Effects of isorhamnetin on liver injury in heat stroke-affected rats under dry-heat environments via oxidative stress and inflammatory response
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Isorhamnetin is a natural flavonoid compound, rich in brass, alkaloids, and sterols with a high medicinal value. This study investigated the effects of isorhamnetin on liver injury
and oxidative and inflammatory responses in heat-stroke-affected rats in a dry-heat environment. Fifty Sprague Dawley rats were randomly divided into five groups: normal temperature control
(NC, saline), dry-heat control (DHC, saline), low-dose isorhamnetin-pretreated (L-AS, 25 mg/Kg), medium-dose isorhamnetin-pretreated (M-AS, 50 mg/Kg), and high-dose isorhamnetin-pretreated
(H-AS, 100 mg/Kg) group. Saline was administered to the NC and DHC groups and corresponding concentrations of isorhamnetin were administered to the remaining three groups for 1 week. Blood
and liver tissue were analyzed for oxidative stress and inflammation. The liver histopathological injury score, serum liver enzyme (alanine transaminase, aspartate transaminase, and lactate
dehydrogenase), liver oxidative stress index (superoxide dismutase [SOD], catalase [CAT], and malondialdehyde), and inflammation index (tumor necrosis factor α [TNF-α], interleukin [IL]-1β,
IL-6, and lipopolysaccharides) were significantly higher in the DHC group than in the NC group (_P_ < 0.05). These index values in the L-AS, M-AS, and H-AS groups were significantly lower
than those in the DHC group (_P_ < 0.05). The index values decreased significantly with an increase in the concentration of isorhamnetin (_P_ < 0.05), while the index values of CAT
and SOD showed the opposite tendency (_P_ < 0.05). The expression of liver tissue nuclear factor kappa B (NF-κB), caspase-3, and heat shock protein (HSP-70) was higher in the DHC group
than in the NC group (_P_ < 0.05). Comparison between the isorhamnetin and DHC groups revealed that the expression of NF-кB and caspase-3 was decreased, while that of HSP-70 continued to
increase (_P_ < 0.05). The difference was significant for HSP-70 among all the isorhamnetin groups (_P_ < 0.05); however, the NF-кB and caspase-3 values in the L-AS and H-AS groups did
not differ. In summary, isorhamnetin has protective effects against liver injury in heat-stroke-affected rats. This protective effect may be related to its activities concerning
antioxidative stress, anti-inflammatory response, inhibition of NF-кB and caspase-3 expression, and enhancement of HSP-70 expression. SIMILAR CONTENT BEING VIEWED BY OTHERS EFFECT OF
CHITOSAN ON BLOOD PROFILE, INFLAMMATORY CYTOKINES BY ACTIVATING TLR4/NF-ΚB SIGNALING PATHWAY IN INTESTINE OF HEAT STRESSED MICE Article Open access 18 October 2021 MECHANISM OF SECONDARY
RENAL INJURY IN TRAUMATIC HEMORRHAGIC SHOCK MODEL UNDER A DRY AND HEAT DESERT ENVIRONMENT Article Open access 28 April 2025 TREATMENT WITH HYDROGEN-RICH WATER PROTECTS AGAINST
THIOACETAMIDE-INDUCED HEPATIC ENCEPHALOPATHY IN RATS THROUGH STABILIZING LIVER–BRAIN DISTURBANCE Article Open access 23 May 2025 INTRODUCTION Prolonged exposure to temperatures exceeding 40
°C can disrupt the body’s natural temperature regulation, causing an imbalance that elevates the core body temperature and increases the risk of heat stroke. Heat stroke can induce a range
of physiological and behavioral disorders, such as nerve damage, immune disorders, and multiple organ dysfunction1. The liver is involved in important functions in the body, including
maintaining energy metabolism homeostasis, synthesizing bile, storing glycogen, and clearing toxins2. Heat stroke can cause severe liver damage. Although the precise mechanism of liver
injury in heat stroke remains unclear, emerging research suggests that oxidative stress, inflammatory responses, and energy metabolism disorders are key factors3. Therefore, identifying
substances capable of alleviating oxidative stress and inflammatory responses is important for the control and treatment of heat stroke-induced liver injury. Bioflavonoids are the most
abundant phytochemical substances in numerous fruits and vegetables4. These compounds exhibit a wide range of pharmacological effects, including anti-oxidative stress, anti-inflammatory
response, immune regulation, anti-diabetes, anti-hypertension, and anti-cancer activities5. Isorhamnetin, a naturally occurring bioflavonoid, causes fewer side effects and has a higher
safety profile compared with conventional drugs. Isorhamnetin can inhibit oxidative stress, ameliorate paracetamol-induced liver injury by modulating the NLRP3/NF-κB/Nrf2 pathway6, and
mitigate oxidative stress in diabetic mice7. Moreover, isorhamnetin exhibits protective effects against lung injury in heat stroke-affected rats by reducing inflammatory responses and
oxidative stress8. Additionally, isorhamnetin plays a protective role in myocardial injury induced by ischemia/reperfusion by reducing apoptosis and oxidative stress9. To date, the effect of
isorhamnetin on liver injury in heat stroke-affected rats under dry-heat environments has not been evaluated. This study was conducted to examine the effects of isorhamnetin on oxidative
stress and inflammatory responses in the liver injury of heat stroke-affected rats in a dry-heat environment and to determine the mechanism of its protective effect on the liver in a rat
model with early-stage heat stroke10. By revealing insights into the prevention and control of environmental heat stroke, this study provides a theoretical basis for studies of ethnomedicine
transformation and applications. METHODS MATERIALS Fifty male Sprague–Dawley rats weighing 240–260 g were purchased from the Experimental Animal Center of Xinjiang Medical University
(license number SCXK (Xin) 2018–0002). The rats had free access to food and water. They were housed in specific pathogen-free animal laboratories for 1 week at ambient temperature (22 ± 1
°C) and humidity (50% ± 5% relative humidity). All animal procedures were performed in strict accordance with international ethical standards and the guidelines for the care and use of
experimental animals issued by the National Institutes of Health. Animal procedures were approved by the Animal Ethics Committee of the Xinjiang Military Region General Hospital (approval
number: DWLL20230323) and reported in accordance with ARRIVE guidelines. Isorhamnetin (CAT no: I811872) was provided by Shanghai Macklin Biochemical (Shanghai, China). Test kits for catalase
(CAT; CAT no: A007-1-1), malondialdehyde (MDA; CAT no: A003-1), and superoxide dismutase (SOD; CAT no: A001-3) were purchased from the Nanjing Jiancheng Bioengineering Institute (Nanjing,
China). Rat tumor necrosis factor (TNF)-α (CAT no: JER-06), interleukin (IL)-1β (CAT no: JER-01), and IL-6 (CAT no: JER-04) enzyme-linked immunosorbent assays (ELISA) kits were purchased
from Qiaoyi Biotechnology Co., Ltd. (Anhui, China). The sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) kit (CAT no: P1200) and RIPA buffer (high) (CAT no: R0010) were
from Beijing Solarbio Science & Technology (Beijing, China). The BCA protein detection kit (CAT no: 23227) was purchased from Thermo Fisher Scientific, Inc. (Waltham, MA, USA). Goat
anti-rabbit antibody (ab97051) was purchased from Abcam (Cambridge, UK), and antibodies against β-actin (rabbit mAb #4970), nuclear factor (NF)-κB (rabbit mAb #8242), caspase-3 (rabbit pAb
#9662), and heat shock protein (HSP)-70 (rabbit pAb #4872) were purchased from Cell Signaling Technology (Danvers, MA, USA). METHODS After 1 week of specific pathogen-free laboratory
adaptive feeding, the sample size was determined based on our previous research. Based on a study by Dong et al.8 of the effect of isorhamnetin on heat stroke-induced lung injury in rats,
the 50 Sprague–Dawley rats were divided into the following five groups using a random number table: normal temperature control group (NC group), dry-heat control group (DHC group), low-dose
isorhamnetin-pretreated group (L-AS group, 25 mg/kg), medium-dose isorhamnetin- pretreated group (M-AS group, 50 mg/kg), and high-dose isorhamnetin-pretreated group (H-AS group, 100 mg/kg).
Saline was administered to the NC and DHC groups for 1 week, and the concentrations of isorhamnetin described above were administered to the other three groups. On day 8, except for rats in
the NC group, all rats were transferred in The Simulated Climate Cabin for the Special Environment of Northwest Chin, deprived of food, and drinking water, and the rats were placed in the
same location to ensure consistent exposure to the ambient temperature. The rectal temperature of the rats was measured 150 min after the initiation of the experiment. The rectal temperature
of the DHC group reached above 40.5 °C, indicating that the dry-heat heat stroke rat model was successfully established. All rats that survived the 150-min heat exposure were included in
the experiment and subjected to further analysis. After abdominal anesthesia with 3% pentobarbital, the vena cava blood was removed, the liver tissue was extracted, and blood was drawn prior
to euthanasia. The serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), and lipopolysaccharide (LPS) were measured. Part of the liver tissue was removed for
histopathological examination and electron microscopy. The remaining liver tissue was frozen in liquid nitrogen. CAT, MDA, and SOD were detected according to the kit instructions. TNF-α,
IL-1β, and IL-6 levels were detected using ELISA, and HSP-70, NF-кB, and caspase-3 levels were detected using western blotting. HISTOPATHOLOGY The middle lobe of the right liver lobe was
fixed in 4% paraformaldehyde for 1 week, dehydrated, paraffin embedded, sectioned 5 μm slices, and stained with hematoxylin and eosin. After staining, histopathological changes in the liver
were observed under an optical microscope, and the degree of liver injury was evaluated using the liver injury scoring method11. CHANGES IN LIVER TISSUE UNDER ELECTRON MICROSCOPY Kidney
specimens were cut into 2 mm fragments, soaked in 2.5% glutaraldehyde overnight at 4 °C, washed three times with 0.1 M phosphate buffer, and fixed with 1% osmium tetroxide at 4 °C for 2 h.
The sample was placed in increasing concentrations of ethanol for dehydration. Using epoxy resin for tissue penetration and embedding and heated at 70 °C for 9 h. Trimming and slicing the
sample. The sections were stained with uranyl acetate and lead citrate at 25 °C for 15 min and observed under a transmission electron microscope (JEM-1230; JEOL, Tokyo, Japan). DETERMINATION
OF SERUM LIVER ZYMOGRAM Venous blood was centrifuged at 1500×_g_ for 10 min, and the supernatant was collected. The plasma ALT, AST, and LDH levels were measured using a fully automated
biochemical analyzer (XA-46000996, Shenzhen Mindary Biomedical Electronics, Shenzhen, China). PREPARATION OF LIVER TISSUE HOMOGENATES Approximately 0.1 g of liver tissue was placed in a
centrifuge tube and cut into small pieces with sharp scissors. After adding 900 µL of Phosphate Buffer Solution (PBS) to each centrifuge tube, an electric homogenizer was used to grind the
liver tissue and obtain 10% homogenization. The homogenate was left to stand for 2 h and centrifuged at 12,000×_g_ for 10 min in a low-temperature centrifuge (Heraeus Fresco 21, Thermo
Fisher Scientific); the supernatant was collected to detect inflammatory factors and oxidative stress indicators. All procedures were performed on ice. DETERMINATION OF INFLAMMATORY FACTORS
AND OXIDATIVE STRESS INDICATORS The TNF-α, IL-1β, IL-6, LPS, CAT, MDA, and SOD levels in liver tissue homogenates were determined using ELISA kits. Optical densities were read with a
microplate reader (550, Bio-Rad Laboratories, Hercules, CA, USA). The data were recorded, a standard curve was plotted, and protein concentrations were calculated. WESTERN BLOTTING The liver
tissue (0.1 g) was cut with scissors, ground in RIPA buffer (0.9 mL) with an electric homogenizer, and placed on ice for 2 h. After centrifuging the homogenate at 12,000×_g_ and 4 °C for 15
min, the protein concentration was measured using the BCA method. RIPA buffer was then added to balance the protein concentration in the liver tissue homogenate. All samples were mixed with
2 × loading buffer, boiled for 10 min, and separated using SDS–PAGE at 50 V for 30 min, followed by 100 V for 90 min using an electrophoresis instrument (PowerPac HC, Bio-Rad Laboratories).
Proteins in the gel were transferred the protein to a polyvinylidene fluoride (PVDF) membrane for 20–40 min. The PVDF membrane was blocked with 5% skimmed milk at 25 °C ± 1 °C for 2 h;
antibodies against NF-κB (1:1000), HSP70 (1:1000), caspase-3 (1:1000), and β-actin (1:1000) were added and incubated overnight at 4 °C. The membranes were washed five times with TBST (5 min
each) and incubated with the corresponding second antibody (1:20,000) to 25 °C ± 1 °C. After washing the PVDF membrane three times with TBST solution, bands were visualized via
chemiluminescence (ChemDoc-IT®510 Imager; Ultra-Violet Products Ltd., Cambridge, UK). The membranes were scanned, and band intensities were analyzed using Visionworks LS (version 8.1.2;
Ultra-Violet Products Ltd.). STATISTICAL ANALYSIS Data were analyzed using SPSS 23.0 statistical software (SPSS, Inc., Chicago, IL, USA). The data were expressed as the x ± s, and one-way
analysis of variance and the least significant difference test were used; a difference of _P_ < 0.05 was considered to indicate a statistically significant difference. RESULTS RAT LIVER
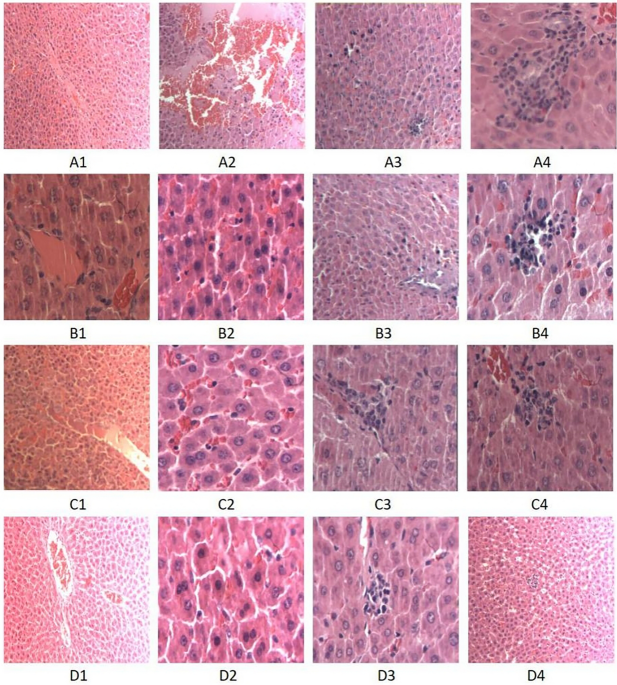
STRUCTURAL CHANGES AND LESION HISTOPATHOLOGY SCORE Light microscopy revealed that the hepatocyte structure of the NC group was intact. Liver cells in the DHC group showed edema, eosinophilic
changes, cell death, liver cell cytoplasmic red staining, unclear cell boundary, central venous thrombosis, and other histopathological changes. The degree of histopathological hepatocyte
damage across all isorhamnetin-pretreated groups was lower than that in the DHC group (Fig. 1). The histopathological injury scores of the NC, DHC, and three isorhamnetin-pretreated (L-AS,
M-AS, and H-AS) groups were 0.3 ± 0.48, 7.90 ± 0.32, 6.70 ± 0.48, 5.80 ± 0.42, and 4.90 ± 0.32, respectively (Fig. 2). ULTRASTRUCTURAL CHANGES IN HEPATOCYTES IN EACH GROUP Electron
microscopy was used to examine the liver tissue of the NC group and revealed that the cell membrane structure of the hepatocytes, the nuclear membrane, and the mitochondrial structure were
intact (A). In the DHC group, the euchromatin ultrastructure of the nucleus gradually disappeared and more heterochromatin-like structures appeared; the nuclear membrane pores were blurred.
The nuclear membrane was incomplete. The mitochondrial structure was disordered and disappeared. The cell membrane structure was destroyed and the cells underwent lysis or necrosis (B). In
the L-AS group, there was less chromosomal heterochromatin in the nucleus, no obvious damage to the mitochondrial structure, and occasional destruction of cell membrane structure (C). In the
M-AS group, there was intact nuclear structure and no obvious chromatin heterochromatin. The mitochondrial structure was clear and the cell membrane was intact (D). In the H-AS group, the
hepatocyte structure was intact, the nuclear structure was intact, and mitochondria were increased (E) (Fig. 3). ALT AND AST LEVELS AS WELL AS MDA, SOD, AND CAT CONTENTS IN EACH GROUP
Compared with those in the NC group, the levels of ALT, AST, and LPS in the DHC group were significantly higher (_P_ < 0.05). The levels of ALT, AST, and LPS in the blood of the
isorhamnetin-pretreated groups were lower than those in the DHC group (_P_ < 0.05). These differences were significant across the three isorhamnetin-pretreated groups (_P_ < 0.05)
(Table 1). LPS, TNF-Α; IL-1Β, AND IL-6 LEVELS IN EACH GROUP The levels of TNF-α, IL-1β, and IL-6 in the livers of the DHC group were higher than those in the NC group (_P_ < 0.05).
Compared with those in the DHC group, the levels of TNF-α, IL-1β, and IL-6 in the isorhamnetin-pretreated groups were lower (_P_ < 0.05). The difference was significant across all
isorhamnetin-pretreated groups (_P_ < 0.05) (Table 2). In the ELISA test, all indicator tests were conducted on all samples three times independently. The inter-assay coefficient
variances of the ELISA test are shown in Table 3. WESTERN BLOTTING TO DETECT HSP-70; NF-КB, AND CASPASE-3 LEVELS IN THE LIVER TISSUE The expression of HSP-70, NF-ĸB, and caspase-3 was
significantly higher in the DHC group than in the NC group (_P_ < 0.05). Compared with that in the DHC group, the expression levels of NF-ĸB and caspase-3 were significantly lower after
treatment with isorhamnetin, and the expression of HSP-70 was significantly lower in the H-AS group than that in the L-AS group (_P_ < 0.05). The protein expression levels of HSP-70 were
significantly higher after treatment with isorhamnetin (_P_ < 0.05), with significant differences across all isorhamnetin-pretreated groups (_P_ < 0.05) (Fig. 4). DISCUSSION Heat
stroke refers to a disruption in the regulation of body temperatures induced by high temperature and dry environments. In this condition, heat accumulation surpasses the body’s ability to
dissipate it, resulting in varying degrees of impairment to cellular and multiple organ function, which can be life-threatening12. The pathogenesis of heat stroke disease remains poorly
understood. Available evidence suggests that heat stroke is often caused by heat stress resulting from exposure to high temperatures or strenuous exercise. The key mediators of heat stress
activation are the pro-inflammatory cytokine cascade and oxidative stress13. Animal experiments showed that direct heat damage, the inflammatory response, and oxidative stress can cause
liver damage and, in severe cases, liver cell necrosis11,14,15. Isorhamnetin are natural flavonoids widely found in plants and exert antioxidant, anti-inflammatory, anti-mutagenic, and
anti-cancer abilities, which regulate key cellular enzyme functions5. Our study confirms the above research results. We analyzed the characteristics of liver injury caused by heat stroke and
found that isorhamnetin protects against liver injury in heat stroke-affected rats. This protective effect may be related to the anti-oxidative stress and anti-inflammatory effects of
isorhamnetin and involved inhibition of NF-кB and caspase-3 expression and enhancement of HSP-70 expression. We observed that heat radiation not only liver caused tissue damage but also led
to alterations in the ultrastructure of the liver. In the isorhamnetin-pretreated groups of rats, liver tissue damage decreased with increasing doses of isorhamnetin, suggesting that
isorhamnetin protects against liver damage. Various transaminases are present at different levels in different tissue types. Hepatocytes contain large amounts of ALT and AST. When liver
cells are stimulated and damaged, ALT and AST are released into the bloodstream16. Hence, monitoring of blood ALT and AST levels serves as an important indicator for evaluating liver
function. Heat stroke often triggers fluctuations in ALT and AST levels17. In the DHC group, the levels of ALT and AST significantly increased, indicating liver damage in these rats.
Although the isorhamnetin-pretreated groups also exhibited elevated ALT and AST levels, the increases were not significant compared with those in the DHC group. Thus, isorhamnetin can reduce
liver damage, thereby reducing ALT and AST levels in the bloodstream. During heat stroke, the body generates large quantities of highly reactive molecules, such as reactive oxygen species
(ROS) and reactive nitrogen species, disturbing the balance of thermal oxidative stress. This disruption leads to direct and indirect damage to liver cells. ROS can damage macromolecules
such as proteins, lipids, and DNA by altering their structures and functions, thus impacting overall cellular function18. Additionally, extracellularly produced ROS can induce protease
damage. Anti-proteases are proteins that function to control and regulate proteolytic enzymes. Damage to the protease barrier not only disrupts the protease-antiprotease balance but also
promotes tissue damage through uncontrolled proteolysis at the injury site. Furthermore, most of the body’s redox reactions occur in the mitochondria. Excessive ROS production can damage the
mitochondrial membrane, impairing cellular energy supply and resulting in liver cell damage. In addition, the accompanying increase in activation of the mitochondrial apoptosis pathway
leads to apoptosis19. MDA, commonly used as an indicator of lipid peroxidation, reflects ROS levels20. Antioxidant enzymes such as SOD and GSH scavenge ROS21, but elevated ROS levels cause
peroxidation of lipids and other molecules. SOD and CAT protect cells from oxidative stress by detoxifying carcinogens or reducing stress; overproduction of ROS alters the
oxidant‐antioxidant balance. Excessive ROS disrupts the membrane lipid composition through lipid peroxidation, consequently increasing the levels of MDA, a final metabolite product of lipid
peroxidation22. The peroxidation reaction increases free radical production, exacerbating cell damage. The main function of this reaction is to catalyze the decomposition of H2O2 into H2O
and O2, preventing H2O2 from reacting with O2 under the action of iron chelate to form harmful –OH23. We observed significant decreases in CAT and SOD levels of the DHC group and a
significant increase in the MDA content in the liver tissues compared to those in the NC group, indicating increased oxide and antioxidant levels in response to heat radiation. This increase
in oxidative stress, reflected by increased MDA levels, indirectly reflects elevated ROS levels in the liver. In contrast, CAT and SOD levels increased, whereas the MDA content decreased,
in the liver tissues compared with those in the DHC group. Moreover, with increasing doses of isorhamnetin, CAT and SOD levels increased and the MDA content decreased in the liver tissues of
the isorhamnetin-pretreated group; the difference between groups was significant. These results indicate that isorhamnetin attenuated the oxidative stress response of the liver, thereby
reducing liver damage. HSP-70 is a molecular chaperone that is constitutively expressed under normal conditions to maintain protein homeostasis and is induced during environmental
stress24,25. HSP-70 interacts with unfolded proteins to prevent irreversible polymerization and catalyze refolding of its substrates in an ATP- and co-chaperone-dependent manner26. The
HSP-70 family, which acts on various substrates, including newly synthesized and denatured proteins, combines with other chaperones to stabilize existing proteins against aggregation and
facilitate the folding of newly translated polypeptides in the cytosol and organelles27. These chaperones recognize non-native conformations of proteins, contributing to these processes.
Beyond its chaperone activity, HSP-70 is important for the maturation and inactivation of nuclear hormones and other signal transduction molecules. Under high-temperature and other
stimulating conditions, HSP-70 expression in the body significantly increases, enhancing the body’s ability to withstand heat and stress28, which aligns with our findings. In the DHC group,
HSP-70 expression in the liver tissue was increased, likely induced by self-protection mechanisms in response to stress. We also observed significantly increased HSP-70 expression in the
isorhamnetin-pretreated groups, suggesting that isorhamnetin exerts a protective effect on the rat liver by increasing HSP-70 expression in the liver. With increasing isorhamnetin doses,
HSP-70 expression increased. However, once HSP-70 expression reached a specific threshold, increasing the isorhamnetin dosage did not significantly increase HSP-70 expression. Following
exposure to heat radiation, the damage caused by inflammatory reactions on tissues to organisms becomes notable. TNF-α, produced by numerous immune cells including T cells, B cells, natural
killer cells, and macrophages29, mediates cellular responses via interactions with TNF-R1 and TNF-R2 receptors, activating apoptotic pathways depending on the cell type and physiological
background30,31. TNF-α plays a key regulatory role in inflammation and host defense against bacterial infections32. Furthermore, TNF-α damages endothelial cells in blood vessels, leading to
vascular dysfunction, thrombosis, and local blood flow blockage in tissues, thereby causing hemorrhage and hypoxic necrosis33. TNF-α also serves as an endogenous pyrogen, stimulating acute
phase protein synthesis in hepatocytes under heat stress and inducing IL-6 production in other cells33. Our results confirm those of previous studies; the levels of TNF-α, IL-1β, and IL-6 in
the liver tissue of rats in the DHC group were significantly increased. In contrast, the levels of TNF-α, IL-1β, and IL-6 in the isorhamnetin-pretreated group were lower than those in the
DHC group, indicating that isorhamnetin can reduce the expression of these inflammatory factors, subsequently inhibiting some cascades reducing liver tissue damage. In addition, TNF-α
activates the expression of upstream NF-кB34, a multi-effect transcription factor present in almost all cell types and involved in various biological processes such as inflammation,
immunity, differentiation, cell growth, tumorigenesis, and apoptosis. NF-кB plays a complex and important role in the regulation of the immune response and inflammation35. NF-κB can function
as an inflammatory factor, endotoxin, and oxidative stress factor and is an important mediator of oxidative stress36. Our research findings also indicate significantly increased NF-κB
expression in the liver of the DHC group compared with that in the NC group, indicating that heat stroke caused inflammatory responses in the body in a process involving TNF-α and IL-6.
These elevated levels confirmed that inflammatory factors and upstream factors participate in the early and intermediate stages of heat stroke-induced inflammatory responses, triggering
cascades that aggravated tissue damage. Additionally, NF-κB expression was decreased in the isorhamnetin-pretreated groups compared with that in the DHC group, suggesting isorhamnetin’s
capacity to inhibit NF-κB expression and the ability of NF-κB to regulate inflammatory factor expression, thus suppressing the inflammatory response. Whether through thermal direct damage,
inflammatory reactions induced by internal environment changes, or oxidative stress responses, the result is liver cell damage. To maintain internal environment stability and reduce damage,
the body actively undergoes apoptosis, a strictly regulated mode of cell suicide characterized by nuclear pyknosis, cell shrinkage, cell membrane blebbing, and DNA fragmentation37. Caspases
form a family of cysteine proteases that are central regulators of apoptosis. Inflammatory factors and pro-apoptotic stimuli activate the promoter (caspase-11), which in turn activates
caspase-1, which functions directly with caspase-3 to promote cellular inflammatory responses and apoptosis. Caspase-3 is a reliable indicator of apoptosis38,39,40 and an important
peripheral and intrinsic apoptotic pathway for the activation of apoptotic proteases41. In agreement with these findings, we observed increased caspase-3 expression in the rat liver tissue
in the DHC group, indicating liver tissue apoptosis due to heat radiation. In contrast, caspase-3 expression in the isorhamnetin-pretreated groups was lower than that in the DHC group at
high doses of isorhamnetin. This may be because isorhamnetin inhibits the expression of caspase-3 or inhibits a factor upstream of the caspase family, or because isorhamnetin reduces
inflammatory responses and caspase family activation by inflammatory factors. These factors induce the expression of caspase-3, which plays a role in liver protection. The liver exhibits a
close relationship with the intestines, and the role of “intestinal-hepatic axis” should be considered. Previous studies have demonstrated that heat stroke changes intestinal mucosa
permeability, causing mucosal barrier damage42,43,44. Subsequently, intestinal bacteria entering the bloodstream through the portal vein induce flora shifts, leading to bacteremia, sepsis,
and systemic inflammatory response syndrome, ultimately resulting in multiple organ dysfunction syndrome that directly threatens life43,45. Various inflammatory factors and endotoxins can
also activate NF-κB, triggering amplification of cascade reactions46; combined with our results, isorhamnetin may protect intestinal mucosa permeability. CONCLUSION This study provides a
theoretical basis for effective prevention and control of heat and disease in desert dry-heat environments and provides a foundation for the application of ethnic medicine to new fields of
research. However, further research is needed to elucidate the potential mechanisms and practical significance of using isorhamnetin to treat heat stroke and related diseases. DATA
AVAILABILITY The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request. REFERENCES * Bouchama, A. _et al._ Classic and
exertional heatstroke. _Nat. Rev. Dis. Primers_ 8(1), 8 (2022). Article PubMed Google Scholar * Zhang, X. _et al._ Sheng Mai San ameliorated heat stress-induced liver injury via
regulating energy metabolism and AMPK/Drp1-dependent autophagy process. _Phytomedicine_ 97, 153920 (2022). Article CAS PubMed Google Scholar * Wang, F. _et al._ The pathogenesis and
therapeutic strategies of heat stroke-induced liver injury. _Crit Care_ 26(1), 391 (2022). Article PubMed PubMed Central Google Scholar * Al-Khayri, J. M. _et al._ Flavonoids as
potential anti-inflammatory molecules: A review. _Molecules_ 27(9), 2901 (2022). * Gong, G. _et al._ Isorhamnetin: A review of pharmacological effects. _Biomed. Pharmacother._ 128, 110301
(2020). Article CAS PubMed Google Scholar * Rousta, A. M. _et al._ Therapeutic potential of isorhamnetin following acetaminophen-induced hepatotoxicity through targeting
NLRP3/NF-kappaB/Nrf2. _Drug Res. (Stuttg)_ 72(5), 245–254 (2022). Article CAS PubMed Google Scholar * Alqudah, A. _et al._ Isorhamnetin reduces glucose level, inflammation, and oxidative
stress in high-fat diet/streptozotocin diabetic mice model. _Molecules_ 28(2) (2023). * Dong, X. _et al._ Anti-inflammatory and anti-oxidative effects of isorhamnetin for protection against
lung injury in a rat model of heatstroke in a dry-heat environment. _Med. Sci. Monit._ 28, e935426 (2022). Article CAS PubMed PubMed Central Google Scholar * Xu, Y. _et al._
Cardioprotective effect of isorhamnetin against myocardial ischemia reperfusion (I/R) injury in isolated rat heart through attenuation of apoptosis. _J. Cell. Mol. Med._ 24(11), 6253–6262
(2020). Article CAS PubMed PubMed Central Google Scholar * Ou, Z. R. _et al._ Heatstroke model for desert dry-heat environment and observed organ damage. _Am. J. Emerg. Med._ 32(6),
573–579 (2014). Article Google Scholar * Du, D. _et al._ Camel whey protein alleviates heat stress-induced liver injury by activating the Nrf2/HO-1 signaling pathway and inhibiting HMGB1
release. _Cell Stress Chaperones_ 27(4), 449–460 (2022). Article CAS PubMed PubMed Central Google Scholar * Yezli, S. _et al._ Classic heat stroke in a desert climate: A systematic
review of 2632 cases. _J. Intern. Med._ 294(1), 7–20 (2023). Article PubMed Google Scholar * Xia, X. _et al._ Bovine lactoferrin alleviates pulmonary lipid peroxidation and inflammatory
damage in heat stroke rats. _Ther. Hypothermia Temp. Manag._ 12(4), 223–228 (2022). Article PubMed Google Scholar * Hu, J. M. _et al._ Ethyl pyruvate ameliorates heat stroke-induced
multiple organ dysfunction and inflammatory responses by induction of stress proteins and activation of autophagy in rats. _Int. J. Hyperthermia_ 38(1), 862–874 (2021). Article CAS PubMed
Google Scholar * Du, D. _et al._ Hydrolyzed camel whey protein alleviated heat stress-induced hepatocyte damage by activated Nrf2/HO-1 signaling pathway and inhibited NF-kappaB/NLRP3
axis. _Cell Stress Chaperones_ 26(2), 387–401 (2021). Article CAS PubMed PubMed Central Google Scholar * Zhang, M. _et al._ Liquiritigenin protects against arsenic trioxide-induced
liver injury by inhibiting oxidative stress and enhancing mTOR-mediated autophagy. _Biomed. Pharmacother_ 143, 112167 (2021). Article CAS PubMed Google Scholar * Liu, K. _et al._
L-Theanine mediates the p38MAPK signaling pathway to alleviate heat-induced oxidative stress and inflammation in mice. _Food Funct._ 13(4), 2120–2130 (2022). Article CAS PubMed Google
Scholar * Zhang, M. _et al._ AVE 0991 Attenuates pyroptosis and liver damage after heatstroke by inhibiting the ros-nlrp3 inflammatory signalling pathway. _Biomed. Res. Int._ 2019, 1806234
(2019). PubMed PubMed Central Google Scholar * Liu, H. _et al._ Copper induces oxidative stress and apoptosis in the mouse liver. _Oxid. Med. Cell. Longev._ 2020, 1359164 (2020). PubMed
PubMed Central Google Scholar * Ding, Y., Yu, Z. & Zhang, C. Diallyl trisulfide protects against concanavalin A-induced acute liver injury in mice by inhibiting inflammation, oxidative
stress and apoptosis. _Life Sci._ 278, 119631 (2021). Article CAS PubMed Google Scholar * Yue, S. _et al._ Hepatoprotective effect of apigenin against liver injury via the non-canonical
nf-kappab pathway in vivo and in vitro. _Inflammation_ 43(5), 1634–1648 (2020). Article CAS PubMed Google Scholar * Shi, H. _et al._ Schisandrin B diet inhibits oxidative stress to
reduce ferroptosis and lipid peroxidation to prevent pirarubicin-induced hepatotoxicity. _Biomed. Res. Int._ 2022, 5623555 (2022). Article PubMed PubMed Central Google Scholar * Netto,
L. E. & Antunes, F. The roles of peroxiredoxin and thioredoxin in hydrogen peroxide sensing and in signal transduction. _Mol. Cells_ 39(1), 65–71 (2016). Article CAS PubMed PubMed
Central Google Scholar * Pereira, B. _et al._ Immunodetection of heat shock protein 70 and cell death in liver of a neotropical fish acutely and chronically exposed to acetaminophen and
propranolol. _Environ. Sci. Pollut. Res. Int._ 28(9), 11233–11244 (2021). Article CAS PubMed Google Scholar * Rutledge, B. S., Choy, W. Y. & Duennwald, M. L. Folding or holding?
Hsp70 and Hsp90 chaperoning of misfolded proteins in neurodegenerative disease. _J. Biol. Chem._ 298(5), 101905 (2022). Article CAS PubMed PubMed Central Google Scholar * Elmallah, M.
_et al._ Membrane-anchored heat-shock protein 70 (Hsp70) in cancer. _Cancer Lett._ 469, 134–141 (2020). Article CAS PubMed Google Scholar * Hagymasi, A. T., Dempsey, J. P. &
Srivastava, P. K. Heat-shock proteins. _Curr. Protoc._ 2(11), e592 (2022). Article CAS PubMed Google Scholar * Boyko, A. A. _et al._ HSP70 in human polymorphonuclear and mononuclear
leukocytes: Comparison of the protein content and transcriptional activity of HSPA genes. _Cell Stress Chaperones_ 22(1), 67–76 (2017). Article CAS PubMed Google Scholar * Sethi, J. K.
& Hotamisligil, G. S. Metabolic messengers: Tumour necrosis factor. _Nat. Metab._ 3(10), 1302–1312 (2021). Article CAS PubMed Google Scholar * Alijotas-Reig, J. _et al._ Tumor
necrosis factor-alpha and pregnancy: Focus on biologics. An updated and comprehensive review. _Clin. Rev. Allergy Immunol._ 53(1), 40–53 (2017). Article CAS PubMed Google Scholar *
Zhang, H. & Xiao, W. TNFR1 and TNFR2 differentially mediate TNF-α-induced inflammatory responses in rheumatoid arthritis fibroblast-like synoviocytes. _Cell. Biol. Int._ 41(4), 415–422
(2017). Article CAS PubMed Google Scholar * van Loo, G. & Bertrand, M. Death by TNF: A road to inflammation. _Nat. Rev. Immunol._ 23(5), 289–303 (2023). Article PubMed Google
Scholar * Shang, G. S., Liu, L. & Qin, Y. W. IL-6 and TNF-α promote metastasis of lung cancer by inducing epithelial-mesenchymal transition. _Oncol. Lett._ 13(6), 4657–4660 (2017).
Article CAS PubMed PubMed Central Google Scholar * Zhou, W. _et al._ Jiangzhi Granule attenuates non-alcoholic steatohepatitis by suppressing TNF/NFkappaB signaling pathway-a study
based on network pharmacology. _Biomed. Pharmacother._ 143, 112181 (2021). Article CAS PubMed Google Scholar * Barnabei, L. _et al._ NF-kappaB: At the borders of autoimmunity and
inflammation. _Front. Immunol._ 12, 716469 (2021). Article CAS PubMed PubMed Central Google Scholar * Verma, A. _et al._ Triterpenoids principle of _Wedelia calendulacea_ attenuated
diethynitrosamine-induced hepatocellular carcinoma via down-regulating oxidative stress, inflammation and pathology via NF-kBpathway. _Inflammopharmacology_ 26(1), 133–146 (2018). Article
CAS PubMed Google Scholar * Chehade, H. _et al._ Determination of caspase activation by western blot. _Methods Mol. Biol._ 2255, 1–12 (2021). Article CAS PubMed Google Scholar *
Yokose, K. _et al._ TNF-α potentiates uric acid-induced interleukin-1β (IL-1β) secretion in human neutrophils. _Mod. Rheumatol._ 28(3), 513–517 (2018). Article CAS PubMed Google Scholar
* Dong, Y. P. _et al._ Electrochemiluminescent sensing for caspase-3 activity based on Ru(bpy)3(2+)-doped silica nanoprobe. _Anal Chem_ 88(3), 1922–1929 (2016). Article CAS PubMed Google
Scholar * Crowley, L. C. & Waterhouse, N. J. Detecting cleaved caspase-3 in apoptotic cells by flow cytometry. _Cold Spring Harb. Protoc._ 11, 1101–1106 (2016). Google Scholar * Asadi,
M. _et al._ Caspase-3: Structure, function, and biotechnological aspects. _Biotechnol. Appl. Biochem._ 69(4), 1633–1645 (2022). Article CAS PubMed Google Scholar * Wei, L. _et al._
Effects of chitosan oligosaccharides on intestinal oxidative stress and inflammation response in heat stressed rats. _Exp Anim_ 70(1), 45–53 (2021). Article CAS PubMed Google Scholar *
Tang, Z. _et al._ Heat stress-induced intestinal barrier impairment: Current insights into the aspects of oxidative stress and endoplasmic reticulum stress. _J. Agric. Food Chem._ 71(14),
5438–5449 (2023). Article CAS PubMed Google Scholar * Zhou, J. Y. _et al._ Wnt/beta-catenin-mediated heat exposure inhibits intestinal epithelial cell proliferation and stem cell
expansion through endoplasmic reticulum stress. _J. Cell. Physiol._ 235(7–8), 5613–5627 (2020). Article CAS PubMed Google Scholar * Wang, Z. _et al._ Heat stress-induced intestinal
barrier damage and dimethylglycine alleviates via improving the metabolism function of microbiota gut brain axis. _Ecotoxicol. Environ. Saf._ 244, 114053 (2022). Article CAS PubMed Google
Scholar * Yang, G. _et al._ Heatstroke induces liver injury via IL-1β and HMGB1-induced pyroptosis. _J Hepatol_ 63(3), 1688–1688 (2015). Google Scholar Download references FUNDING This
work was partially supported by the Open Project of Key Laboratory of Xinjiang Uygur Autonomous Region (Grant number 2020D04029). AUTHOR INFORMATION Author notes * These authors contributed
equally: Xinyue Yang, Hongwei Wang, and Caifu Shen. AUTHORS AND AFFILIATIONS * Key Laboratory of Special Environmental Medicine of Xinjiang, General Hospital of Xinjiang Military Command,
Urumqi, 830000, China Xinyue Yang, Caifu Shen, Xiang Dong, Jiajia Li & Jiangwei Liu * Shandong Provincial Third Hospital, Jinan, 25000, China Hongwei Wang * Graduate School, Xinjiang
Medical University, Urumqi, 830000, China Xinyue Yang Authors * Xinyue Yang View author publications You can also search for this author inPubMed Google Scholar * Hongwei Wang View author
publications You can also search for this author inPubMed Google Scholar * Caifu Shen View author publications You can also search for this author inPubMed Google Scholar * Xiang Dong View
author publications You can also search for this author inPubMed Google Scholar * Jiajia Li View author publications You can also search for this author inPubMed Google Scholar * Jiangwei
Liu View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS All authors contributed to the study’s conception and design. Material preparation,
data collection, and analysis were performed by X.Y.Y., H.W.W., C.F.S., X.D., J.J.L., and C.F.S. The first draft of the manuscript was written by X.Y.Y., H.W.W., and C.F.S., and all authors
commented on previous versions of the manuscript. All authors read and approved the final manuscript. CORRESPONDING AUTHOR Correspondence to Jiangwei Liu. ETHICS DECLARATIONS COMPETING
INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and
institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY FIGURE 1. SUPPLEMENTARY FIGURE 2. SUPPLEMENTARY FIGURE 3. SUPPLEMENTARY FIGURE 4. RIGHTS AND PERMISSIONS OPEN ACCESS This
article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as
you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party
material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the
article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the
copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Yang, X., Wang, H., Shen,
C. _et al._ Effects of isorhamnetin on liver injury in heat stroke-affected rats under dry-heat environments via oxidative stress and inflammatory response. _Sci Rep_ 14, 7476 (2024).
https://doi.org/10.1038/s41598-024-57852-y Download citation * Received: 05 August 2023 * Accepted: 22 March 2024 * Published: 29 March 2024 * DOI: https://doi.org/10.1038/s41598-024-57852-y
SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy
to clipboard Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * Isorhamnetin * Heat shock disease * Liver injury * Oxidative stress * Inflammation