL RESEARCH AND
FIELD STUDIES Authors complied with the IUCN Policy Statement on Research Involving Species at Risk of Extinction and the Convention on the Trade in Endangered Species of Wild Fauna and
Flora. RESULTS MORPHOLOGICAL CHARACTERISTIC Studying the morphological characteristic among different cultivars is a fundamental aspect of agricultural research. Specifically, researching
into the differences in harvest timing and other growth characteristics among these cultivars plays an important role in improving agricultural productivity and resource efficiency. The
morphological traits of the nine leaf mustard cultivars were presented in Table 1. Two groups, early harvested cultivar and late harvested cultivar were determined according to bolting
period. On the 60th day of growth, which on April 30, four cultivars including ‘Dolsan (control)’, ‘Alsami’, ‘Sundongii’, ‘Kkottolii’ were harvested. These cultivars displayed bolting rates
exceeding 80% while the other five cultivar does not bolt. All four cultivars exhibited immature growth, with insufficient leaf number and leaf length development. The early harvest
cultivars displayed a significantly lower average fresh weight, measuring 47.38 ± 7.09 g (_t_-test _p_ < 0.05), compared to the late harvest cultivars. The reduction in fresh weight was
influenced by early bolting, where reproductive development initiated before sufficient vegetative growth, resulting in a lower fresh weight. On the other hand, the five cultivars harvested
on May 21st showed a lower bolting rate than the early-harvested cultivars, despite being harvested later. ‘Nuttongii’ did not initiate bolting, yet it was harvested due to its extended
growth period exceeding the recommended 90 days, which had the potential to influence the final quality of the leaf mustard. Among the cultivar, in leaf number, ‘Sindongii’ had the highest
number of 73 ± 9, and for fresh weight, ‘Zzangdolii’ had the highest at 968 ± 138.2 g. However, while other cultivars exhibit bolting after an average number of leaf mustard growth days,
‘Nuttongii’ does not show any floret formation. In this study, we harvested ‘Nuttongii’ for comparison with other cultivars. ALLELOPATHY EFFECT OF VOLATILE COMPOUNDS Bioassays assessing
germination rate and initial growth of lettuce seedlings were conducted to evaluate the allelopathic potential of volatile compounds in the leaves of each mustard cultivar (Fig. 2). These
volatiles significantly inhibited both germination and initial growth of lettuce seedlings, as shown in Fig. 2A, even in the absence of direct contact. The bioactive compound in mustard is
known as glucosinolate, specifically sinigrin28. Despite the indirect contact with sinigrin, an allelopathic effect was observed in all mustard cultivar29. As shown in Fig. 2B, leaf mustard
significantly inhibited the germination of lettuce seeds, especially with the treatments of ‘Nuttongii’, ‘Sindongii’, ‘Mekomi’, ‘Alsami’, ‘Sundongii’, and ‘Zzangdolii'. Moreover, these
six cultivars also inhibit growth of lettuce seedlings (Fig. 2C). The result shows that leaf mustard powder treatments resulted in delayed seed germination and inhibited shoot and root
growth, compared with non-treatment. The leaf mustard powder, initiating hydrolysis by absorbing moisture on the agar gel, released hydrolysis products into the dish. The inhibition of seed
germination and growth by these hydrolysis products may be attributed to principles such as cell cycle arrest30,31. GLUCOSINOLATES AND GLUCOSINOLATES HYDROLYSIS PRODUCTS In this study, we
analyzed the glucosinolate (GSL) concentration and their glucosinolate hydrolysis products (GSHP) in nine mustard cultivars to understand the allelopathic effect of leaf mustard, as shown in
Fig. 3. Investigating both GSL and GSHP concentrations is crucial to comprehend the correlation and composition of these substances and their hydrolysis products32. This analysis provides
insights into the compounds responsible for allelopathy. Figure 3A presents the total GSL concentration and sinigrin concentration, with error bars indicating the standard deviation of the
total GSL. Sinigrin was the predominant GSL in leaf mustard, accounting for 79% of all detected GSLs. The identified GSLs include glucoiberin, glucoraphanin, sinigrin, gluconapin,
4-hydroxyglucobrassicin, glucoiberverin, glucobrassicin, 4-methoxyglucobrassicin, gluconasturin, and neoglucobrassicin. Figure 3B shows the total concentration of ten GSHPs, including AITC
and CETP, with error bars for the total GSHP standard deviation. Among the GSHPs, AITC and CETP were the primary compounds, constituting 36% and 45% of the total GSHP, respectively. Detected
GSHPs also include 1-cyano-2,3-epithiopropane, 1-cyano-3,4-epithiobutane, 1-cyano-4,5-epithiopentane, 3-butenyl isothiocyanate, 3-phenylpropionitrile, allyl isothiocyanate, allyl
thiocyanate, iberverin, iberverin nitrile, and phenethyl isothiocyanate. ‘Mekomi’, ‘Nuttongii’, ‘Sundongii’, and ‘Sindongii’ exhibited significantly higher GSL concentrations than ‘Dolsan’
(control), with these four cultivars also showing higher sinigrin concentrations. ‘Mekomi’, ‘Nuttongii’, ‘Sundongii’, and ‘Sindongii’ showed significantly higher GSL concentration than
‘Dolsan (control)’. Those four cultivars also showed significantly higher sinigrin concentration. ‘Mekomi’ showed the highest sinigrin concentration as 43.13 ± 9.51 μmole·g−1 DW. GSL
concentrations were highest in order of ‘Mekomi’, ‘Nuttongii’, ‘Sundongii’, ‘Sindongii’, ‘Zzangdolii’, ‘Kkottolii’, ‘Ssamdoli’, ‘Alsami’, and ‘Dolsan (Control)’. On the other hand, the order
of cultivars with GSHP concentration did not correspond to the order of cultivars with GSL concentration. GSHP concentrations were highest in order of ‘Nuttongii’, ‘Sindongii’, ‘Alsami’,
‘Sundongii’, ‘Mekomi, ‘Ssamdoli’, ‘Dolsan (Control)’, ‘Zzangdolii’, and ‘Kkottolii’. ‘'Nutdongii’ exhibited the highest AITC concentration at 27.47 ± 6.46 µmole g−1 and CETP
concentrations at 35.65 ± 10.89 µmole g−1 which is most significantly higher among the cultivars. ‘Mekomi’ exhibited the highest sinigrin concentration, while the content of GSHP was
relatively low. This suggests that factors other than the substrate may influence the quantity of hydrolysis products. MYROSINASE AND ITS CO-FACTOR ENZYME ANALYSIS The results presented in
Fig. 4 suggest that myrosinase and ESP enzyme activities significantly influence GSHP concentrations. In Fig. 4A, the nitrile formation (%) indicates the conversion rate of sinigrin into
AITC or CETP, while Fig. 4B shows how myrosinase activity affects the overall concentration of GSHPs. This analysis was conducted to identify the factors contributing to the variation in
GSHP concentration among cultivars that have similar levels of GSL concentrations. Each cultivar demonstrates unique myrosinase activity and nitrile formation percentage. The order of
cultivars based on the highest nitrile formation (%) is as follows: ‘Dolsan (Control)’, ‘Kkottolii’, ‘Ssamdolii’, ‘Sundongii’, ‘Zzangdolii’, ‘Nuttongii’, ‘Alsami’, ‘Sindongii’, and ‘Mekomi’.
Except for ‘Ssamdolii’ and ‘Kkottolii’, all other cultivars showed lower nitrile formation compared to ‘Dolsan (Control)’. Regarding myrosinase activity, the order from highest to lowest
is: ‘Zzangdolii’, ‘Kkottolii’, ‘Mekomi’, ‘Sindongii’, ‘Nuttongii’, ‘Dolsan (Control)’, ‘Alsami’, ‘Sundongii’, and ‘Ssamdolii’. CORRELATION BETWEEN LEAF MUSTARD AND THEIR ALLELOPATHY EFFECT
Pearson correlation analysis was conducted to examine the relationship between the allelopathic effects, and the compounds analyzed in leaf mustard. It is known that the Brassicaceae family
contains allelochemicals in ITC (isothiocyanate) form, which can inhibit weed growth33. The study examined the impact of different compounds (GSL, other GSL, CETP, AITC, total GSHP, and
other GSHP) on lettuce seed parameters (shoot length, root length, germination rate) and visualized their interactions through a heat map (Fig. 5). The analysis revealed a strong positive
correlation between 'sinigrin' and 'total GSL' (_r_ = 0.964, _p_ < 0.001) and, between 'CETP' and 'total GSHP' (_r_ = 0.830, _p_ < 0.01),
indicating these are major contributors to GSL and GSHP, respectively, across all cultivars. However, 'sinigrin', 'total GSL', 'CETP', and 'total
GSHP' did not show allelopathic effects on lettuce seeds. The study also confirmed that 'AITC,' derived from sinigrin, significantly inhibits lettuce seed growth even at lower
concentrations than CETP. AITC concentrations were found to be significantly negatively correlated with 'shoot length' (_r_ = − 0.87, _p_ < 0.01), 'root length' (_r_
= − 0.88, _p_ < 0.01), and 'germination rate' (_r_ = − 0.84, _p_ < 0.01) of lettuce. Additionally, a positive correlation between 'nitrile formation' and
'shoot length' (_r_ = 0.78, _p_ < 0.05), 'root length' (_r_ = 0.71, _p_ < 0.05), and 'germination rate' (_r_ = 0.08, _p_ < 0.01) indicates that higher
nitrile formation leads to a lower rate of ITC generation with allelopathic activity from the substrate GSL. DETERMINATION OF HERITABILITY OF ALLELOPATHIC EFFECT AND ALLELOCHEMICALS The
impact of cultivars, which is genetic factors, on lettuce seed allelopathy has not been investigated18. To understand the genetic influence on allelopathic effect and compounds of leaf
mustard, we conducted an analysis of variance (ANOVA) to calculate and quantify each factor and trait. Table 2 revealed highly significant differences in all 11 traits analyzed through ANOVA
at the _p_ < 0.001 level, with _h_2 exceeding 0.7. This research suggests that allelopathic effects are influenced by genetic factors. Furthermore, the composition of each cultivar was
also found to be determined by genetic factors. These results indicate that the cultivar is a key determinant of both compounds and allelopathic effects. DISCUSSION POTENTIAL ALLELOPATHIC
MUSTARD CULTIVAR BASED ON AITC: IMPLICATIONS FOR FUTURE CULTIVATION AND BREEDING AITC a biofumigant recognized in the United States34, is a key component released by leaf mustard, making it
a valuable resource in sustainable weed management practices35. The adoption of allelopathic plants, like leaf mustard, represents a significant step toward environmentally friendly and
sustainable weed control36. Utilizing the researched leaf mustard water potential values37, we calculated the concentration of AITC per 100 g of leaf mustard fresh weight in this study. In
the late-harvested group, ‘Nuttongii’ had the highest AITC concentration per 100 g fresh weight (246.16 ± 46.79 µmole 100 g−1), surpassing ‘Zzangdolii’, which had the highest fresh weight
but the lowest AITC content at 83.29 ± 2.92 µmole 100 g−1 (FW). Remarkably, ‘Nuttongii’ not only exhibited elevated AITC levels but also significantly inhibited germination rates and root
and shoot lengths, corroborating previous studies on AITC's allelopathic effects and its correlation with AITC concentrations38. This suggests that ‘Nuttongii’ holds potential as an
effective herbicide based on its AITC concentration. Previous study found that the mechanism of allelopathy in horseradish involves the release of allyl isothiocyanate compounds from its
root extract, which, at increasing concentrations, inhibit onion root growth, disrupt the cell cycle, and increase cell death, thereby demonstrating a concentration-dependent inhibitory
effect39. Additionally, ‘Nuttongii’ may achieve higher fresh weight if left unharvested due to late bolting. Harvesting practices for ‘Nuttongii’ differed from other cultivars, potentially
leading to an underestimated biomass. Future studies should consider cultivating ‘Nuttongii’ until bolting for more accurate sinigrin measurements. Our research revealed that the precursor
of sinigrin had minimal impact on allelopathic activity, contrary to previous studies suggesting its influence through indirect contact. Previous research has been unable to deduce a precise
cause-and-effect relationship through statistical correlation analysis18. Given these results, Consequently, in the future, cultivation and breeding efforts should focus on AITC, given its
physiological significance as a bioactive compound. ENHANCED ALLELOPATHIC EFFECT IN LEAF MUSTARD: SIGNIFICANCE OF NITRILE FORMATION AND ESP ACTIVITY A critical aspect of this study involves
exploring the genetic determinants of AITC production and nitrile formation. Allelochemicals like AITC are produced when sinigrin undergoes hydrolysis in the presence of water, facilitated
by the myrosinase enzyme. This process transforms sinigrin into hydrolysis products including AITC40. However, previous research has largely overlooked the crucial role of nitrile formation
in AITC production and the CEPT mechanism. Our findings underscore the substantial impact of these factors on allelopathic effects, revealing a robust genetic underpinning, as highlighted in
Table 2. While earlier mustard cultivation research primarily focused on sinigrin content41, our investigation into sinigrin and its conversion products reveals a surprising lack of
correlation. Our study has revealed a correlation between AITC levels and the enhancement of allopathic effects, indicating that varieties genetically regulated by the _ESP_ gene may possess
higher allelopathic capabilities. (Fig. 6A). Consequently, breeding for high AITC content should prioritize reducing ESP activity7. Our findings also demonstrate the significant
contribution of AITC to the physiological activity observed in mustard plants. Utilizing advanced AITC measurement techniques and nitrile formation screening assays18,24,42, we have
identified promising candidates mustard leaf ‘Nuttongii’ for enhancing sustainable agricultural production. By manipulating genes related to AITC synthesis and nitrile formation, we can
expedite the development of cultivars with enhanced allelopathic properties. Our research suggests that CRISPR-Cas9 is an efficient tool for enhancing desired glucosinolates, including
targeting the ESP gene to increase bioactive allelochemicals from sinigrin. This method not only enhances desired traits but also minimizes unintended genetic changes, ensuring the safety
and predictability of the breeding process. In the face of sustainability challenges, eco-conscious agricultural practices are increasingly important Cultivars produce higher AITC levels
with reduced nitrile formation, as our research suggests, can significantly lessen the dependence on chemical herbicides (Fig. 6B). This shift can improve soil health, decrease chemical
runoff, and reduce harm to non-target organisms, contributing to more environmentally friendly farming methods. BIOFUMIGATION AGRICULTURAL PRACTICES AND MANAGEMENT STRATEGIES Incorporating
brassica species as cover crops within agricultural rotations presents a dual-functional strategy for soil conservation and sustainable pest and weed management43. This practice, termed
biofumigation, necessitates strategic planning and precise execution for optimal efficacy. Central to this strategy is the selection of appropriate biofumigant crops, such as mustard, which
are known for their ability to release volatile compounds with pesticidal properties. A meticulously designed crop rotation scheme is imperative for diversifying the production cycle,
thereby mitigating the risk of pest and pathogen build-up and enhancing the biofumigation effect. The effective use of cover crops for biofumigation, particularly during their decomposition
phase, requires meticulous planning regarding the timing of their incorporation into the soil and an understanding of existing soil conditions. This careful approach is crucial for
maximizing their biofumigation potential. Notably, the concentration of glucosinolates, key compounds in biofumigation, is affected by variables such as the growing season44,45 and potential
insect damage, as indicated in studies46,47,48,49,50,51. These factors underscore the importance of strategic crop management to enhance biofumigation efficacy for ecofriendly cultivation.
BEYOND FUMIGATION: UNVEILING THE MULTIFACETED POTENTIAL OF AITC IN FOOD AND MEDICINE AITC treatment has been reported to be effective against various pathogens and weeds under warm
conditions in sandy soil when applied before planting crops52,53. It possesses notable fumigant properties effective against a range of soil-borne pathogens and weeds54, as demonstrated in
Fig. 6C. The United States Food and Drug Administration has designated AITC as a 'Generally Recognized as Safe' (GRAS) substance, underscoring its potential as a benign food
additive. This compound's diverse physiological properties open up a multitude of applications in agricultural practices. One of the primary advantages of AITC is its function as a
natural herbicide, offering an environmentally sustainable alternative to traditional synthetic herbicides. This aspect of AITC is elaborated further in the preceding sections of this paper.
Additionally, AITC has been observed to possess significant antipathogenic properties, particularly in the context of postharvest plant disease management. Studies have indicated its
efficacy in combatting fungal infections, notably reducing decay in perishable fruits such as strawberries, blackberries, blueberries, raspberries, and other similar crops38,55,56. In the
field of food engineering, the utilization of AITC emerges as a promising strategy for augmenting both food preservation and flavor enhancement. This approach is in line with the current
trend towards developing food products that are both safer and more flavorful. A key element in harnessing AITC's potential involves the targeted manipulation of the ESP gene, a
critical factor in the biosynthesis of AITC from its precursor, sinigrin. Advances in genetic engineering techniques enable the complete conversion of sinigrin into AITC, which is notable
for its potent aroma, estimated to be around 300 times stronger than that produced by CETP57. This technology is crucial for foods like sauces and condiments that need distinct aromas. It
enables healthier, naturally flavored products, reducing artificial additives and aligning with consumer trends towards natural ingredients. Additionally, AITC shows promise as an
antibacterial agent, potentially improving food safety. This is especially relevant in food safety research, where the prevention of pathogen outbreaks in salad vegetables is a significant
concern58. AITC's application could enhance the safety of these vegetables, contributing to safer food consumption. In the medical field, AITC has garnered attention due to its
promising medicinal properties, particularly its potential as an anticancer agent. highlighted by its ability to induce apoptosis, inhibit carcinogenesis, enhancing detoxifying phase II
enzymes24 and exert anti-inflammatory effects, make it a compound of considerable interest in the medical community59. The AITC-enhanced mustard, with its potential as a therapeutic agent in
cancer treatment, can be linked to further research, particularly through clinical studies. Additionally, in the field of ecology, AITC may play a crucial role in deepening our
understanding of its ecological impact, particularly concerning plant–insect interactions and ecosystem dynamics. This compound, naturally present in cruciferous plants, serves as a
bioactive substance with potential influences on various ecological processes. AITC's role in plant–insect interactions is significant. It functions as a natural pest deterrent,
offering an ecological advantage to plants that produce it. This characteristic can alter the and feeding preferences and growth of insect pests60. This is evident in the modulation of
feeding behaviors and growth patterns in insect pests, as indicated in previous studies examining the effects of glucosinolates, a class of compounds to which AITC belongs, on insect
feeding56. In conclusion, our research provides a clear target for precision breeding techniques, leveraging genetic factors influencing AITC production and nitrile formation, ultimately
advancing eco-conscious agriculture and promoting sustainability in farming practices, while opening doors to cross-disciplinary applications of AITC modulation. CONCLUSION In conclusion,
'Nuttongii' cultivars exhibit notably elevated levels of AITC and pronounced allelopathic effects compared to other varieties. Our research underscores the superior allelopathic
properties of selected 'Nuttongii' plant varieties, which can be attributed to genetic diversity influencing enzyme activities. In summary, this approach promotes environmentally
friendly agriculture and sustainable farming practices, while also facilitating interdisciplinary applications in AITC modulation. DATA AVAILABILITY Data is provided within the manuscript or
supplementary information files. REFERENCES * Cheng, F. & Cheng, Z. Research progress on the use of plant allelopathy in agriculture and the physiological and ecological mechanisms of
allelopathy. _Front. Plant Sci._ https://doi.org/10.3389/fpls.2015.01020 (2015). Article PubMed PubMed Central Google Scholar * Einhellig, F. A. Mechanisms and modes of action of
allelochemicals. _The science of allelopathy_, 171–188 (1986). * Walsh, M. J. & Powles, S. B. Management strategies for herbicide-resistant weed populations in Australian dryland crop
production systems. _Weed Technol._ 21, 332–338 (2007). Article CAS Google Scholar * Ghidoli, M. _et al._ Camelina sativa (L.) Crantz as a promising cover crop species with allelopathic
potential. _Agronomy_ 13, 2187 (2023). Article CAS Google Scholar * Ofosu, R. _et al._ Herbicide resistance: Managing weeds in a changing world. _Agronomy_ 13, 1595 (2023). Article CAS
Google Scholar * Baysal-Gurel, F., Liyanapathiranage, P. & Mullican, J. Biofumigation: Opportunities and challenges for control of soilborne diseases in nursery production. _Plant.
Health Prog._ 19, 332–337. https://doi.org/10.1094/php-08-18-0049-rv (2018). Article Google Scholar * Bell, L., Oloyede, O. O., Lignou, S., Wagstaff, C. & Methven, L. Taste and flavor
perceptions of glucosinolates, isothiocyanates, and related compounds. _Mol. Nutr. Food Res._ 62, 1700990 (2018). Article Google Scholar * De Cauwer, B., Vanbesien, J., De Ryck, S. &
Reheul, D. Impact of Brassica juncea biofumigation on viability of propagules of pernicious weed species. _Weed Res._ 59, 209–221 (2019). Article Google Scholar * Singh, A. P., Kishore, P.
S., Kar, S. & Dewanjee, S. Secondary Metabolites of Brassica juncea (L.) Czern and Coss: Occurence, Variations and Importance. (2022). * Kang, S. Isolation and antimicrobial activity of
antimicrobial substance obtained from leaf mustard (Brassica juncea). _J. Korean Soc. Food Nutr. (Korea Republic)_ (1995). * Oh, S., Kim, K., Bae, S. & Choi, M. R. Sinigrin content of
different parts of Dolsan leaf mustard. _Korean J. Food Preserv._ 22, 553–558 (2015). Article Google Scholar * Åsberg, S. E., Bones, A. M. & Øverby, A. Allyl isothiocyanate affects the
cell cycle of Arabidopsis thaliana. _Front. Plant Sci._ 6, 364 (2015). PubMed PubMed Central Google Scholar * Petropoulos, S., Di Gioia, F. & Ntatsi, G. Vegetable organosulfur
compounds and their health promoting effects. _Curr. Pharm. Design_ 23, 2850–2875 (2017). Article CAS Google Scholar * Zhang, Y. Allyl isothiocyanate as a cancer chemopreventive
phytochemical. _Mol. Nutr. Food Res._ 54, 127–135 (2010). Article CAS PubMed PubMed Central Google Scholar * Henderson, D. R., Riga, E., Ramirez, R. A., Wilson, J. & Snyder, W. E.
Mustard biofumigation disrupts biological control by Steinernema spp. nematodes in the soil. _Biological Control_ 48, 316–322 (2009). Article Google Scholar * Simpson, T., Chiu, Y.-C.,
Richards-Babb, M., Blythe, J. M. & Ku, K.-M. Demonstration of allelopathy of horseradish root extract on lettuce seed. _Biochem. Mol. Biol. Educ._ 47, 333–340.
https://doi.org/10.1002/bmb.21219 (2019). Article CAS PubMed Google Scholar * Ku, K. M., Jeffery, E. H. & Juvik, J. A. Influence of seasonal variation and methyl jasmonate mediated
induction of glucosinolate biosynthesis on quinone reductase activity in broccoli florets. _J. Agric. Food Chem._ 61, 9623–9631 (2013). CAS PubMed Google Scholar * Frazie, M. D., Kim, M.
J. & Ku, K. M. Health-promoting phytochemicals from 11 mustard cultivars at baby leaf and mature stages. _Molecules_ https://doi.org/10.3390/molecules22101749 (2017). Article PubMed
PubMed Central Google Scholar * Reddy, P. P. _Recent advances in crop protection_ 37–60 (Springer India, Delhi, 2013). Book Google Scholar * Huang, H. _et al._ Variation characteristics
of glucosinolate contents in leaf mustard (Brassica juncea). _Agronomy_ 12, 2287 (2022). Article CAS Google Scholar * ISO, E. 9167-1 (1992). _Rapeseed-Determination of glucosinolates
content-Part_ 1. * Moon, H. W. & Ku, K. M. The effect of additional shading utilizing agriphotovoltaic structures on the visual qualities and metabolites of broccoli. _Front. Plant Sci._
14, 1111069. https://doi.org/10.3389/fpls.2023.1111069 (2023). Article PubMed PubMed Central Google Scholar * Kim, M. J. _et al._ Cultivar-specific changes in primary and secondary
metabolites in pak choi (Brassica rapa, Chinensis group) by methyl jasmonate. _Int. J. Mol. Sci._ 18, 1004 (2017). Article PubMed PubMed Central Google Scholar * Ku, K.-M., Jeffery, E.
H., Juvik, J. A. & Kushad, M. M. Correlation of quinone reductase activity and allyl isothiocyanate formation among different genotypes and grades of horseradish roots. _J. Agric. Food
Chem._ 63, 2947–2955. https://doi.org/10.1021/jf505591z (2015). Article CAS PubMed Google Scholar * Singh, M., Ceccarelli, S. & Hamblin, J. Estimation of heritability from varietal
trials data. _Theor. Appl. Genetics_ 86, 437–441 (1993). Article CAS Google Scholar * Rameeh, V. Multivariate regression analyses of yield associated traits in rapeseed (Brassica napus
L.) genotypes. _Adv. Agric._ 2014, 1–5. https://doi.org/10.1155/2014/626434 (2014). Article Google Scholar * Abu-Ellail, F. F. B., Ghareeb, Z. & Grad, W. Sugarcane family and
individual clone selection based on best linear unbiased predictors (BLUPS) analysis at single stool stage. _J. Sugarcane Res._ 8 (2021). * Lietzow, J. Biologically active compounds in
mustard seeds: A toxicological perspective. _Foods_ 10, 2089 (2021). Article CAS PubMed PubMed Central Google Scholar * Griffiths, D., Birch, A. & Hillman, J. Antinutritional
compounds in the brasi analysis, biosynthesis, chemistry and dietary effects. _J. Horticult. Sci. Biotechnol._ 73, 1–18 (1998). Article CAS Google Scholar * Wang, N. _et al._ Analysis of
the isothiocyanates present in three Chinese Brassica vegetable seeds and their potential anticancer bioactivities. _Eur. Food Res. Technol._ 231, 951–958 (2010). Article CAS Google
Scholar * Traka, M. & Mithen, R. Glucosinolates, isothiocyanates and human health. _Phytochem. Rev._ 8, 269–282 (2009). Article CAS Google Scholar * Fahey, J. W., Zalcmann, A. T.
& Talalay, P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. _Phytochemistry_ 56, 5–51. https://doi.org/10.1016/S0031-9422(00)00316-2 (2001).
Article CAS PubMed Google Scholar * Bangarwa, S. K. & Norsworthy, J. K. Glucosinolate and isothiocyanate production for weed control in plasticulture production system. In
_Glucosinolates (Mérillon, JM_ (ed. Ramawat, K. G.) 201–235 (Springer, Cham, 2017). Chapter Google Scholar * Isagro USA, I. Vol. 2023 (ed Inc. Technology Sciences Group) Page 4 of 17
(2014). * Gimsing, A. & Kirkegaard, J. Glucosinolate and isothiocyanate concentration in soil following incorporation of Brassica biofumigants. _Soil Biol. Biochem._ 38, 2255–2264
(2006). Article CAS Google Scholar * Shahzad, B. _et al._ Utilizing the allelopathic potential of brassica species for sustainable crop production: A review. _J. Plant Growth Regul._
https://doi.org/10.1007/s00344-018-9798-7 (2019). Article Google Scholar * USDA. (2019). * Park, D., Park, S.-Y., Liu, K.-H. & Ku, K.-M. Optimal allyl isothiocyanate concentration on
Botrytis cinerea during the postharvest storage of blackberries and mechanism of blackberry color changes at high concentration of allyl isothiocyanate. _Postharvest Biol. Technol._ 199,
112292 (2023). Article CAS Google Scholar * Simpson, T. & Ku, K. M. Metabolomics and physiological approach to understand allelopathic effect of horseradish extract on onion root and
lettuce seed as model organism. _Plants (Basel)._ https://doi.org/10.3390/plants10101992 (2021). Article PubMed PubMed Central Google Scholar * Shakour, Z. T., Shehab, N. G., Gomaa, A.
S., Wessjohann, L. A. & Farag, M. A. Metabolic and biotransformation effects on dietary glucosinolates, their bioavailability, catabolism and biological effects in different organisms.
_Biotechnol. Adv._ 54, 107784 (2022). Article CAS PubMed Google Scholar * Teklehaymanot, T. _et al._ Variation in plant morphology and Sinigrin content in Ethiopian mustard (Brassica
carinata L.). _Horticult. Plant J._ 5, 205–212. https://doi.org/10.1016/j.hpj.2019.07.005 (2019). Article Google Scholar * Ku, K.-M., Kim, M. J., Jeffery, E. H., Kang, Y.-H. & Juvik,
J. A. Profiles of glucosinolates, their hydrolysis products, and quinone reductase inducing activity from 39 arugula (Eruca Sativa Mill.) accessions. _J. Agric. Food Chem._ 64, 6524–6532
(2016). Article CAS PubMed Google Scholar * Haramoto, E. & Gallandt, E. Brassica cover cropping for weed management: A review. _Renew. Agric. Food Syst._ 19, 187–198.
https://doi.org/10.1079/RAFS200490 (2004). Article Google Scholar * Chae, S.-H. _et al._ Kimchi cabbage (Brassica rapa subsp. pekinensis [Lour.]) Metabolic changes during growing seasons
in the Republic of Korea. _Horticult. Environ. Biotechnol._ https://doi.org/10.1007/s13580-023-00546-7 (2023). Article Google Scholar * Chae, S. H., Lee, O. N., Park, H. Y. & Ku, K. M.
Seasonal effects of glucosinolate and sugar content determine the pungency of small-type (Altari) radishes (Raphanus sativus L.). _Plants (Basel)_ https://doi.org/10.3390/plants11030312
(2022). Article PubMed PubMed Central Google Scholar * Kim, M. J. _et al._ Cultivar-specific changes in primary and secondary metabolites in pak choi (Brassica Rapa, Chinensis Group) by
methyl jasmonate. _Int. J. Mol. Sci._ https://doi.org/10.3390/ijms18051004 (2017). Article PubMed PubMed Central Google Scholar * Ku, K. M., Becker, T. M. & Juvik, J. A.
Transcriptome and metabolome analyses of glucosinolates in two broccoli cultivars following jasmonate treatment for the induction of glucosinolate defense to Trichoplusia ni (Hübner). _Int.
J. Mol. Sci._ https://doi.org/10.3390/ijms17071135 (2016). Article PubMed PubMed Central Google Scholar * Ku, K. M., Jeffery, E. H. & Juvik, J. A. Exogenous methyl jasmonate
treatment increases glucosinolate biosynthesis and quinone reductase activity in kale leaf tissue. _PLoS One_ 9, e103407. https://doi.org/10.1371/journal.pone.0103407 (2014). Article ADS
CAS PubMed PubMed Central Google Scholar * Ku, K. M., Jeffery, E. H. & Juvik, J. A. Optimization of methyl jasmonate application to broccoli florets to enhance health-promoting
phytochemical content. _J. Sci. Food. Agric._ 94, 2090–2096. https://doi.org/10.1002/jsfa.6529 (2014). Article CAS PubMed Google Scholar * Ku, K. M. _et al._ Methyl jasmonate and
1-methylcyclopropene treatment effects on quinone reductase inducing activity and post-harvest quality of broccoli. _PLoS One_ 8, e77127. https://doi.org/10.1371/journal.pone.0077127 (2013).
Article ADS CAS PubMed PubMed Central Google Scholar * Ku, K. M., Choi, J. H., Kushad, M. M., Jeffery, E. H. & Juvik, J. A. Pre-harvest methyl jasmonate treatment enhances
cauliflower chemoprotective attributes without a loss in postharvest quality. _Plant. Foods Hum. Nutr._ 68, 113–117. https://doi.org/10.1007/s11130-013-0356-y (2013). Article CAS PubMed
Google Scholar * Gao, J., Pei, H. & Xie, H. Influence of allyl isothiocyanate on the soil microbial community structure and composition during pepper cultivation. _J. Microbiol.
Biotechnol._ 31, 978–989. https://doi.org/10.4014/jmb.2012.12016 (2021). Article CAS PubMed PubMed Central Google Scholar * Hanschen, F. S., Yim, B., Winkelmann, T., Smalla, K. &
Schreiner, M. Degradation of biofumigant isothiocyanates and allyl glucosinolate in soil and their effects on the microbial community composition. _PLoS One_ 10, e0132931.
https://doi.org/10.1371/journal.pone.0132931 (2015). Article CAS PubMed PubMed Central Google Scholar * Narwal, S. Allelopathy: Future role in weed control. _Allelopathy in Agriculture
and Forestry, Scientific Publishers, Jodhpur, India_, 245–272 (1994). * Song, H. J. & Ku, K. M. Optimization of allyl isothiocyanate sanitizing concentration for inactivation of
Salmonella Typhimurium on lettuce based on its phenotypic and metabolome changes. _Food Chem._ 364, 130438. https://doi.org/10.1016/j.foodchem.2021.130438 (2021). Article CAS PubMed
Google Scholar * Ugolini, L. _et al._ Postharvest application of brassica meal-derived allyl-isothiocyanate to kiwifruit: Effect on fruit quality, nutraceutical parameters and physiological
response. _J. Food Sci. Technol._ 54, 751–760. https://doi.org/10.1007/s13197-017-2515-x (2017). Article CAS PubMed PubMed Central Google Scholar * Chin, H. W., Zeng, Q. & Lindsay,
R. C. Occurrence and flavor properties of sinigrin hydrolysis products in fresh cabbage. _J. Food Sci._ 61, 101–104. https://doi.org/10.1111/j.1365-2621.1996.tb14735.x (2006). Article
Google Scholar * Fuzawa, M. _et al._ Roles of vegetable surface properties and sanitizer type on annual disease burden of rotavirus illness by consumption of rotavirus-contaminated fresh
vegetables: A quantitative microbial risk assessment. _Risk Anal._ 40, 741–757. https://doi.org/10.1111/risa.13426 (2020). Article PubMed Google Scholar * Wagner, A. E.,
Boesch-Saadatmandi, C., Dose, J., Schultheiss, G. & Rimbach, G. Anti-inflammatory potential of allyl-isothiocyanate–role of Nrf2, NF-(κ) B and microRNA-155. _J. Cell Mol. Med._ 16,
836–843. https://doi.org/10.1111/j.1582-4934.2011.01367.x (2012). Article CAS PubMed PubMed Central Google Scholar * Ratzka, A., Vogel, H., Kliebenstein, D. J., Mitchell-Olds, T. &
Kroymann, J. Disarming the mustard oil bomb. _Proc. Natl. Acad. Sci._ 99, 11223–11228. https://doi.org/10.1073/pnas.172112899 (2002). Article ADS CAS PubMed PubMed Central Google
Scholar Download references FUNDING This work was supported by the National Research Foundation of Korea (NRF) Grant funded by the Korea government. (MSIT) (2021R1C1C1007733). AUTHOR
INFORMATION AUTHORS AND AFFILIATIONS * Department of Plant Biotechnology, College of Life Sciences and Biotechnology, Korea University, 145 Anam-Ro, Seongbuk-Gu, Seoul, 02841, Republic of
Korea Da-Yeong Ko, Hojoung Lee & Kang-Mo Ku * Department of Horticulture, Chonnam National University, Gwangju, Republic of Korea, 61186 Su-Mi Seo * Agricultural Technology Center of
Yeosu City, Yeosu, 59633, Republic of Korea Yong-Hyuk Lee * Department of Horticulture, College of Industrial Science, Kongju National University, Yesan, 32439, Republic of Korea Chan Saem
Gil Authors * Da-Yeong Ko View author publications You can also search for this author inPubMed Google Scholar * Su-Mi Seo View author publications You can also search for this author
inPubMed Google Scholar * Yong-Hyuk Lee View author publications You can also search for this author inPubMed Google Scholar * Chan Saem Gil View author publications You can also search for
this author inPubMed Google Scholar * Hojoung Lee View author publications You can also search for this author inPubMed Google Scholar * Kang-Mo Ku View author publications You can also
search for this author inPubMed Google Scholar CONTRIBUTIONS D.-Y.K. and S.-M.S.: experiment design, lab work, data production, data interpretation, writing first draft of manuscript;
Y.-.H.L., C.S.G., and H. L.: results interpretation, data interpretation, writing first draft of manuscript; K.-M.K.: project design, experiment design, data interpretation, manuscript final
edit. All authors reviewed and confirmed the final draft. CORRESPONDING AUTHOR Correspondence to Kang-Mo Ku. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing
interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. RIGHTS AND
PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any
medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The
images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is
not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission
directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Ko, DY.,
Seo, SM., Lee, YH. _et al._ Turning glucosinolate into allelopathic fate: investigating allyl isothiocyanate variability and nitrile formation in eco-friendly _Brassica juncea_ from South
Korea. _Sci Rep_ 14, 15423 (2024). https://doi.org/10.1038/s41598-024-65938-w Download citation * Received: 13 January 2024 * Accepted: 25 June 2024 * Published: 04 July 2024 * DOI:
https://doi.org/10.1038/s41598-024-65938-w SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * Glucosinolate * Sinigrin * Allyl isothiocyanate *
Mustard * Epithiospecifier protein