Durability performance of alkali-activated concrete with pre-treated coarse recycled aggregates for pavements
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
This study examines the effect of coarse recycled aggregates (CRAs) and processed coarse recycled aggregates (PCRAs) on the behaviour of alkali-activated concrete (AAC) before and after
exposure to marine seawater and acidic environments (5% HCl and 5% H2SO4 solutions). Measurements of compressive strength and the microstructure changes were conducted over periods of 56 and
90 days to assess these effects. The experimental design included varying the replacement levels of NAs with CRAs and PCRAs from (0–100%) and using ground-granulated blast furnace slag and
fly ash as constant components. In addition to durability tests, sorptivity assessments were conducted to gauge the material’s porosity and water absorption capabilities. Advanced
microstructure techniques, such as scanning electron microscopy (SEM) and X-ray diffraction (XRD), were employed to detail the pre and post-exposure mineralogical and microstructural
transformations within the AAC blends. The AAC mixtures incorporating PCRAs emerged as durable, showcasing better strength and a denser, more compact matrix facilitated by the synergistic
formation of NASH and CASH gels after exposure to aggressive agents compared to untreated CRAs. In addition, the results show that the samples exposed to marine seawater exhibited improved
mechanical performance compared to those exposed to acidic environments. The novelty of this study lies in its exploration of the effects of recycling plant-based CRAs and PCRAs on AAC for
marine and acid exposure.
The growing demand for concrete due to urbanization has increased the production of Ordinary Portland Cement (OPC) by over four billion tons in 2018–20201,2,3,4. Natural coarse aggregate,
comprising 65% of the volume of concrete, is a significant component. Nevertheless, the combined stockpiles are diminishing rapidly. The global natural aggregate (NA) output is around 4.5
billion tonnes. The greenhouse gas emissions associated with producing natural coarse aggregate are estimated to be around 7.4 to 8.0 kg CO2-e per tonne5,6. Furthermore, releasing dust and
particulate matter from trucks and crushers is an additional factor contributing to the escalation of global warming. Moreover, the emissions of dust and particulate matter from trucks and
crushers also contribute to the rise in global warming7.
This has resulted in using coarse recycled aggregates (CRAs) from constructing and demolishing waste (CDW) to progress towards environmental sustainability and a society with zero carbon
emissions. To reduce the need for OPC as a binder, geological source materials with a high silicon and aluminium content or an industrial by-product such as silica fume, nano silica, ground
granulated blast furnace slag (GGBFS) and fly ash (FA) react with an alkali activated solution (AAS) called alkali-activated concrete (AAC). A significant portion of CO2 emissions during the
cement process comes from the calcination of limestone (calcium carbonate, CaCO3). When limestone is heated in a cement kiln to produce lime (calcium oxide, CaO), a key ingredient of
cement, CO2 is released as a byproduct of this chemical reaction8. In contrast, producing commercial through-products emits fewer greenhouse gases than OPC. FA emits 80–90%, while GGBFS
emits 80% less greenhouse gases than OPC9,10,11,12,13.
The use of a mixture of sodium or potassium-based hydroxide and silicate as an alkaline activator has been utilized in research14,15. High calcium-based components have been used to enhance
the compressive strength of geopolymer concrete, leading to AAC production. Similar to geopolymer concrete, AAC is produced with the addition of calcium carbonate compounds, resulting in the
formation of calcium silicate hydrate (CSH) gel, and other gels such as calcium aluminate silicate hydrate (CASH) and sodium aluminate silicate hydrate (NASH)16,17,18,19,20,21.
The potential of CDW as an alternative to NA suggests that CRA can effectively substitute for coarse and fine aggregates22,23. Previous research24,25, identified the adhered mortar on CRAs
as a significant concern due to a weak interfacial transition zone (ITZ), compromising the binder-CRA bond and affecting the concrete’s strength and durability. Studies have shown that
completely replacing NA with CRAs in AAC might decrease compressive strength by up to 30%26,27. The additional water utilised in the process of CRAs decreases the speed at which the
aluminosilicate precursors of an alkali-activated matrix dissolve28. Sata et al.29 demonstrated that including CRAs results in AAC’s compromised interfacial transition zone. In addition, the
sorptivity, water absorption, and volume of permeable voids of AAC rose as the CRA content increased30,31. Hu et al.31 found strong relationships between the volume of permeable voids,
sorptivity and water absorption in AAC with CRAs. Research has shown that the drying shrinkage of AAC steadily increases over time but at a reduced pace after 28 days of curing32. Zhang et
al.33 demonstrated that the drying shrinkage doubled when 100% of the NA was replaced with CRAs. The chemical durability of AAC was examined by subjecting it to seawater, magnesium sulphate,
and sulfuric acid. The study indicated that sulfuric acid posed the most risk to the AAC34. To address this, a novel and concise hybrid pre-treatment process for CRAs involving mild
chemical treatment followed by mechanical treatment was developed, as documented in a prior study by the current research team35.
This study investigates AAC’s durability and microstructural characterization performance developed from Na2O dosage of 4% and Ms of 1.25. The influence of varying CRAs and PCRA on the AAC
sample’s performance was investigated. Various experiments, such as sorptivity, acid, and seawater resistance, are conducted. In addition, microstructural characterization like X-ray
diffraction (XRD), Fourier transform infrared spectroscopy (FTIR), stereomicroscopy, scanning electron microscopy (SEM), and energy-dispersive X-ray (EDX) are also conducted to correlate the
corresponding observations with the durability performance of the AAC specimens.
The significance of this research lies in its focused examination of AAC incorporating CRAs sourced from CDW recycling plants, particularly under marine and acid exposure conditions.
Previous studies have concentrated mainly on the partial substitution of natural aggregates (NAs) with laboratory-controlled CRAs, often overlooking the heterogeneity of CRAs derived from
recycling plants and their effect on the durability of AAC. This gap highlights a critical need for an in-depth analysis of AAC’s performance when integrated with recycling plant-based CRAs
and PCRAs, extending beyond the conventional laboratory settings to include rigorous marine and acidic environmental testing. This study also aims to assess the sorptivity of AAC with NA,
CRAS and PCRAs. This study sheds light on the interaction within AAC mixes, including CDW plant-based CRAs and PCRs, offering valuable insights into their behaviour and performance in
challenging environments. The investigation extends to a detailed comparison of AAC’s properties when mixed with CRAs and PCRAs, aiming to delineate the material’s resilience, structural
integrity, and longevity. The outcomes of this research are poised to significantly contribute to the construction industry’s knowledge base, providing a robust framework for understanding
the potential of recycling plant-based CRAs and PCRAs in AAC applications. By elucidating AAC’s durability and microstructural attributes in marine and acidic conditions, this work paves the
way for more sustainable construction practices, encouraging the adoption of environmentally friendly materials that reduce reliance on natural resources. Furthermore, the findings from
this study are expected to increase confidence among industry practitioners and researchers alike, fostering the integration of sustainable aggregates into construction processes and
promoting the broader application of AAC in infrastructure development.
This section briefly describes the materials employed in the current investigation. The binding materials comprise a combination of GGBFS and FA, with GGBFS and FA being significant parts of
the total binder. Astraa Chemicals in Chennai supplied the GGBFS, while the FA was obtained from the Ramagundam Thermal Power Plant in Telangana. The chemical analysis revealed that GGBFS
consists of 35.37% lime (CaO), 33.06% silica (SiO2), and 16.81% alumina (Al2O3) as major components, with the rest being minor ingredients. The chemical analysis of FA showed 48.81% silica,
3.80% lime, and 31.4% alumina. The Blaine’s fineness of GGBFS and FA was measured at 390 m2/kg and 327 m2/kg, respectively. The specific gravity of GGBFS is 2.85 and that of FA is 2.04. All
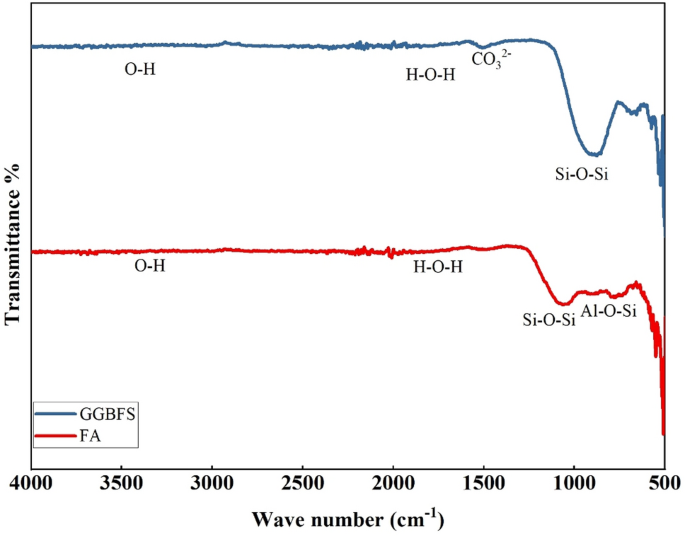
these material qualities meet the criteria set by ASTM C618 and BS EN 15167-136,37. The FTIR analysis of FA and GGBFS used in the study are depicted in Fig. 1. It shows that the range of
wavelengths from 3500 to 1600 cm−1 indicates the stretching of the (–OH) bonds and the bending vibrations of (H–O–H) in water molecules that are absorbed on the surface and trapped in the
spaces of the polymeric structure. The band between 1600 and 1000 cm−1 shows that Si–O–Si bonds are present, characteristic of quartz. At about 1470 cm−1, the frequencies of CO3–2 were found
to indicate stretching vibrations of the C–O groups. The 900–500 cm−1 band indicates that the Al–O–Si and Si–O–Si bonds stretch symmetrically. This transition from amorphous to
semi-crystalline alumino-silicate solids occurs. The band seen at frequencies below 500 cm−1 consists of the bending waves of the bonds Si–O–Si and O–Si–O.
The SEM images and average particle size distribution of binders (i.e., GGBFS and FA) used in the study are illustrated in Fig. 2. It depicts that the particles of GGBFS have sharper,
denser, and more irregular crystal structures. On the other hand, FA particles primarily display a spherical morphology. The particle size distribution of the binders is shown in Fig. 2. The
measurements obtained from ImageJ software show that approximately 90% of particles are in a range of 0.5–10 µm and 0.11–30 µm for FA and GGBFS, respectively. The histogram illustrates that
the mean particle size of FA and GGBFS were 2.36 µm and 9.34 µm, respectively.
The locally accessible quarry river sand that satisfied IS 238638 requirements for Zone-II grading and had a specific gravity of 2.56 was employed for the study. The coarse aggregates for
the reference mix were crushed granite stones procured from a local quarry. For the other combinations, the CRAs were obtained from the M/s Re Sustainability Limited, Hyderabad recycling
plant; the obtained CRAs were sieved to remove particles larger than 20 mm. As per the previous research by Khan et al.35, PCRAs were made by subjecting CRAs to a hybrid pre-treatment method
(i.e., chemical pre-treatment with a 0.1 M CRCFS-1500 acidic solution followed by a short mechanical pre-treatment process). A crushed angular NA, CRAs and PCRAs with a specific gravity of
2.67, 2.34 and 2.62, respectively, and water absorption rates of 0.26, 4.67, and 0.87, respectively, and a size passing through a 20 mm and held on a 4.75 mm mesh were adopted, meeting the
requirements as per IS 238638. The available tap water was used to prepare the AAC mixes. It was ensured that it was free of any impurities as per the requirements of IS 45639.
NaOH solid flakes and Na2SiO3 solution were used as the AAS. The supplier of both compounds was M/s Amrutha Organics in Hyderabad, India. As per IS 1421240, the composition of Na2SiO3 was
ascertained. It was found that 0.094 kg of Na2O, 0.301 g of SiO2, and 0.605 kg of H2O constitute 1 kg of Na2SiO3 solution. Na2SiO3 was discovered to have a Ms value of 3.20. After dissolving
NaOH in H2O to develop the AAS, Na2SiO3 was added to the mixture to get the desired modulus (Ms = 1.25). Based on the literature, the activator solution was adjusted to yield a 4% dosage of
Na2O (by weight of total binder content) with a set Ms value of 1.2541,42. The solution was mixed thoroughly and transferred to a closed container. It was left for one day before being used
in the AAC. The w/b ratio of 0.45 was attained by adding more water.
An air-entraining agent would be beneficial when mixing the AAC with different coarse aggregates, as identified from the previous work43. Therefore, the present study used an air-entraining
agent, i.e., MASTERAIR 721, to prepare the AAC. The local supplier provides the chemical properties of AEA (i.e., the pH, relative density, chloride ion content, and aspect are 6, 1.020,