Heavy metals immobilization and bioavailability in multi-metal contaminated soil under ryegrass cultivation as affected by zno and mno2 nanoparticle-modified biochar
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Pollution by heavy metals (HMs) has become a global problem for agriculture and the environment. In this study, the effects of pristine biochar and biochar modified with manganese
dioxide (BC@MnO2) and zinc oxide (BC@ZnO) nanoparticles on the immobilization and bioavailability of Pb, Cd, Zn, and Ni in soil under ryegrass (_Lolium perenne_ L.) cultivation were
investigated. The results of SEM–EDX, FTIR, and XRD showed that ZnO and MnO2 nanoparticles were successfully loaded onto biochar. The results showed that BC, BC@MnO2 and BC@ZnO treatments
significantly increased shoots and roots dry weight of ryegrass compared to the control. The maximum dry weight of root and shoot (1.365 g pot−1 and 4.163 g pot−1, respectively) was reached
at 1% BC@MnO2. The HMs uptake by ryegrass roots and shoots decreased significantly after addition of amendments. The lowest Pb, Cd, Zn and Ni uptake in the plant shoot (13.176, 24.92,
32.407, and 53.88 µg pot−1, respectively) was obtained in the 1% BC@MnO2 treatment. Modified biochar was more successful in reducing HMs uptake by ryegrass and improving plant growth than
pristine biochar and can therefore be used as an efficient and cost effective amendment for the remediation of HMs contaminated soils. The lowest HMs translocation (TF) and bioconcentration
factors were related to the 1% BC@MnO2 treatment. Therefore, BC@MnO2 was the most successful treatment for HMs immobilization in soil. Also, a comparison of the TF values of plant showed
that ryegrass had a good ability to accumulate all studied HMs in its roots, and it is a suitable plant for HMs phytostabilization. SIMILAR CONTENT BEING VIEWED BY OTHERS EXTRACTION OF HEAVY
METALS FROM COPPER TAILINGS BY RYEGRASS (_LOLIUM PERENNE_ L.) WITH THE ASSISTANCE OF DEGRADABLE CHELATING AGENTS Article Open access 01 April 2024 ACIDIFIED BIOCHAR IMPROVES LEAD TOLERANCE
AND ENHANCES MORPHOLOGICAL AND BIOCHEMICAL ATTRIBUTES OF MINT IN SALINE SOIL Article Open access 30 May 2023 CHEMICAL FORMS OF CADMIUM IN SOIL AND ITS DISTRIBUTION IN FRENCH MARIGOLD
SUB-CELLS IN RESPONSE TO CHELATOR GLDA Article Open access 20 October 2022 INTRODUCTION Heavy metal (HMs) pollution caused by industrialization and economic development has become a major
environmental, agricultural, and public health problem worldwide1,2. Heavy metal are non-degradable and stable in nature, and at concentrations of less than 1 ppm, they are considered toxic
to plants and animals3,4,5,6. Moreover, due to their high mobility, these metals pose a major threat to human and animal health as they accumulate in water bodies and agricultural soils and
enter the food chain7,8,9,10. Therefore, in order to clean and restore soil health and eliminate or limit the bioavailability of HMs to plants and humans, it is essential to use
environmentally sound and economically viable technologies11,12,13. To date, various methods have been used to remediate soils contaminated with HMs, including electrokinetic remediation,
bioremediation, soil washing, and chemical precipitation14,15,16,17, but the application of these technologies is limited due to their high cost, environmental contamination from the
generation of secondary chemicals, and damage to soil structure15,16,18,19. The Environmental Protection Agency (EPA) has identified soil immobilization as the most effective, economical,
and environmentally safe technique for remediation of HM-contaminated soils on a broad scale14,20,21. Immobilization using ion exchange reactions changes the adsorption, complexation, and
precipitation of HMs from active to stable phases and reduces the bioavailability of HMs in soil22,23,24,25. In situ immobilization of HMs requires careful consideration of the choice of
immobilization substance26. Many substances such as montmorillonite, limestone, compost, metal oxides, and biochar have been used to immobilize HMs in the soil7,27,28. Biochar is a
carbon-rich adsorbent produced from the pyrolysis of various raw materials in the absence or limited presence of oxygen29,30,31. Due to its high porosity, active functional groups, and high
CEC, biochar has been considered an ideal amendment for the immobilization of HMs in24,32,33. Therefore, biochar can reduce the availability, mobility and leaching of HMs in soil, and
limiting their bioavailability for the plants15,30. Biochar immobilizes HMs in soil through various mechanisms such as complexation, cation exchange, electrostatic interactions, reduction
and precipitation30. Biochar also protects the plant from HMs by increasing antioxidant activity31. However, unmodified biochar has a limited capacity to immobilize and adsorb HM in
soil23,24,34,35. Therefore, it is important to modify biochar to improve its ability to immobilize HMs in soil. Various physical, chemical, and biological techniques have been used to
improve the properties of biochar, e.g. specific surface area, porosity, number of adsorption sites on the surface, and number of surface functional groups36,37,38. Recently, the
modification of biochar with nanomaterials, especially nanometals, has attracted attention due to their high efficiency, low toxicity and environmental compatibility39,40,41. Nanomaterials
are particles ranging in size from 1 to 100 nm42,43. In the last decade, nanomaterials have been used extensively for the removal of HMs from water and soil due to their tiny size, strong
reactivity, and high surface activity44,45,46. However, these materials tend to aggregate and deactivate due to their high surface energy, which limits their ability to remove
contaminants33,41,44,45,46,47. To overcome these problems, biochar can serve as a carrier for metal oxide nanoparticles due to its porosity and high ion exchange capacity48,49. The composite
of biochar and metal nanoparticles eliminates their deficiencies and creates a material that combines the advantages of both properties50,51. The functional surface groups, the specific
surface area, and the cation exchange capacity increase by modifying biochar with metal nanoparticles. This increase leads to improvement of ion exchange, complexation and thus the
stabilization of HMs36,49,52. Among metal nanoparticles, zinc oxide nanoparticles have attracted attention due to their low toxicity, compatibility, high binding energy, low production cost
and biodegradability compared to other nanoparticles44,53,54. They are also widely used for the removal of HMs from water and soil39. In addition, manganese oxide nanoparticles have been
shown to be well suited for stabilization of HMs due to their high specific surface area, high affinity for HMs, abundance of hydroxyl functional groups on their surface, stability in a wide
pH range, and high negative surface charges55,56,57,58. Numerous studies have demonstrated the effect of biochar modified with various nanoparticles on the immobilization of HMs in
soil37,59,60,61,62. However, studies on the effect of biochar modified with ZnO and MnO2 nanoparticles on HMs immobilization in soil under plant cultivation is still limited and need to be
explored in detail. Ryegrass (_Lolium perenne_ L.) was selected as the study plant in our research due to its fast growth, high biomass, high potential for accumulation of metals, and high
tolerance to these metals63,64,65. In this study, we tested the hypothesis that MnO2 and ZnO nanoparticle-modified biochars outperforms the pristine biochar in terms of their ability to
immobilize HMs, reduce their bioavailability, and improve plant development. Therefore, the objectives of this study include: (i) Synthesis of modified biochar with MnO2 and ZnO
nanoparticles and comparison their morphology, physicochemical properties (ii) Evaluation and comparison of the efficiency of pristine biochar (BC), biochar modified with ZnO nanoparticles
(BC@ZnO), and biochar modified with MnO2 nanoparticles (BC@MnO2) in term of immobilization of Pb, Cd, Ni and Zn in multi-metal contaminated soils and (iii) Comparing the effects of pristine
and ZnO and MnO2 nanoparticle modified biochar on the HMs uptake and ryegrass (_Lolium perenne_ L.) growth. RESULTS CHARACTERISTICS OF THE BC, BC@MNO2 AND BC@ZNO FT-IR ANALYSIS FTIR analysis
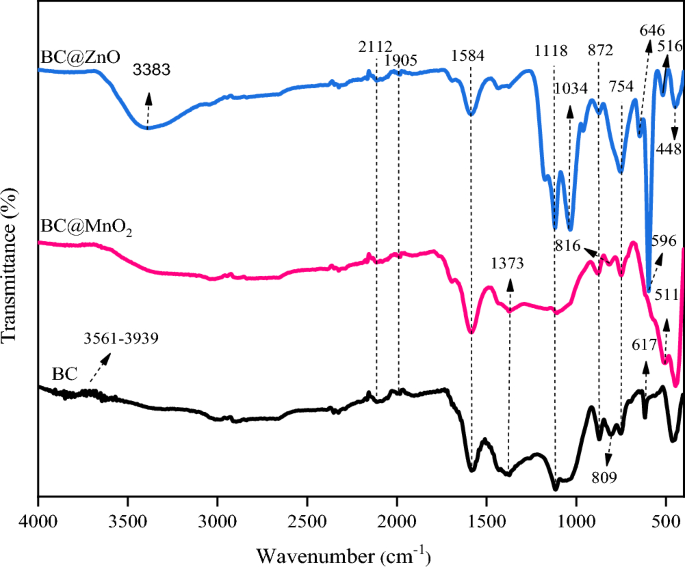
was carried out to investigate the functional groups of BC, BC@MnO2, and BC@ZnO (Fig. 1). The broad band 3300–3940 cm−1 represents the strong and broad O–H stretching of alkyne66,67. The
peaks at 2700–3000 cm−1 corresponded to the C–H stretching vibration68,69. The existence of the C ≡ C triple or C = C = C double bonds of alkene was confirmed by the peaks at 1905 and 2112
cm−170. The peak at 1584 cm−1 is attributed to the stretching vibration of C = C71. The peak at 1373 cm−1 was the characteristic peak of –C–H of the methyl group72. The peaks at 1116 cm−1
are assigned to C–O–C stretching vibrations in ether or saturated chain anhydrides73,74. The peak at 1034 cm−1 is assigned to C–O stretching75. The peaks at 729–880 cm−1 were characteristic
of the bending vibration of aromatic C–H groups76. The peak at 617 cm−1 is attributed to the bending vibration of the O–H group77. The peak at 511 cm−1 is associated with the Mn–O stretching
vibration, shows that the MnO2 nanoparticles were successfully loaded onto the surface of the biochar66,73. Peaks at 646, 596, 516 and 448 cm−1 confirmed the presence of a Zn–O stretching
vibration77,78,79. XRD ANALYSIS To determine the crystal structure and phase composition of BC, BC@MnO2, and BC@ZnO, the X-ray diffraction (XRD) pattern was used (Fig. 2). The peaks of 26.6°
in BC, 27.3° in BC@ZnO and 23° in BC@MnO2 were attributed to graphite, indicating that a stable and regular graphite structure was formed during the pyrolysis process80. The peak at 40.6°
in BC was assigned to quartz or sylvite81,82. The peaks at 50.2° and 58.72° in BC and BC@ZnO and the peaks at 28.4° and 73.7° in BC, corresponded to sylvite83,84,85. The peaks at 29.4° and
30.9° in BC were attributed to calcite74. The peak at 66.4° in BC was assigned to quartz74. The peaks at 26.3°, 32.93°, 34.8°, 37.5°, 41.2° and 60.2° reflect the crystal planes of the
hexagonal structure of wurtzite ZnO recorded in the card (JCPDS card No. 36–1451)78,86,87,88. The 21.4° and 24.5° peaks in BC@ZnO are also indicative of zinc silicate78,86,87,88,89. The XRD
pattern of BC@ZnO is consistent with the results of other related studies90,91,92,93. The 36.7° peak in BC@MnO2 was assigned to amorphous MnO273,94. Compared to the patterns of BC and
BC@ZnO, the XRD pattern of BC@MnO2 revealed weaker peaks. These weak peaks were probably caused by the charge of the amorphous MnO2, which had an impact on the XRD patterns of other
crystals66. In general, the XRD of MnO2-loaded biochar did not show many peaks for phase identification. This could be due to the unfavorable effect of the high reaction rate between Mn(II)
and Mn(VII), in the case of MnO2 synthesized by the co-precipitation method73,95. According to the results of previous studies, amorphous materials have effective adsorption properties due
to their high specific surface area and the large number of active sites on their surfaces73,96. These results are consistent with the observations of this study, which showed that BC@MnO2
plays a more effective role in the surface adsorption of heavy metals compared to other adsorbents. SEM–EDX AND BET ANALYSIS The surface morphology and elemental composition of BC, BC@MnO2
and BC@ZnO were investigated using SEM–EDX analysis (Fig. 3). According to Fig. 3a, the biochar contains honeycomb and porous structures, which are probably due to the removal of volatile
substances during the pyrolysis process. Figure 3b shows that the surface of biochar is uniformly covered with fine and dense particles, indicating the deposition of MnO2 nanoparticles.
Similar results were obtained by20,66,97. The SEM of the BC@ZnO nanocomposite showed spherical and white particulates of ZnO in the form of clusters on the biochar surface. These spherical
particles are probably due to the irregular growth of the ZnO crystals98. These results have also been obtained by other researchers88,93,99,100,101. In general, the surface of the biochar
modified with ZnO and MnO2 nanoparticles was irregular, non-uniform and more uneven compared to pristine biochar confirming the successful loading of ZnO and MnO2 nanoparticles onto the
biochar. Previous research has shown that a rough surface leads to an increase in the specific surface area and increase in the positive charge thereby increasing the adsorption of
pollutant20,99,100,102,103. EDX analysis of the biochar showed the presence of C, O, Ca, Mg, K, Si and Cl elements. The presence of Mn and Zn and the significant increase in O content in
BC@MnO2 and BC@ZnO confirmed the successful loading of the biochar surface with MnO2 and ZnO nanoparticles. Therefore, according to the results of XRD, FTIR and SEM–EDX analyses, it is
likely that the ZnO and MnO2 nanoparticles were successfully loaded onto the BC. The BET analysis showed that the addition of MnO2 and ZnO nanoparticles increased the specific surface area
of pristine biochar from 60.52 m2 g-1 to 430.71 and 341.2 m2 g-1, respectively. This increase is most likely the result of the deposition of ZnO and MnO2 nanoparticles on the surface of
biochar, which led to an increase in surface roughness and consequently an increase in specific surface area82,104. It can be concluded that biochar modified with nanoparticles has
appropriate active sites for the adsorption of HMs, making it a high-potential adsorbent. In similar studies, Liang et al. (2017) and Wang et al. (2020), reported that loading biochar with
MnO2 and ZnO nanoparticles led to a significant increase in specific surface area of nanocomposites66,88. According to the results, biochar modified with MnO2 nanoparticles can absorb heavy
metals more effectively compared to other adsorbents. This superiority can be attributed to the amorphous structure, more exchangeable cations and a higher specific surface area133. EFFECT
OF THE ADSORBENTS ON RYEGRASS GROWTH To investigate the effects of BC, BC@ZnO, and BC@MnO2 at doses of 0.5 and 1% on the growth of ryegrass under Cd, Pb, Zn, and Ni stress, the dry weight of
the roots and shoots of ryegrass was evaluated (Fig. 4). Compared to the controls, the application of BC, BC@MnO2, and ZnO significantly (_p_ < 0.05) increased the dry weight of the
ryegrass shoots and roots. The application of biochar at 0.5% and 1% increased the dry weight of ryegrass shoots and roots by about 50.83–61% and 60.56–49.17%, respectively, compared to the
control treatment. The addition of BC@ZnO and BC@MnO2 at 0.5% and 1% also increased the dry weight of the aerial parts by 78–81.9% and 84.28–85.83%, respectively, compared to the control.
The addition of BC@MnO2 and BC@ZnO at 0.5% and 1% increased the root dry weight by 78.11–79.2% and 81–82.13%, respectively, compared to the control treatment. The dry weight of ryegrass
roots and shoots in biochar treatments modified with ZnO and MnO2 nanoparticles differed significantly (_p_ < 0.05) compared to the pristine biochar treatment. Moreover, the addition of
BC@MnO2 to the soil significantly increased the dry weight of the root and shoot biomass of ryegrass compared to the BC@ZnO treatments (_p_ < 0.05). The type and dose of adsorbent also
had a significant effect on the dry weight of roots and shoots. Increasing the dose from 0.5 to 1% significantly increased the dry weight of the roots and shoots. The results from Fig. 4
show that the BC@MnO2 treatment resulted in the highest dry weight of roots and shoots, while the control treatment gave the lowest. BC@MnO2 was found to be more effective than BC@ZnO in
increasing the dry weight of roots and shoots. In addition, a positive correlation was found between the shoot and root dry weights of the ryegrass plants in all treatments. After increasing
the adsorbent dosage from 0.5 to 1%, the dry weight of the roots and shoots increased. This increase is probably the result of increased HMs immobilization in the soil and improved nutrient
supply at higher doses. In agreement with previous studies, a linear relationship was found between the amount of adsorbent used and the dry weight of the root and shoot of plant11,105,106.
EFFECTS OF BC, BC@MNO2, AND BC@ZNO ON THE UPTAKE OF NI, ZN, PB, AND CD BY RYEGRASS SHOOTS AND ROOTS Table 1 shows data on HM uptake by ryegrass shoots treated with BC, BC@MnO2, and BC@ZnO.
Compared to the control treatment, the addition of pristine biochar and biochar modified with nanoparticles significantly reduced (_p_ < 0.05) the uptake of Pb, Ni, and Cd in the ryegrass
shoot. The application of 0.5% and 1% BC, BC@ZnO, and BC@MnO2 reduced Pb uptake in the shoots by 22.29–47.31%, 67.55–74.79%, and 83.82–89.33%, respectively, compared to the control.
Application of BC, BC@ZnO, and BC@MnO2 at two levels of 0.5% and 1% reduced uptake of Cd in shoot parts by 18.97–45.11%, 75.29–78.37%, and 78.73–82.83%, respectively, compared to control.
Table 1 also shows that the application of 0.5 and 1% BC, BC@ZnO and BC@MnO2 decreased the Ni uptake in the shoots by 11.55–32.58%, 52.76–61.67%, and 68.44–71.88%, respectively, compared to
the control. Although Zn uptake in shoots was significantly (_p_ < 0.05) decreased by different doses of BC (17.86–40.20%) and BC@MnO2 (74.98–79.85%) compared to control, BC@ZnO
significantly (_p_ < 0.05) increased Zn uptake in shoots (4.91–16.61%). Compared to application of BC treatments, the application of BC@MnO2 and BC@ZnO treatments resulted in a greater
reduction in the uptake of HMs in the shoots, with the exception of ZnO nanoparticle-modified biochar, which increased the uptake of Zn in the shoots. In addition, BC@MnO2 was more effective
than BC@ZnO in reducing HMs uptake by the shoots. In addition to the type of adsorbent, the amount of adsorbent also affected the uptake of HMs by the shoots of ryegrass. When the rates of
BC, BC@ZnO, and BC@MnO2 increased from 0.5 to 1%, the Pb, Ni and Cd uptake in shoots decreased. Zn uptake in shoots increased by increasing the amount of BC@ZnO from 0.5 to 1%. Also, BC@MnO2
was more effective than BC@ZnO in decreasing the uptake of HMs by the shoots. According to our results, the lowest uptake of HMs by ryegrass shoots was observed in 1% of BC@MnO2 treatment.
Moreoveer, all adsorbents showed a strong propensity to uptake Pb than other HMs. Table 2 shows the effect of BC, BC@MnO2, and BC@ZnO at 0.5 and 1% on the uptake of HMs by ryegrass roots.
Compared to the control treatment, the addition of BC, BC@MnO2, and BC@ZnO significantly reduced the uptake of Ni, Pb, and Cd by plant roots (_p_ < 0.05). Compared to the control, Zn
uptake was significantly reduced (_p_ < 0.05) by the BC and BC@MnO2 treatments but increased by BC@ZnO. With the exception of the BC@ZnO treatment, which increased the Zn uptake, the
application of biochar modified with nanoparticles decreased HMs uptake in roots more than pristine biochar. Moreover, the dosage of the adsorbents proved to be influential in reducing the
HMs uptake by the ryegrass roots. When the amount of BC was increased from 0.5 to 1%, uptake of Pb, Cd, Zn, and Ni in root decreased by 16.74, 16.74, 15.71, and 22.19%, respectively.
Similarly, increasing the BC@MnO2 dosage from 0.5 to 1% decreased the root uptake of Cd, Pb, Zn, and Ni by 16.46%, 25.2%, 20.2%, and 11.18%, respectively. . The absorption of Cd, Ni, and Pb
in the roots decreased by 16.27%, 19.7%, and 16.15%, respectively, when in the BC@ZnO dose was increased, while Zn uptake increased by 12.79%. As the results show, the roots of ryegrass had
higher concentrations of HMs compared to the shoots. In general, the tendency for uptake HMs by BC and BC@MnO2 was as Pb > Cd > Zn > Ni and for BC@ZnO as Pb > Cd > Ni > Zn
in the roots and shoots of ryegrass; in reality, the plant absorbs these heavy metals to a lesser extent. The lower uptake of heavy metals by the plant leads to a higher immobilization of
heavy metals in the soil, and thus to lower availability for the plant. BIO-CONCENTRATION (BCF) AND TRANSLOCATION FACTORS (TF) To better understand the effect of different amendments on the
uptake and translocation of heavy metals in ryegrass, the bioaccumulation factor (BCF) and translocation factor (TF) were calculated and presented in Figs. 5 and 6. According to the results
shown in Fig. 5, the addition of all adsorbents to the soil, significantly decreased (_p_ < 0.05) the TF for all HMs compared to the control treatment. Therefore, addition of 0.5% and 1%
biochar led to reductions in TF values for Pb, Cd, Zn, and Ni by 13.75–18.75%, 6.17–7.53%, 6.90–12.64%, and 7.63–8.87%, respectively, compared to the control. The effect of BC treatment in
reducing the TF of HMs compared to the control was as follows: Pb > Zn > Ni > Cd. Also, addition of BC@ZnO at two levels of 0.5% and 1% decreased the TF values for Pb, Cd, Zn and Ni
compared to the control treatment from 45 to 52.50%, 27.78–37.90%, 20.69–32.18%, and 16.23–28.06% respectively,. Compared to the control, BC@ZnO treatment reduced the TF of HMs in the
following order: Pb > Cd > Zn > Ni. Similarly, the addition of BC@MnO2 at 0.5 and 1% decreased the TF values for Pb, Cd, Zn, and Ni from 53.75 to 61.25%, 50.49–54.44%, 49.43–50.57%,
and 33.13–35.93%, respectively, compared to the control treatment. The effect of biochar treatment on reduction of TF values compared to the control were as follows: Pb > Cd > Zn >
Ni. Increasing the amount of adsorbent led to a further reduction in TF. As can be seen in Fig. 5, the TF values of HMs in the nanoparticle-modified biochar treatments were significantly
lower (_p_ < 0.05) than those in the pristine biochar treatment. In addition, BC@MnO2 reduced the TF of HMs in ryegrass better than BC@ZnO. The treatment with 1% BC@MnO2 had the lowest TF
of HMs, while the control treatment had the highest TF. In general, according to the obtained results, the TF of HMs in ryegrass was as follows: Ni > Zn > Cd > Pb, indicating
greater transfer of Ni from the roots to the shoots. Similar to TF, the addition of adsorbent reduced the BCF of HMs compared to the control, and this decrease became greater when the
adsorbent dose was increased. According to the results, the control treatment and the treatment with 1% BC@MnO2 had the highest and lowest BCF values for HMs, respectively. Thus, the BCF
values of Pb, Cd, Zn, and Ni decreased by 96.98, 95.29, 95.08, and 94.35%, respectively in the treatment of 1% BC@MnO2 compared to the control. The BCF in the biochar modified with
nanoparticles was significantly lower compared to the treatment of pristine BC. In general, the BCF in ryegrass in all treatments was as follows: Ni > Zn > Cd > Pb. DISCUSSION
EFFECT OF BC, BC@MNO2 AND BC@ZNO ON PLANT BIOMASS Abiotic stress, such as heavy metals, can lead to physiological changes in plants, including a decrease in plant biomass107,108,109. The
lower dry weight of roots and shoots observed in the control treatment compared to the other treatments may be attributed to the high concentration of HMs in the plants (Fig. 4). This
results is consistent with previous studies108,109. Shoot and root dry weight increased significantly in comparison to the control treatment when biochar and modified biochar were added to
metal-contaminated soils. This might be due to the fact that the addition of biochar and modified biochar stabilize of HMs in the soil and limit their bioavailability to the plants, and
reduce the uptake of metals leading to an improvement in root and shoot growth of the plant. Similar results were obtained by Ali et al. (2019)110 and Shahbaz et al. (2018)111. They reported
that the application of biochar prevents the transfer of HMs in the plant and increases the dry weight and grain yield of wheat, sunflower, and maize. In addition, biochar increases plant
growth by providing macro and micronutrients. In addition, biochar improves the physicochemical properties of the soil, which produces the optimal conditions for better plant growth. These
conclusions have been supported by numerous reports.112,113,114,115. Research by Wang et al. (2021) also aligns with these results and shows that the presence of various biochars improves
nutrient availability116 and HMs immobilization117, resulting in increased ryegrass biomass18. The results of the present study have obviously shown that loading of biochar with ZnO and MnO2
nanoparticles plays a role in increasing the dry weight of ryegrass roots and shoots (Fig. 4). The increase in the dry weight of roots and shoots in modified biochar treatments may be due
to the dual effect of biochar and nanoparticles on the plants. Indeed, the application of biochar immobilizes the HMs in the soil and nanoparticles increase the zinc and manganese content in
the plant. These results are in line with published studies showing that NPs promoted the growth of the plants when exposed to metal stress53,118,119. These results are consistent with
other findings108,109. In one study, Kareen et al. (2023) observed that incorporation of biochar with zinc oxide nanoparticles resulted in improved alfalfa growth in Cd-contaminated soil.
They attributed this result to the competition between nutrients and heavy metals at the root surface and to the immobilization of Cd in the soil109. Lin et al. (2017) and Yu et al. (2017)
reported that the application of MnO2 modified biochar in an arsenic-contaminated soil significantly increased the grain weight and dry weight of rice roots and shoots compared to pristine
biochar120,121. In addition, the BC@MnO2 treatment performed better compared to other treatments in terms of increasing the dry biomass of ryegrass roots and shoots. This is most likely due
to the fact that BC@MnO2, due to its higher specific surface area, amorphous structure, and greater number of exchangeable cations, reduced the bioavailability of HMs in the soil more
effectively than other treatments thus minimizing their impact on ryegrass development. EFFECT OF BC, BC@MNO2 AND BC@ZNO ON PLANT HMS CONCENTRATION Various organic treatments have been
employed to reduce the bioavailability and toxicity of HMs in plants and soils,122,123. As reported by Azim-Zadeh et al. (2014), organic amendments can alter soil properties, ultimately
affecting the bioavailability of HMs124. In this study, the presence of HMs in ryegrass shoots and roots was used as an indicator of the HMs bioavailability in soils (Tables 1 and 2). In the
present study, addition of BC, BC@MnO2 and BC@ZnO significantly decreased the uptake of HMs in the roots and shoots and as the dose was increased, the reduction effect increased. This might
be due to the fact that biochar immobilizes HMs in soil and reduces their bioavailability. Previous research has shown that oxygen-containing functional groups on biochar surfaces
facilitate the immobilization of metal ions through the formation of precipitates and complexes27,125,126. The interaction with the mineral and crystal lattices on the BC surface also allows
the HM ions to form to form insoluble inorganic compounds (such as metal phosphate, metal carbonate, or metal silicate)127,128. EDX analysis revealed (Fig. 3) that the presence of divalent
cations such as Ca (II) and Mg (II) in biochar can replace HM ions on biochar surfaces129. In fact, it can be argued that biochar decreased the bioavailability of HMs by immobilizing HMs in
the soil through the mechanisms of ion exchange, complexation, and precipitation, which in turn reduced uptake by ryegrass roots and shoots. The silica in wheat straw biochar can also lead
to a reduction in bioavailability in the soil, a reduction in adsorption by the plant, and a reduction in the transfer of HMs from the roots to the shoots130. Similar results have also been
reported by other researchers. Based on the research of Wang et al. (2018), biochar significantly reduced the solubility of HMs in soil131. Awad et al. (2020) reported that the HM contents
of soil and plant tissues were significantly reduced after biochar applications132. The results showed that loading MnO2 and ZnO nanoparticles on the biochar led to a more noticeable
decrease in the plant's absorption of HMs, suggesting that BC@ZnO and BC@MnO2 have a greater ability to immobilize HMs than pristine biochar. BC@NP composites have better
physicochemical properties compared to pristine biochar due to the combination of both constituent particles. Therefore, the substantial reduction in HMs uptake by ryegrass with
nanoparticle-modified biochar application may be attributed to the combined effects of biochar and nanoparticles on soil and plant surfaces. In addition to the benefits of biochar in
reducing the bioavailability of HMs, nanoparticles can also reduce the adverse effects of HMs on plant growth and productivity27. Also, according to the results of BET analysis, biochar
modified with nanoparticles had a higher specific surface area compared to pristine biochar. This suggests that these treatments have been more effective in immobilizing HMs133. According to
previous research, cation channels of Ca2+, Zn2+, Mn2+, and Fe2+ are the pathways through which Cd, Pb, Ni, and other divalent heavy metals enter plant cells134,135. When BC@MnO2 and BC@ZnO
are applied, Mn2+ and Zn2+ levels increase, which can compete with HMs in the transport pathway and obstruct their transfer through plant membranes. In similar reports,2,136,137 the
addition of MnSO4 and ZnSO4 amendments could greatly reduce the buildup of HMs by increasing the amount of accessible Mn and Zn content. In the study of Wang et al. (2019) the capacities of
biochar derived from tea branches and Fe–Mn-modified biochar (MnFe2O4-biochar) to immobilize Sb and Cd in contaminated soils and reduce the bioavailability of Sb and Cd in _Lolium
multiflorum_ Lam were assessed. Their results demonstrated that MnFe2O4-biochar application significantly reduced extractable Sb and Cd concentrations, transformed exchangeable Sb and Cd
into less accessible forms, and decreased the accumulation of Sb and Cd in plants138. Furthermore, Suleiman et al. (2020) investigated the biochar with ZnO nanoparticles treatment on
sunflowers grown in wastewater contaminated with HMs. They reported that biochar and ZnO significantly reduced the availability of HMs and decreased their uptake by sunflower compared to
pristine biochar27. As shown in Tables 1 and 2, the BC@ZnO treatment increased Zn2+ uptake by the ryegrass roots and shoots. This is probably due to dissolving of the ZnO nanoparticles in
soil and releasing ionic Zn, and increasing its bioavailability and uptake by plants. Similar results were obtained by Hossein et al. (2018), Shafqat Ali et al. (2019), and Rizwan et al.
(2019). They reported that ZnO nanoparticles increased the bioavailability of Zn2+ and increased its uptake by different plant organs118,139,140. The data in Tables 1 and 2 show that there
was a significant difference between BC@ZnO and BC@MnO2 treatments in reducing the uptake of Pb, Ni, and Zn for the roots and shoots of plants, but no significant difference was observed for
Cd. Previous research has demonstrated that ZnO nanoparticles decrease Cd levels in a variety of plant species44,141,142. Due to the antagonistic effects of both metals, it is possible to
link this decrease in Cd uptake, at least in part, to the rise in Zn levels143. Zn and Cd compete with each other because both are transported to the root surface plasma membrane by a common
carrier144. Since the adsorption of Zn is easier than that of Cd, Cd phytoaccumulation is reduced when Zn is present145. Numerous studies have shown that plants with higher Zn levels
absorbed less Cd22,141,146,147. According to our results, the amount of Pb uptake by the plant was lower than that of other metals. This demonstrates that Pb is more adsorbed and immobilized
onto the adsorption sites than Cd, Ni, and Zn. Zn, Pb, Cd, and Ni are cations with similar valency, therefore, they are able to compete for the same sites and functional groups on the
adsorbent’s surfaces. According to Houben et al. (2013), Pb may have a stronger affinity for carboxylic and phenolic functional groups that are present on the surface of BC148. Similar
findings were observed by Jiang et al. (2020), Norini et al. (2019), and Namgay et al. (2010), who showed that biochar immobilizes Pb more strongly than other cationic HMs and removes it
from the reach of plants149,150,151. Therefore, according to the results of our study, it can be stated that BC, BC@MnO2, and BC@ZnO can effectively decrease the accumulation of HMs in
ryegrass, consequently reducing phytotoxicity. Also, biochar modified with nanoparticles were more effective than pristine biochar in reducing the uptake of HMs by ryegrass. EFFECT OF BC,
BC@MNO2 AND BC@ZNO ON BIO-CONCENTRATION (BCF) AND TRANSLOCATION FACTORS (TF) OF HM IN RYEGRASS TISSUES The two most important metrics for assessing the potential risks of metal ions for
plant growth in contaminated soils are bioconcentration (BCF) and translocation factors (TF). TF indicates the transport of HMs from the roots to the shoots of ryegrass. The TF value (Fig.
5) of all HMs was less than 1, indicating that the HMs mainly accumulate in the roots. A low TF value also indicates a lower transport of HMs from roots to the shoots. Thus, all adsorbents
were successful in preventing the HMs transport from the roots to the shoots. Compared to pristine biochar, nanoparticle-modified biochar was more effective in reducing the transfer of HMs.
The results presented in Fig. 6 indicated that the BCF values of all HMs except Pb (in the control treatment) were greater than 1 and with the addition of adsorbents reached below 1.
Therefore, the results confirm that in this study, soil amendment with BC, BC@MnO2, and BC@ZnO was effective in reducing the uptake of HMs by ryegrass. In addition, the modified adsorbents
were found to be more effective in reducing the bioavailability of HMs. Similar findings to this study were reported by others18,149,150,151,152,153. The results of this study show that the
adsorbents are able to efficiently immobilize HMs in the soil, reduce their bioavailability to the plant and, on the other hand, prevent their translocation to the aerial parts of the plant.
As a result, ryegrass is less dangerous for primary consumers. Zhang et al. (2016) reported that high BCF and TF values indicate higher HMs concentrations in soil, higher uptake by plants
and higher translocation to aboveground organs, resulting in increased risk to consumers154. These findings suggest that ryegrass has the ability to bind HMs in its roots. For this reason,
ryegrass is considered as a suitable phyto-stabilizing plant155. According to our results, the BCF and TF values of Pb were lower than those of the other HMs in all treatments, indicating
less uptake by the plant roots and less transfer to the aerial parts. This result supports the findings on metal uptake by plants. CONCLUSION The main purposes of this study were to
synthesis modified biochar with MnO2 and ZnO nanoparticles and evaluate and compare their efficiency to remediate HMs contaminated soil and reduce their bioavailability, and improve plant
growth. Results showed that ZnO and MnO2 nanoparticles were successfully loaded onto biochar (BC). The addition of BC, BC@MnO2 and BC@ZnO immobilized Pb, Zn, Cd and Ni in soil, reduced their
uptake by the ryegrass and improved the dry weight of the ryegrass, but BC@MnO2 and BC@ZnO were more effective than unmodified BC in immobilizing HMs in the soil and reduced their uptake by
ryegrass. In addition, the efficiency of immobilization and the reduction of HMs uptake by the plant were higher in treatments with 1% adsorbents than with 0.5%. Overall, our results showed
that the application of 1% BC@MnO2 was better than other treatments in increasing the ryegrass dry weight, improving immobilization and reducing the availability of HMs to ryegrass. The
results showed that adsorbents had a stronger affinity for Pb than the other HMs. It can be concluded that each effectiveness of treatment depends not only on its dose and type but also on
the type of HMs. Results of present study showed that biochar modified with MnO2 and ZnO nanoparticles had more benefit effect on soils remediation compared to pristine biochar, and they can
be used as efficient and low-cost amendments to remediate HMs-contaminated soils and improve plant growth. Also, a comparison of the TF in ryegrass showed that it had a good ability to
accumulate all studied HMs in its roots; therefore, it is a suitable plant for HMs phytostabilization. METHODS MATERIALS AND CHEMICALS All chemicals, lead nitrate (Pb(NO3)2), Zinc nitrate
(Zn(NO3)2), Cadmium nitrate (Cd(NO3)2), Nickel nitrate (Ni(NO3)2), potassium permanganate (KMnO4), Hydrogen Peroxide (H2O2), Sodium hydroxide (NaOH), nitric acid (HNO3), Zinc sulfate
heptahydrate (ZnSO4.7H2O), and potassium hydroxide (KOH) were provided by Merck company (Germany) and used without any further purification. PREPARATION OF BIOCHAR Wheat straw, selected as
the raw material for preparing biochar and obtained from the animal husbandry station of Shiraz University Faculty of Agriculture. Wheat straw was first washed several times with deionized
water to remove impurities, dried at 60°C for 48 h, and then ground. The crushed samples were pyrolyzed at 500°C in an electric furnace with limited oxygen for three hours130. The produced
biochar (BC) was passed through a 2-mm sieve and stored in plastic containers. PREPARATION OF BIOCHAR-LOADED WITH MNO_2_ NANOPARTICLES Biochar modified with MnO2 nanoparticles prepared
according to the Zhang et al. (2020) and Liang et al. (2017) methods. Briefly, 15 g of BC and 3.16 g of KMnO4 (the manganese source) were combined with 150 mL of deionized water and stirred
at 25 °C for 30 min. While stirring, 40 ml of 30% H2O2 was added to the mixture dropwise. After adding 1 M HNO3 and NaOH to bring the pH of 7.0, the mixture was stirred for another 30 min
and allowed to stand at room temperature for three hours. After filtering, the mixture was repeatedly purified with distilled water and dried at 105 °C for 12 h66,156. PREPARATION OF
BIOCHAR-LOADED WITH ZNO NANOPARTICLES Biochar modified with ZnO nanoparticles was synthesized by the precipitation method. 0.15 M KOH and 0.1 M ZnSO4.7H2O solutions were prepared in
distilled water. 100 mL of 0.1 M ZnSO4 solution and 1.0 g of BC were well mixed for 10 min. Then 100 mL of KOH solution was added to the mixture while being vigorously stirred continuously
to ensure homogeneity. The mixture was then left to stand for 1 h and then filtered using a Whatman filter. Finally to remove the moisture, the filtered nanocomposite (BC@ZnO) was oven dried
at 100°C for 30 h100. ADSORBENT CHARACTERIZATION Scanning electron microscopy (SEM) (TESCAN-Vega3, Czechia) with scattered X-ray spectroscopy (EDX) was used to study the morphology and
composition of the adsorbent’s elements. The surface functional groups and the crystal structures of metal minerals of the adsorbents before and after HMs adsorption was characterized by
Fourier transform infrared spectroscopy (FTIR) (Tensor II, Bruker, Germany) and X-ray diffractometer (XRD) (Rigaku Ultima IV, Japan) in the 2θ range of 20° to 80°, respectively. The specific
surface area of the adsorbents was investigated by Brunauer, Emmett,—Teller (BET) (Belsorp mini II, Microtrac Bel Corp, Japan). SOIL SAMPLING The studied soil sample was collected from a
depth of 0–30 cm from the Shiraz University Faculty of Agriculture, Bajgah, Fars Province, Iran. The collected soil was air-dried, ground, and passed through a 2 mm sieve. Some
physicochemical properties of the studied soil such as particle size by hydrometric method157, pH using a pH meter in saturated paste158, electrical conductivity (EC) in the extraction using
an electrical conductivity meter159, cation exchange capacity (CEC) by the replacing cations with NaOAc160, organic matter (OM) by the Walkley–Black method161. DTPA extraction was used to
evaluate the available form of iron (Fe), manganese (Mn), zinc (Zn), copper (Cu), lead (Pb), cadmium (Cd) and nickel (Ni),162 and their concentration was determined by the Shimadzu AA-670G
atomic absorption spectroscopy(Table 3). SOIL CONTAMINATION AND INCUBATION The soil was divided into four equal sections, and 100 mg kg-1 of Zn, Cd, Ni, and Pb (as a nitrate source) were
added to each section. The contaminated soil was carefully mixed and incubated for one month at 25°C in a greenhouse condition. Soil samples were irrigated with distilled water during the
incubation period, and the soil moisture content was kept at field capacity by adding distilled water163. To evaluate the effect of biochar and biochar modified with ZnO and MnO2
nanoparticles on the immobilization of Pb, Ni, Cd, and Zn and their bioavailability for ryegrass, a completely randomized design with three replications was conducted under greenhouse
conditions. The treatments consisted of: HMs polluted soil (Control) (CK), pristine biochar (0.5% (BC-0.5%), 1% (BC-1%)), ZnO NPs-modified biochar (0.5% ([email protected]%), 1% (BC@ZnO-1%)) and
MnO2 NPs-modified biochar (0.5% ([email protected]%), 1% (BC@MnO2-1%)). Adsorbents were added to the contaminated soil, and then they were completely mixed with the soil and incubated for two
weeks at a 25 °C temperature and the field capacity moisture. POT EXPERIMENTS AND HMS MEASURMENTS IN PLANT Ryegrass seeds were selected for cultivation and sterilized in a 1% (_V/V)_ NaOCl
solution for five minutes. Then, it was washed several times with deionized water and soaked overnight in deionized water. The intubated soils with adsorbents were transferred to pots and
100 perennial ryegrass seeds were planted in each pot138.The greenhouse temperature was set steady during the day and night at 25 ± 5 °C. Soil moisture was kept at field capacity by adding
distilled water during the growth period (without any leaching). After 7 weeks aerial parts of the plant were harvested and the roots were separated from the soils. To remove any dust
particles, the collected shoots were first properly rinsed with tap water and then with distilled water. the roots were washed with tap water, EDTA, and distilled water. A dilute EDTA
solution was used to remove HMs that may be present on the surface of the roots. The aerial parts and roots were completely dried at 60 °C for 72 h. After weighting, the dried samples were
ground. The concentrations of Cd, Ni, Pb and Zn in the samples were measured by atomic absorption spectrophotometry (AA-6800, Shimadzu) after wet digestion. Equations 1 and 2 were used to
determine the HMs uptake by the root and shoot 163: $${\text{Root uptake }}\left( {\mu {\text{g pot}}^{{ - { 1}}} } \right) = {\text{root concentration }}\left( {\mu {\text{g g}}^{{ - { 1}}}
} \right) \, \times {\text{ root dry matter }}\left( {{\text{g pot}}^{{ - { 1}}} } \right)$$ (1) $${\text{Shoot uptake }}\left( {\mu {\text{g pot}}^{{ - { 1}}} } \right) \, = {\text{ shoot
concentration }}\left( {\mu {\text{g g}}^{{ - { 1}}} } \right) \, \times {\text{ shoot dry matter }}\left( {{\text{g pot}}^{{ - { 1}}} } \right)$$ (2) BIO CONCENTRATION AND TRANSLOCATION
FACTORS The effectiveness of biochar and biochar modified with MnO2 and ZnO nanoparticles to adsorb HMs and minimize their absorption by plant roots and shoots was determined by transport
factor (TF) and bio-concentration factor (BCF) indexes18. $${\text{TF}}=\frac{\mathrm{Concentration\,of\,metal\,in\,shoots }(\mathrm{mg kg}-1)}{\mathrm{Concentration\,of\,metal\,in\,roots
}(\mathrm{mg kg}-1)}$$ (3) $${\text{BCF}}=\frac{Concentration\,of\,metal\,in\,roots\,and\,shoots (mg kg-1)}{concentration\,of\,metal\,in\,soil (mg kg-1)}$$ (4) STATISTICAL ANALYSIS Data were
analyzed using SAS 9.4 software. Differences between treatments were determined following Duncan’s Multiple Range Test (DMRT), (_P_ ≤ 0.05). Probability levels of 1% and 5% (_P_ ≤ 0.01 or
0.05) were selected to test the significance of the differences. Figures were drawn using Excel 2018 software. ETHICAL APPROVAL No ethical issues were violated in this study. CONSENT TO
PUBLISH All the authors gave their consent to publishing this manuscript in your journal. DATA AVAILABILITY All data generated or analyzed during this study is included in this article.
REFERENCES * Bandara, T. _et al._ Chemical and biological immobilization mechanisms of potentially toxic elements in biochar-amended soils. _Crit. Rev. Environ. Sci. Technol._ 50, 903–978
(2020). Article CAS Google Scholar * Lu, T. _et al._ Remediation of cadmium-polluted weakly alkaline dryland soils using iron and manganese oxides for immobilized wheat uptake. _J. Clean.
Prod._ 365, 132794 (2022). Article CAS Google Scholar * Zhang, L., Tang, S., Jiang, C., Jiang, X. & Guan, Y. Simultaneous and efficient capture of inorganic nitrogen and heavy metals
by polyporous layered double hydroxide and biochar composite for agricultural nonpoint pollution control. _ACS Appl. Mater. Interfaces_ 10, 43013–43030 (2018). Article CAS PubMed Google
Scholar * Campos, P. & De la Rosa, J. M. Assessing the effects of biochar on the immobilization of trace elements and plant development in a naturally contaminated soil.
_Sustainability_ 12, 6025 (2020). Article CAS Google Scholar * Gupta, S., Sireesha, S., Sreedhar, I., Patel, C. M. & Anitha, K. L. Latest trends in heavy metal removal from wastewater
by biochar based sorbents. _J. Water Process Eng._ 38, 101561 (2020). Article Google Scholar * Pandey, L. M. Surface engineering of nano-sorbents for the removal of heavy metals:
Interfacial aspects. _J. Environ. Chem. Eng._ 9, 104586 (2021). Article CAS Google Scholar * Bandara, T. _et al._ Chemical and biological immobilization mechanisms of potentially toxic
elements in biochar-amended soils. _Crit. Rev. Environ. Sci. Technol._ 50, 903–978 (2020). Article CAS Google Scholar * Wang, Y. M. _et al._ Simultaneous immobilization of soil Cd(II) and
As(V) by Fe-modified biochar. _Int. J. Environ. Res. Public Health_ 17, 1–12 (2020). CAS Google Scholar * Gupta, S., Sireesha, S., Sreedhar, I., Patel, C. M. & Anitha, K. L. Latest
trends in heavy metal removal from wastewater by biochar based sorbents. _J. Water Process Eng._ 38, 101561 (2020). Article Google Scholar * Gueret Yadiberet Menzembere, E. R. _et al._
Insight into modified biochars and their immobilizing effects on heavy metal(loids) in contaminated soils—potentials and influencing factors: A review. _Pedosphere_
https://doi.org/10.1016/J.PEDSPH.2022.06.030 (2022). Article Google Scholar * Azeem, M. _et al._ Effects of sheep bone biochar on soil quality, maize growth, and fractionation and
phytoavailability of Cd and Zn in a mining-contaminated soil. _Chemosphere_ 282, 131016 (2021). Article CAS PubMed Google Scholar * Qiu, M. _et al._ Biochar for the removal of
contaminants from soil and water: A review. _Biochar_ 4, 1–25 (2022). Article Google Scholar * Zhang, G. _et al._ Positive and negative effects of nanoscale zero-valent iron-enriched
biochar on sulfamethoxazole remediation in contaminated soil. _Ecotoxicol. Environ. Saf._ 246, 114133 (2022). Article CAS PubMed Google Scholar * Alaboudi, K. A., Ahmed, B. & Brodie,
G. Effect of biochar on Pb, Cd and Cr availability and maize growth in artificial contaminated soil. _Ann. Agric. Sci._ 64, 95–102 (2019). Article Google Scholar * Gong, H., Zhao, L.,
Rui, X., Hu, J. & Zhu, N. A review of pristine and modified biochar immobilizing typical heavy metals in soil: Applications and challenges. _J. Hazard. Mater._ 432, 128668 (2022).
Article CAS PubMed Google Scholar * Rahim, H. U., Akbar, W. A. & Alatalo, J. M. A comprehensive literature review on cadmium (Cd) status in the soil environment and its
immobilization by biochar-based materials. _Agronomy_ 12(4), 877 (2022). Article CAS Google Scholar * Wu, P. _et al._ Unraveling the molecular mechanisms of Cd sorption onto MnOx-loaded
biochar produced from the Mn-hyperaccumulator Phytolacca americana. _J. Hazard. Mater._ 423, 127157 (2022). Article CAS PubMed Google Scholar * Wang, Z., Shen, R., Ji, S., Xie, L. &
Zhang, H. Effects of biochar derived from sewage sludge and sewage sludge/cotton stalks on the immobilization and phytoavailability of Pb, Cu, and Zn in sandy loam soil. _J. Hazard. Mater._
419, 126468 (2021). Article CAS PubMed Google Scholar * Jiang, J., Xu, R. K., Jiang, T. Y. & Li, Z. Immobilization of Cu(II), Pb(II) and Cd(II) by the addition of rice straw derived
biochar to a simulated polluted Ultisol. _J. Hazard. Mater._ 229–230, 145–150 (2012). Article PubMed Google Scholar * Zhang, Y. _et al._ Enhanced remediation of cadmium-polluted soil and
water using facilely prepared MnO2-coated rice husk biomass. _Chem. Eng. J._ 457, 141311 (2023). Article ADS CAS Google Scholar * Li, Q. _et al._ Simultaneous immobilization of arsenic,
lead and cadmium by magnesium-aluminum modified biochar in mining soil. _J. Environ. Manage._ 310, 114792 (2022). Article CAS PubMed Google Scholar * Wu, F. _et al._ Effects of zinc
oxide nanoparticles on arsenic stress in rice (_Oryza sativa_ L.): germination, early growth, and arsenic uptake. _Environ. Sci. Pollut. Res._ 27, 26974–26981 (2020). Article CAS Google
Scholar * Zhang, H. _et al._ Efficient removal of heavy metal ions from wastewater and fixation of heavy metals in soil by manganese dioxide nanosorbents with tailored hollow mesoporous
structure. _Chem. Eng. J._ 459, 141583 (2023). Article ADS CAS Google Scholar * Meng, Z., Huang, S., Xu, T., Lin, Z. & Wu, J. Competitive adsorption, immobilization, and desorption
risks of Cd, Ni, and Cu in saturated-unsaturated soils by biochar under combined aging. _J. Hazard. Mater._ 434, 128903 (2022). Article CAS PubMed Google Scholar * Miandad, R. _et al._
Journal of Colloid and Interface Science Untapped conversion of plastic waste char into carbon-metal LDOs for the adsorption of Congo red. _J. Colloid Interface Sci._ 511, 402–410 (2018).
Article ADS CAS PubMed Google Scholar * Fan, Z. _et al._ Co-pyrolysis technology for enhancing the functionality of sewage sludge biochar and immobilizing heavy metals. _Chemosphere_
317, 137929 (2023). Article CAS PubMed Google Scholar * Seleiman, M. F. _et al._ Effects of ZnO nanoparticles and biochar of rice straw and cow manure on characteristics of contaminated
soil and sunflower productivity, oil quality, and heavy metals uptake. _Agronomy_ 10, 1–21 (2020). Article Google Scholar * Xu, D. M., Fu, R. B., Wang, J. X., Shi, Y. X. & Guo, X. P.
Chemical stabilization remediation for heavy metals in contaminated soils on the latest decade: Available stabilizing materials and associated evaluation methods-A critical review. _J.
Clean. Prod._ 321, (2021). * Baragaño, D. _et al._ Application of biochar, compost and ZVI nanoparticles for the remediation of As, Cu, Pb and Zn polluted soil. _Environ. Sci. Pollut. Res._
27, 33681–33691 (2020). Article Google Scholar * Tu, C. _et al._ Biochar and bacteria inoculated biochar enhanced Cd and Cu immobilization and enzymatic activity in a polluted soil.
_Environ. Int._ 137, (2020). * Tang, H. _et al._ Biochar: A promising soil amendment to mitigate heavy metals toxicity in plants. _Not. Bot. Horti Agrobot. Cluj-Napoca_ 50, 1–24 (2022).
Google Scholar * Batool, M. _et al._ Microbial-assisted soil chromium immobilization through zinc and iron-enriched rice husk biochar. _Front. Microbiol._ 13, (2022). * Mandal, S. _et al._
Synergistic construction of green tea biochar supported nZVI for immobilization of lead in soil: A mechanistic investigation. _Environ. Int._ 135, 105374 (2020). Article CAS PubMed Google
Scholar * Qu, J. _et al._ Stabilization of lead and cadmium in soil by sulfur-iron functionalized biochar: Performance, mechanisms and microbial community evolution. _J. Hazard. Mater._
425, (2022). * Fan, J. _et al._ Remediation of cadmium and lead polluted soil using thiol-modified biochar. _J. Hazard. Mater._ 388, 122037 (2020). Article CAS PubMed Google Scholar *
Gholizadeh, M. & Hu, X. Removal of heavy metals from soil with biochar composite: A critical review of the mechanism. _J. Environ. Chem. Eng._ 9, 105830 (2021). Article CAS Google
Scholar * Tan, W. T. _et al._ Simultaneous alleviation of Cd availability in contaminated soil and accumulation in rice (Oryza sativa L.) by Fe-Mn oxide-modified biochar. _Sci. Total
Environ._ 858, 159730 (2023). Article ADS CAS PubMed Google Scholar * Wu, Z., Chen, X., Yuan, B. & Fu, M. L. A facile foaming-polymerization strategy to prepare 3D MnO2 modified
biochar-based porous hydrogels for efficient removal of Cd(II) and Pb(II). _Chemosphere_ 239, 124745 (2020). Article CAS PubMed Google Scholar * Kamali, N., Rashidi, A., Mirabi, M. &
Ali, M. Journal of Water Process Engineering Comparison of micro and nano MgO-functionalized vinasse biochar in phosphate removal: Micro-nano particle development, RSM optimization, and
potential fertilizer. _J. Water Process Eng._ 39, 101741. https://doi.org/10.1016/j.jwpe.2020.101741 (2020). Article Google Scholar * Hussain, T. _et al._ In-situ stabilization of
potentially toxic elements in two industrial polluted soils ameliorated with rock phosphate-modified biochars. _Environ. Pollut._ 309, 119733 (2022). Article CAS PubMed Google Scholar *
Ahuja, R., Kalia, A., Sikka, R. & Chaitra, P. Nano Modifications of Biochar to Enhance Heavy Metal Adsorption from Wastewaters: A Review. _ACS Omega_
https://doi.org/10.1021/acsomega.2c05117 (2022). Article PubMed PubMed Central Google Scholar * Cai, C., Zhao, M., Yu, Z., Rong, H. & Zhang, C. Utilization of nanomaterials for
in-situ remediation of heavy metal(loid) contaminated sediments: A review. _Sci. Total Environ._ 662, 205–217 (2019). Article ADS CAS PubMed Google Scholar * Antonangelo, J. A. &
Zhang, H. Heavy metal phytoavailability in a contaminated soil of northeastern Oklahoma as affected by biochar amendment. _Environ. Sci. Pollut. Res._ 26, 33582–33593 (2019). Article Google
Scholar * Hussain, F., Hadi, F. & Rongliang, Q. Effects of zinc oxide nanoparticles on antioxidants, chlorophyll contents, and proline in Persicaria hydropiper L. and its potential for
Pb phytoremediation. _Environ. Sci. Pollut. Res._ 28, 34697–34713 (2021). Article CAS Google Scholar * Li, J. _et al._ Passivation of multiple heavy metals in lead–zinc tailings
facilitated by straw biochar-loaded N-doped carbon aerogel nanoparticles: Mechanisms and microbial community evolution. _Sci. Total Environ._ 803, 149866 (2022). Article ADS CAS PubMed
Google Scholar * Chen, J., Dong, H., Tian, R. & Li, R. Remediation of Trichloroethylene-Contaminated Groundwater by Sulfide-Modified Nanoscale Zero-Valent Iron Supported on Biochar :
Investigation of Critical Factors. (2020). * Zhang, J. Y. _et al._ Nano-Fe3O4-modified biochar promotes the formation of iron plaque and cadmium immobilization in rice root. _Chemosphere_
276, 130212 (2021). Article CAS PubMed Google Scholar * Xing, L.-B. _et al._ Three dimensional nitrogen-doped graphene aerogels functionalized with melamine for multifunctional
applications in supercapacitors and adsorption. _J. Solid State Chem._ 230, 224–232 (2015). Article ADS CAS Google Scholar * Yuan, P., Wang, J., Pan, Y., Shen, B. & Wu, C. Review of
biochar for the management of contaminated soil: Preparation, application and prospect. _Sci. Total Environ._ 659, 473–490 (2019). Article ADS CAS PubMed Google Scholar * Qiu, Y. _et
al._ Immobilization of manganese dioxide nanoparticles on modified poly 2,4-dichlorostyrene microspheres: A highly efficient and recyclable catalyst for borrowing hydrogen reactions. _Org.
Chem. Front._ 6, 3420–3427 (2019). Article CAS Google Scholar * Zhang, Y., Zhang, Y., Akakuru, O. U., Xu, X. & Wu, A. Research progress and mechanism of nanomaterials-mediated in-situ
remediation of cadmium-contaminated soil: A critical review. _J. Environ. Sci. (China)_ 104, 351–364 (2021). Article CAS PubMed Google Scholar * Xiao, F. _et al._ Production of granular
activated carbon by thermal air oxidation of biomass charcoal/biochar for water treatment in rural communities: A mechanistic investigation. _Chem. Eng. J. Adv._ 4, 100035 (2020). Article
CAS Google Scholar * Ali, S. _et al._ Combined use of biochar and zinc oxide nanoparticle foliar spray improved the plant growth and decreased the cadmium accumulation in rice (Oryza
sativa L.) plant. _Environ. Sci. Pollut. Res._ 26, 11288–11299 (2019). Article CAS Google Scholar * Rizwan, M., Ali, S., Rehman, M. Z. ur, Javed, M. R. & Bashir, A. Lead toxicity in
cereals and its management strategies: a critical review. _Water. Air. Soil Pollut._ 229, (2018). * Faheem, _et al._ Adsorption performance and possible mechanism. _J. Taiwan Inst. Chem.
Eng._ 66, 313–320 (2016). Article CAS Google Scholar * Luo, Y. _et al._ Development of phosphorus composite biochar for simultaneous enhanced carbon sink and heavy metal immobilization in
soil. _Sci. Total Environ._ 831, 154845 (2022). Article ADS CAS PubMed Google Scholar * Zhou, P. _et al._ Application of nanoparticles alleviates heavy metals stress and promotes plant
growth: An overview. _Nanomaterials_ 11, 1–18 (2021). Google Scholar * Gao, R. _et al._ Remediation of Pb, Cd, and Cu contaminated soil by co-pyrolysis biochar derived from rape straw and
orthophosphate: Speciation transformation, risk evaluation and mechanism inquiry. _Sci. Total Environ._ 730, 139119 (2020). Article ADS CAS PubMed Google Scholar * Wan, X., Li, C. &
Parikh, S. J. Simultaneous removal of arsenic, cadmium, and lead from soil by iron-modified magnetic biochar. _Environ. Pollut._ 261, 114157 (2020). Article CAS PubMed Google Scholar *
Qiao, Y., Wu, J., Xu, Y., Fang, Z. & Zheng, L. Remediation of cadmium in soil by biochar-supported iron phosphate nanoparticles. _Ecol. Eng._ 106, 515–522 (2017). Article Google Scholar
* Qian, W., Liang, J. Y., Zhang, W. X., Huang, S. T. & Diao, Z. H. A porous biochar supported nanoscale zero-valent iron material highly efficient for the simultaneous remediation of
cadmium and lead contaminated soil. _J. Environ. Sci. (China)_ 113, 231–241 (2022). Article CAS PubMed Google Scholar * Li, Y. _et al._ Effects of α-Fe2O3 modified chicken manure biochar
on the availability of multiple heavy metals and soil biochemical properties. _J. Environ. Chem. Eng._ 11, 109922 (2023). Article ADS CAS Google Scholar * Rees, F., Germain, C.,
Sterckeman, T. & Morel, J. L. Plant growth and metal uptake by a non-hyperaccumulating species (Lolium perenne) and a Cd-Zn hyperaccumulator (Noccaea caerulescens) in contaminated soils
amended with biochar. _Plant Soil_ 395, 57–73 (2015). Article CAS Google Scholar * Zhang, J., Yang, N., Geng, Y., Zhou, J. & Lei, J. Effects of the combined pollution of cadmium, lead
and zinc on the phytoextraction efficiency of ryegrass (_Lolium perenne_ L.). _RSC Adv._ 9, 20603–20611 (2019). Article ADS CAS PubMed PubMed Central Google Scholar * Li, G. _et al._
Effect of biochar on Cd and pyrene removal and bacteria communities variations in soils with culturing ryegrass (_Lolium perenne _L.). _Environ. Pollut._ 265, 114887 (2020). Article CAS
PubMed Google Scholar * Liang, J. _et al._ Amorphous MnO2 modified biochar derived from aerobically composted swine manure for adsorption of Pb(II) and Cd(II). _ACS Sustain. Chem. Eng._ 5,
5049–5058 (2017). Article CAS Google Scholar * Wang, H. _et al._ High-efficiency removal capacities and quantitative adsorption mechanisms of Cd2+ by thermally modified biochars derived
from different feedstocks. _Chemosphere_ 272, 129594 (2021). Article CAS PubMed Google Scholar * Lee, H. S. & Shin, H. S. Competitive adsorption of heavy metals onto modified
biochars: Comparison of biochar properties and modification methods. _J. Environ. Manage._ 299, 113651 (2021). Article CAS PubMed Google Scholar * Reddy, N. R. _et al._ Photocatalytic
hydrogen production by ternary heterojunction composites of silver nanoparticles doped FCNT-TiO2. _J. Environ. Manage._ 286, 112130 (2021). Article CAS PubMed Google Scholar * Kończyk,
J., Kluziak, K. & Kołodyńska, D. Adsorption of vanadium (V) ions from the aqueous solutions on different biomass-derived biochars. _J. Environ. Manage._ 313, 114958 (2022). Article
PubMed Google Scholar * Chen, D. _et al._ The mechanism of cadmium sorption by sulphur-modified wheat straw biochar and its application cadmium-contaminated soil. _Sci. Total Environ._
714, 136550 (2020). Article ADS CAS PubMed Google Scholar * Sidiras, D., Batzias, F., Konstantinou, I. & Tsapatsis, M. Simulation of autohydrolysis effect on adsorptivity of wheat
straw in the case of oil spill cleaning. _Chem. Eng. Res. Des._ 92, 1781–1791 (2014). Article CAS Google Scholar * Gao, M., Zhang, Y., Gong, X., Song, Z. & Guo, Z. Removal mechanism
of di-n-butyl phthalate and oxytetracycline from aqueous solutions by nano-manganese dioxide modified biochar. _Environ. Sci. Pollut. Res._ 25, 7796–7807 (2018). Article CAS Google Scholar
* Kumar, T. S. M. _et al._ Characterization, thermal and antimicrobial properties of hybrid cellulose nanocomposite films with in-situ generated copper nanoparticles in tamarindus indica
nut powder. _J. Polym. Environ._ 29, 1134–1142 (2021). Article CAS Google Scholar * Lin, Q. _et al._ Effectively removal of cationic and anionic dyes by pH-sensitive amphoteric adsorbent
derived from agricultural waste-wheat straw. _J. Taiwan Inst. Chem. Eng._ 76, 65–72 (2017). Article CAS Google Scholar * He, X. _et al._ NonDestructive discrimination of ship deck paint
using attenuated total reflection-fourier transform infrared (ATR-FTIR) spectroscopy with chemometric analysis. _Anal. Lett._ 53, 2761–2774 (2020). Article CAS Google Scholar * Tiwari, A.
K. _et al._ Innovative investigation of zinc oxide nanoparticles used in dentistry. _Crystals_ 12(8), 1063 (2022). Article CAS Google Scholar * Hu, H. _et al._ Nano-ZnO functionalized
biochar as a superhydrophobic biosorbent for selective recovery of low-concentration Re ( VII ) from strong acidic solutions. _Miner. Eng._ 142, 105885 (2019). Article CAS Google Scholar
* Wang, Y., Wang, L., Deng, X. & Gao, H. A facile pyrolysis synthesis of biochar/ZnO passivator: immobilization behavior and mechanisms for Cu (II) in soil. _Environ. Sci. Pollut. Res._
27, 1888–1897 (2020). Article CAS Google Scholar * Cui, P., Lee, J., Hwang, E. & Lee, H. One-pot reduction of graphene oxide at subzero temperatures. _Chem. Commun._ 47, 12370–12372
(2011). Article CAS Google Scholar * Kumar, A. _et al._ Performance evaluation of crop residue and kitchen waste-derived biochar for eco-efficient removal of arsenic from soils of the
Indo-Gangetic plain: A step towards sustainable pollution management. _Environ. Res._ 200, 111758 (2021). Article CAS PubMed Google Scholar * Zhang, H. _et al._ Enhanced removal of heavy
metal ions from aqueous solution using manganese dioxide-loaded biochar: Behavior and mechanism. _Sci. Rep._ 10, 1–13 (2020). Google Scholar * Ahmed, W. _et al._ Enhanced adsorption of
aqueous Pb(II) by modified biochar produced through pyrolysis of watermelon seeds. _Sci. Total Environ._ 784, 147136 (2021). Article ADS CAS PubMed Google Scholar * Wan, S. _et al._
Manganese oxide nanoparticles impregnated graphene oxide aggregates for cadmium and copper remediation. _Chem. Eng. J._ 350, 1135–1143 (2018). Article ADS CAS Google Scholar * Mosa, A.,
El-Ghamry, A. & Tolba, M. Functionalized biochar derived from heavy metal rich feedstock: Phosphate recovery and reusing the exhausted biochar as an enriched soil amendment.
_Chemosphere_ 198, 351–363 (2018). Article ADS CAS PubMed Google Scholar * Fernández-González, R., Martín-Lara, M. A., Moreno, J. A., Blázquez, G. & Calero, M. Effective removal of
zinc from industrial plating wastewater using hydrolyzed olive cake: Scale-up and preparation of zinc-Based biochar. _J. Clean. Prod._ 227, 634–644 (2019). Article Google Scholar * Tho, P.
T. _et al._ Enhanced simultaneous adsorption of As(iii), Cd(ii), Pb(ii) and Cr(vi) ions from aqueous solution using cassava root husk-derived biochar loaded with ZnO nanoparticles. _RSC
Adv._ 11, 18881–18897 (2021). Article ADS CAS PubMed PubMed Central Google Scholar * Wang, Y., Wang, L., Deng, X. & Gao, H. A facile pyrolysis synthesis of biochar/ZnO passivator:
immobilization behavior and mechanisms for Cu (II) in soil. _Environ. Sci. Pollut. Res._ 27, 1888–1897 (2020). Article CAS Google Scholar * Nakarmi, A. _et al._ Benign zinc oxide
betaine-modified biochar nanocomposites for phosphate removal from aqueous solutions. _J. Environ. Manage._ 272, 111048 (2020). Article CAS PubMed PubMed Central Google Scholar *
Mustapha, S. _et al._ The role of kaolin and kaolin/ZnO nanoadsorbents in adsorption studies for tannery wastewater treatment. _Sci. Rep._ 10, 1–22 (2020). Article Google Scholar * Mazhar,
Z. _et al._ Efficacy of ZnO nanoparticles in Zn fortification and partitioning of wheat and rice grains under salt stress. _Sci. Rep._ 13, 1–11 (2023). Article Google Scholar * Wang, S.
_et al._ Carboxymethyl cellulose stabilized ZnO/biochar nanocomposites: Enhanced adsorption and inhibited photocatalytic degradation of methylene blue. _Chemosphere_ 197, 20–25 (2018).
Article ADS CAS PubMed Google Scholar * Gabriela, M. _et al._ Relationship of the physicochemical properties of novel ZnO / biochar composites to their ef fi ciencies in the
degradation of sulfamethoxazole and methyl orange. _Sci. Total Environ._ 748, 141381 (2020). Article ADS Google Scholar * Si, W., Wang, Y., Zhao, S., Hu, F. & Li, J. A Facile Method
for in Situ Preparation of the MnO2/LaMnO3 Catalyst for the Removal of Toluene. _Environ. Sci. Technol._ 50, 4572–4578 (2016). Article ADS CAS PubMed Google Scholar * Luo, X. L., Xu, J.
J., Zhao, W. & Chen, H. Y. A novel glucose ENFET based on the special reactivity of MnO2 nanoparticles. _Biosens. Bioelectron._ 19, 1295–1300 (2004). Article CAS PubMed Google
Scholar * Regmi, P. _et al._ Removal of copper and cadmium from aqueous solution using switchgrass biochar produced via hydrothermal carbonization process. _J. Environ. Manage._ 109, 61–69
(2012). Article CAS PubMed Google Scholar * Zhou, L. _et al._ Adsorption properties of nano-MnO2-biochar composites for copper in aqueous solution. _Molecules_ 22, 1–13 (2017). Article
Google Scholar * Khezami, L. _et al._ Effect of aluminum loading on structural and morphological characteristics of ZnO nanoparticles for heavy metal ion elimination. _Environ. Sci. Pollut.
Res._ 27, 3086–3099 (2020). Article CAS Google Scholar * Kamaraj, M., Srinivasan, N. R., Assefa, G., Adugna, A. T. & Kebede, M. Facile development of sunlit ZnO
nanoparticles-activated carbon hybrid from pernicious weed as an operative nano-adsorbent for removal of methylene blue and chromium from aqueous solution: Extended application in tannery
industrial wastewater. _Environ. Technol. Innov._ 17, 100540 (2020). Article CAS Google Scholar * Tariq, M. A. _et al._ Effective sequestration of Cr (VI) from wastewater using
nanocomposite of ZnO with cotton stalks biochar: modeling, kinetics, and reusability. _Environ. Sci. Pollut. Res._ 27, 33821–33834 (2020). Article CAS Google Scholar * Alhan, S. _et al._
Potential use of ZnO@activated carbon nanocomposites for the adsorptive removal of Cd2+ ions in aqueous solutions. _Environ. Res._ 173, 411–418 (2019). Article CAS PubMed Google Scholar
* Adorna, J., Borines, M., Dang, V. D. & Doong, R. A. Coconut shell derived activated biochar–manganese dioxide nanocomposites for high performance capacitive deionization.
_Desalination_ 492, 114602 (2020). Article CAS Google Scholar * Kamal, A. _et al._ Ball-milled synthesis of maize biochar-ZnO nanocomposite (MB-ZnO) and estimation of its photocatalytic
ability against different organic and inorganic pollutants. _J. Saudi Chem. Soc._ 26, 101445 (2022). Article CAS Google Scholar * Ashraf, M. A., Peng, W., Zare, Y. & Rhee, K. Y.
Effects of size and aggregation/agglomeration of nanoparticles on the interfacial/interphase properties and tensile strength of polymer nanocomposites. _Nanoscale Res. Lett._ 13, (2018). *
Ali, A. _et al._ Apricot shell-and apple tree-derived biochar affect the fractionation and bioavailability of Zn and Cd as well as the microbial activity in smelter contaminated soil.
_Environ. Pollut._ 264, 114773 (2020). Article CAS PubMed Google Scholar * Palansooriya, K. N. _et al._ Soil amendments for immobilization of potentially toxic elements in contaminated
soils: A critical review. _Environ. Int._ 134, 105046 (2020). Article CAS PubMed Google Scholar * Xu, C. _et al._ Evaluation of biochar pyrolyzed from kitchen waste corn straw and peanut
hulls on immobilization of Pb and Cd in contaminated soil. _Environ. Pollut._ 261, 114133 (2020). Article CAS PubMed Google Scholar * Rizwan, M. _et al._ Alleviation of cadmium
accumulation in maize (_Zea mays _L.) by foliar spray of zinc oxide nanoparticles and biochar to contaminated soil. _Environ. Pollut._ 248, 358–367 (2019). Article CAS PubMed Google
Scholar * Kareem, H. A. _et al._ Antagonistic impact on cadmium stress in alfalfa supplemented with nano-zinc oxide and biochar via upregulating metal detoxification. _J. Hazard. Mater._
443, 130309 (2023). Article CAS PubMed Google Scholar * Ali, S. _et al._ Combined use of biochar and zinc oxide nanoparticle foliar spray improved the plant growth and decreased the
cadmium accumulation in rice (Oryza sativa L.) plant. _Environ. Sci. Pollut. Res._ 26, 11288–11299 (2019). Article CAS Google Scholar * Shahbaz, A. K. _et al._ Improvement in
productivity, nutritional quality, and antioxidative defense mechanisms of sunflower (Helianthus annuus L.) and maize (Zea mays L.) in nickel contaminated soil amended with different biochar
and zeolite ratios. _J. Environ. Manage._ 218, 256–270 (2018). Article CAS PubMed Google Scholar * Novak, J. M. _et al._ Impact of biochar amendment on fertility of a southeastern
coastal plain soil. _Soil Sci._ 174, 105–112 (2009). Article ADS CAS Google Scholar * Hartley, W., Dickinson, N. M., Riby, P. & Lepp, N. W. Arsenic mobility in brownfield soils
amended with green waste compost or biochar and planted with Miscanthus. _Environ. Pollut._ 157, 2654–2662 (2009). Article CAS PubMed Google Scholar * Beesley, L. _et al._ A review of
biochars’ potential role in the remediation, revegetation and restoration of contaminated soils. _Environ. Pollut._ 159, 3269–3282 (2011). Article CAS PubMed Google Scholar * Yu, Z. _et
al._ Effects of manganese oxide-modified biochar composites on arsenic speciation and accumulation in an indica rice (Oryza sativa L.) cultivar. _Chemosphere_ 168, 341–349 (2017). Article
ADS CAS PubMed Google Scholar * Hossain, M. K., Strezov, V. & Nelson, P. F. Comparative assessment of the effect of wastewater sludge biochar on growth, yield and metal
bioaccumulation of cherry tomato. _Pedosphere_ 25, 680–685 (2015). Article CAS Google Scholar * Burd, G. I., Dixon, D. G. & Glick, B. R. Plant growth-promoting bacteria that decrease
heavy metal toxicity in plants. _Can. J. Microbiol._ 46, 237–245 (2000). Article CAS PubMed Google Scholar * Hussain, A. _et al._ Zinc oxide nanoparticles alter the wheat physiological
response and reduce the cadmium uptake by plants. _Environ. Pollut._ 242, 1518–1526 (2018). Article CAS PubMed Google Scholar * Rizwan, M. _et al._ Influence of biochar amendment and
foliar application of iron oxide nanoparticles on growth, photosynthesis, and cadmium accumulation in rice biomass. _J. Soils Sediments_ 19, 3749–3759 (2019). Article CAS Google Scholar *
Yu, Z. _et al._ Effects of manganese oxide-modified biochar composites on arsenic speciation and accumulation in an indica rice (Oryza sativa L.) cultivar. _Chemosphere_ 168, 341–349
(2017). Article ADS CAS PubMed Google Scholar * Lin, J., Sun, M., Su, B., Owens, G. & Chen, Z. Immobilization of cadmium in polluted soils by phytogenic iron oxide nanoparticles.
_Sci. Total Environ._ 659, 491–498 (2019). Article ADS CAS PubMed Google Scholar * Yang, W.-T. _et al._ Effects of a combined amendment on Pb, Cd, and as availability and accumulation
in rice planted in contaminated paddy soil. _Soil Sediment Contam. An Int. J._ 26, 70–83 (2017). Article CAS Google Scholar * Bashir, A. _et al._ Application of co-composted farm manure
and biochar increased the wheat growth and decreased cadmium accumulation in plants under different water regimes. _Chemosphere_ 246, 125809 (2020). Article CAS PubMed Google Scholar *
Azimzadeh, Y., Shirvani, M. & Shariatmadari, H. Green manure and overlapped rhizosphere effects on Pb chemical forms in soil and plant uptake in maize/canola intercrop systems: a
rhizobox study. _Soil Sediment Contam. An Int. J._ 23, 677–690 (2014). Article CAS Google Scholar * Dong, X. _et al._ The sorption of heavy metals on thermally treated sediments with high
organic matter content. _Bioresour. Technol._ 160, 123–128 (2014). Article ADS CAS PubMed Google Scholar * Aborisade, M. A. _et al._ Carbothermal reduction synthesis of
eggshell-biochar modified with nanoscale zerovalent iron/activated carbon for remediation of soil polluted with lead and cadmium. _Environ. Nanotechnology, Monit. Manag._ 18, (2022). *
Taneez, M. & Hurel, C. A review on the potential uses of red mud as amendment for pollution control in environmental media. _Environ. Sci. Pollut. Res._ 26, 22106–22125 (2019). Article
CAS Google Scholar * Xu, Y. _et al._ A further inquiry into co-pyrolysis of straws with manures for heavy metal immobilization in manure-derived biochars. _J. Hazard. Mater._ 380, 120870
(2019). Article CAS PubMed Google Scholar * Li, Z. _et al._ Phytolith-rich biochar increases cotton biomass and silicon-mineralomass in a highly weathered soil. _J. Plant Nutr. Soil
Sci._ 181, 537–546 (2018). Article CAS Google Scholar * Lu, K. _et al._ Effect of bamboo and rice straw biochars on the bioavailability of Cd, Cu, Pb and Zn to Sedum plumbizincicola.
_Agric. Ecosyst. Environ._ 191, 124–132 (2014). Article CAS Google Scholar * Wang, Y. _et al._ Effects of biochar on growth, and heavy metals accumulation of moso bamboo (Phyllostachy
pubescens), soil physical properties, and heavy metals solubility in soil. _Chemosphere_ 219, 510–516 (2019). Article ADS CAS PubMed Google Scholar * Awad, M., Moustafa-Farag, M., Wei,
L., Huang, Q. & Liu, Z. Effect of garden waste biochar on the bioavailability of heavy metals and growth of _Brassica juncea_ (L.) in a multi-contaminated soil. _Arab. J. Geosci._ 13,
(2020). * Algethami, J. S., Irshad, M. K., Javed, W., Alhamami, M. A. M. & Ibrahim, M. Iron-modified biochar improves plant physiology, soil nutritional status and mitigates Pb and
Cd-hazard in wheat (Triticum aestivum L.). _Front. Plant Sci._ 14, 1–12 (2023). * Pittman, J. K. Managing the manganese: molecular mechanisms of manganese transport and homeostasis. _New
Phytol._ 167, 733–742 (2005). Article CAS PubMed Google Scholar * Clemens, S. Toxic metal accumulation, responses to exposure and mechanisms of tolerance in plants. _Biochimie_ 88,
1707–1719 (2006). Article CAS PubMed Google Scholar * Zhou, J. _et al._ Effects of zinc application on cadmium (Cd) accumulation and plant growth through modulation of the antioxidant
system and translocation of Cd in low-and high-Cd wheat cultivars. _Environ. Pollut._ 265, 115045 (2020). Article CAS PubMed Google Scholar * Wang, Y. _et al._ Soil application of
manganese sulfate could reduce wheat Cd accumulation in Cd contaminated soil by the modulation of the key tissues and ionomic of wheat. _Sci. Total Environ._ 770, 145328 (2021). Article ADS
CAS PubMed Google Scholar * Wang, Y. Y. _et al._ Simultaneous alleviation of Sb and Cd availability in contaminated soil and accumulation in Lolium multiflorum Lam. After amendment with
Fe–Mn-Modified biochar. _J. Clean. Prod._ 231, 556–564 (2019). Article ADS CAS Google Scholar * Ali, S. _et al._ Combined use of biochar and zinc oxide nanoparticle foliar spray
improved the plant growth and decreased the cadmium accumulation in rice (_Oryza sativa_ L.) plant. _Environ. Sci. Pollut. Res._ 26, 11288–11299 (2019). Article CAS Google Scholar *
Rizwan, M. _et al._ Alleviation of cadmium accumulation in maize (_Zea mays _L.) by foliar spray of zinc oxide nanoparticles and biochar to contaminated soil. _Environ. Pollut._ 248, 358–367
(2019). Article CAS PubMed Google Scholar * Faizan, M., Faraz, A., Mir, A. R. & Hayat, S. Role of zinc oxide nanoparticles in countering negative effects generated by cadmium in
Lycopersicon esculentum. _J. Plant Growth Regul._ 40, 101–115 (2021). Article CAS Google Scholar * Venkatachalam, P. _et al._ Zinc oxide nanoparticles (ZnONPs) alleviate heavy
metal-induced toxicity in Leucaena leucocephala seedlings: a physiochemical analysis. _Plant Physiol. Biochem._ 110, 59–69 (2017). Article CAS PubMed Google Scholar * Huang, G., Ding,
C., Zhou, Z., Zhang, T. & Wang, X. A tillering application of zinc fertilizer based on basal stabilization reduces Cd accumulation in rice (_Oryza sativa_ L.). _Ecotoxicol. Environ.
Saf._ 167, 338–344 (2019). Article CAS PubMed Google Scholar * Pandey, V. C. Phytoremediation of heavy metals from fly ash pond by Azolla caroliniana. _Ecotoxicol. Environ. Saf._ 82,
8–12 (2012). Article CAS PubMed Google Scholar * Hart, J. J., Welch, R. M., Norvell, W. A., Clarke, J. M. & Kochian, L. V. Zinc effects on cadmium accumulation and partitioning in
near-isogenic lines of durum wheat that differ in grain cadmium concentration. _New Phytol._ 167, 391–401 (2005). Article CAS PubMed Google Scholar * Wu, C. _et al._ Effect of
sulfur-iron modified biochar on the available cadmium and bacterial community structure in contaminated soils. _Sci. Total Environ._ 647, 1158–1168 (2019). Article ADS CAS PubMed Google
Scholar * Wu, C. _et al._ Effect of sulfur-iron modified biochar on the available cadmium and bacterial community structure in contaminated soils. _Sci. Total Environ._ 647, 1158–1168
(2019). Article ADS CAS PubMed Google Scholar * Houben, D., Evrard, L. & Sonnet, P. Beneficial effects of biochar application to contaminated soils on the bioavailability of Cd, Pb
and Zn and the biomass production of rapeseed (Brassica napus L.). _Biomass and Bioenergy_ 57, 196–204 (2013). Article CAS Google Scholar * Namgay, T., Singh, B. & Singh, B. P.
Influence of biochar application to soil on the availability of As, Cd, Cu, Pb, and Zn to maize (Zea mays L.). _Soil Res._ 48, 638–647 (2010). Article CAS Google Scholar * Norini, M. P.
_et al._ Mobility of Pb, Zn, Ba, As and Cd toward soil pore water and plants (willow and ryegrass) from a mine soil amended with biochar. _J. Environ. Manage._ 232, 117–130 (2019). Article
CAS PubMed Google Scholar * Jiang, L., Yi, X., Xu, B. & Lai, K. Effect of wheat straw derived biochar on immobilization of Cd and Pb in single- and binary-metal contaminated soil.
_Hum. Ecol. Risk Assess._ 26, 2420–2433 (2020). Article CAS Google Scholar * Pescatore, A., Grassi, C., Rizzo, A. M., Orlandini, S. & Napoli, M. Effects of biochar on berseem clover
(Trifolium alexandrinum, L.) growth and heavy metal (Cd, Cr, Cu, Ni, Pb, and Zn) accumulation. _Chemosphere_ 287, 131986 (2022). Article CAS PubMed Google Scholar * Chen, L. _et al._
Effects of biochar on the dynamic immobilization of Cd and Cu and rice accumulation in soils with different acidity levels. _J. Clean. Prod._ 372, 133730 (2022). Article CAS Google Scholar
* Zhang, G. _et al._ Effects of biochars on the availability of heavy metals to ryegrass in an alkaline contaminated soil. _Environ. Pollut._ 218, 513–522 (2016). Article CAS PubMed
Google Scholar * Zhang, G. _et al._ Effect of biochar on the presence of nutrients and ryegrass growth in the soil from an abandoned indigenous coking site: The potential role of biochar in
the revegetation of contaminated site. _Sci. Total Environ._ 601–602, 469–477 (2017). Article ADS PubMed Google Scholar * Zhang, H. _et al._ Enhanced removal of heavy metal ions from
aqueous solution using manganese dioxide-loaded biochar: Behavior and mechanism. _Sci. Rep._ 10, 1–13 (2020). Google Scholar * Klute, A. & Page, A. L. _Methods of soil analysis. Part 1.
Physical and mineralogical methods; Part 2. Chemical and microbiological properties_. (American Society of Agronomy, Inc., 1986). * Thomas, G. W. Soil pH and soil acidity. _Methods soil
Anal. Part 3 Chem. methods_ 5, 475–490 (1996). * Rhoades, J. D. Salinity: Electrical conductivity and total dissolved solids. _Methods soil Anal. Part 3 Chem. methods_ 5, 417–435 (1996). *
Jackson, M. L. Soil chemical analysis prentice Hall. _Inc., Englewood Cliffs, NJ_ 498, 183–204 (1958). * Chapman, H. D. Cation‐exchange capacity. _Methods soil Anal. Part 2 Chem. Microbiol.
Prop._ 9, 891–901 (1965). * Lindsay, W. L. & Norvell, Wa. Development of a DTPA soil test for zinc, iron, manganese, and copper. _Soil Sci. Soc. Am. J._ 42, 421–428 (1978). * Razmi, B.,
Ghasemi-Fasaei, R., Ronaghi, A. & Mostowfizadeh-Ghalamfarsa, R. Investigation of factors affecting phytoremediation of multi-elements polluted calcareous soil using Taguchi optimization.
_Ecotoxicol. Environ. Saf._ 207, 111315 (2021). Article CAS PubMed Google Scholar Download references AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Soil Science, School of
Agriculture, Shiraz University, Shiraz, Iran Mahboobeh Varnaseri Ghandali, Sedigheh Safarzadeh & Reza Ghasemi-Fasaei * Faculty of Advanced Technology, Shiraz University, Shiraz, Iran
Sedigheh Zeinali Authors * Mahboobeh Varnaseri Ghandali View author publications You can also search for this author inPubMed Google Scholar * Sedigheh Safarzadeh View author publications
You can also search for this author inPubMed Google Scholar * Reza Ghasemi-Fasaei View author publications You can also search for this author inPubMed Google Scholar * Sedigheh Zeinali View
author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS M.V.G.: Carried out the experiment and analyzed the data. Wrote the manuscript. Discussed the
results and contributed to the final manuscript. S.S.: Conceived of the presented idea. Supervised the project (supervisor). Edited and reviewed the manuscript. Contributed to the final
version of the manuscript. And discussed the results and contributed to the final manuscript. Revised the manuscript. R.G.-F. and S.Z.: Helped review the project (advisor). All authors read
and approved the final manuscript. CORRESPONDING AUTHOR Correspondence to Sedigheh Safarzadeh. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL
INFORMATION PUBLISHER'S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. RIGHTS AND PERMISSIONS OPEN ACCESS
This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as
long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third
party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the
article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the
copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Ghandali, M.V., Safarzadeh,
S., Ghasemi-Fasaei, R. _et al._ Heavy metals immobilization and bioavailability in multi-metal contaminated soil under ryegrass cultivation as affected by ZnO and MnO2 nanoparticle-modified
biochar. _Sci Rep_ 14, 10684 (2024). https://doi.org/10.1038/s41598-024-61270-5 Download citation * Received: 08 December 2023 * Accepted: 03 May 2024 * Published: 09 May 2024 * DOI:
https://doi.org/10.1038/s41598-024-61270-5 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative