Exploring the correlation and causation between alpha oscillations and one-second time perception through eeg and tacs
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Alpha oscillations have been implicated in time perception, yet a consensus on their precise role remains elusive. This study directly investigates this relationship by examining
the impact of alpha oscillations on time perception. Resting-state EEG recordings were used to extract peak alpha frequency (PAF) and peak alpha power (PAP) characteristics. Participants
then performed a time generalization task under transcranial alternating current stimulation (tACS) at frequencies of PAF−2, PAF, and PAF+2, as well as a sham condition. Results revealed a
significant correlation between PAP and accuracy, and between PAF and precision of one-second time perception in the sham condition. This suggests that alpha oscillations may influence
one-second time perception by modulating their frequency and power. Interestingly, these correlations weakened with real tACS stimulations, particularly at higher frequencies. A second
analysis aimed to establish a causal relationship between alpha peak modulation by tACS and time perception using repeated measures ANOVAs, but no significant effect was observed. Results
were interpreted according to the state-dependent networks and internal clock model. SIMILAR CONTENT BEING VIEWED BY OTHERS FROM THE BRAIN’S ENCODING OF INPUT DYNAMICS TO ITS BEHAVIOR:
NEURAL DYNAMICS SHAPE BIAS IN DECISION MAKING Article Open access 19 November 2024 NO SIGNIFICANT RELATIONSHIP FOUND BETWEEN SPONTANEOUS MOTOR TEMPO, HEARTBEAT, AND INDIVIDUAL ALPHA
FREQUENCY: AN ANALYSIS OF INTERNAL TEMPOS Article Open access 17 January 2025 THE ROLE OF ALPHA OSCILLATIONS IN TEMPORAL BINDING WITHIN AND ACROSS THE SENSES Article 24 February 2022
INTRODUCTION From commenting on the duration of a phenomenon to judging which phenomena lasted longer, time perception plays a crucial role in living and surviving in a variable
environment1,2,3. Although humans internally represent time, the neural origin of time perception in the brain, unlike the senses, remains an unsolved problem in cognitive science4. Time is
a property everywhere, from sight to sound and from pain and happiness to fear. However, time is perceived regardless of specific senses and vastly differs from other perceptions in several
respects. First, the lack of specific temporal stimulus in the environment led to the lack of evolutionary specific receptors in the peripheral nervous system to receive a sense of time
(e.g., compared to auditory stimuli in the environment and auditory receptors in the ear)5. Additionally, the brain lacks a localized dedicated region for time perception (e.g., in
comparison to the visual cortex for visual perception), and studies have revealed that time perception recruits many cortical and subcortical regions6. Furthermore, disentangling the role of
cognitive functions, such as attention, memory, and decision-making, from “pure time perception” remains challenging3. To date, different models have been introduced to explain the neural
mechanisms underlying time perception, which can be generally divided into two categories. First, dedicated or centralized approaches7,8 usually consider an internal clock (composed of a
pacemaker and an accumulator)9,10,11,12. Second, intrinsic models that suggest whole brain activity may be involved in our sense of time8,13. Both categories have found pros and cons to
justify time perception in various situations. Despite the existence of models, recent evidence has increasingly pointed towards brain oscillations as promising explanations for the neural
mechanisms involved in time perception. Dedicated models view brain oscillations as the “clock ticks” of an internal pacemaker responsible for timekeeping14. On the other hand, intrinsic
models associate brain oscillations with the activity of state-dependent networks, representing perceived time, or the speed and level of neural information processing and entropy, acting as
indicators of perceived time15,16. Among neural oscillators, the occipital alpha waves have attracted particular attention17,18,19,20,21,22,23,24. The alpha waves are the most dominant
oscillations in the human brain, which have been hypothesized to underlie phasic transmission from the lateral geniculate nucleus (LGN) to the striate and extrastriate cortex. In practice,
alpha oscillation is prominent over the posterior area of the brain, particularly in eyes-closed resting state EEG recordings25. Within the alpha frequency range, a particular frequency
exhibits the greatest power, often resembling a peak. This frequency is referred to as the peak alpha frequency (PAF), while the power associated with this specific frequency is termed the
peak alpha power (PAP)26. PAF varies among individuals (around 10 Hz, equivalent to the period of 100 ms) and is correlated with different cognitive functions such as attention27,28, working
memory29, and decision-making30. On the other hand, an interval of 100 ms forms a building block of immediate perception31,32. Visually identical stimuli presented in this interval are
generally perceived as happening simultaneously33. Since this interval is equal to the period of an oscillator at the frequency of 10 Hz (i.e., circa PAF), it has been speculated that the
internal clock’s pacemaker properties are related to this peak’s frequency: “Faster alpha rhythms would result in longer estimates of time than slower alpha rhythms, considering that more
pulses would accumulate during the same physical time interval34.” The idea of the relationship between alpha oscillations to produce pacemaker pulses was proposed by Treisman21,22,23 and
has been studied by researchers following Treisman’s work. For instance, Glicksohn et al. 118 (2009) considered the left–right asymmetry index for PAF, and Horr et al.110 discussed alpha
band power to be related to time perception through attentional mechanisms. Samaha et al.117 also found a positive correlation between PAF and temporal resolution of visual perception. To
explore this relationship between alpha oscillations and time perception, besides investigating a plausible correlation between these two phenomena, we applied a noninvasive neuromodulation
approach: transcranial alternating current stimulation (tACS). TACS is a transcranial electrical stimulation (tES) method, which passes a weak electric current, typically 1–2 mA, through the
brain (via at least two electrodes, one of which is located on the studied area on the scalp). This current intensity can modulate brain activity regarding changes in the firing threshold
in a population of neurons35,36. Studies have shown that tACS possibly drives the activity of cortical regions to the frequency of stimulation pulses, representing a significant entrainment
of the brain’s cortical oscillations during and after the stimulation37,38. In the realm of transcranial brain stimulation techniques, such as TMS, tDCS, and tRNS, stimulating the occipital
area on time perception has been explored (for a comprehensive review, refer to6). However, the utilization of tACS becomes pertinent when researchers aim to entrain brain oscillation
frequencies toward specific target frequencies, typically serving as the independent variable. Therefore, tACS is recommended for inquiries seeking to assess the repercussions of modulating
brain oscillations on cognitive functions38.The relationship between modulation of brain oscillations and time perception has been previously investigated in a handful of studies. Using a
segregation/integration task, Ronconi et al.111 provided evidence for a causal relationship between the modulation of brain oscillations in the alpha band and the temporal resolution of
perception through visual-auditory stimulation at frequencies around the alpha peak. They showed that the peak alpha frequency determines the integration/segregation of information such that
higher frequencies lead to more segregation (two separated stimuli are perceived as two distinct stimuli). In comparison, lower frequencies cause the temporal integration (two separated
stimuli are perceived as one continuous stimulus) of perceived stimuli. Thus, when the alpha peak is pushed to higher frequencies (but still within the alpha range, normally PAF−2 to PAF+2)
through visual and auditory stimulation, the temporal resolution of perception (segregation) increases. Regarding the use of tACS, Cecere et al.112 used a sound-induced double-flash illusion
task to measure the duration of visual–auditory modality integration. In this task, a white disc appears on the gray background of a monitor for a very short time (12 ms), and a beep sounds
simultaneously. A second beep sounded after a 36–204 ms delay. In the illusion, if the interval between the two beeps is small (less than what is known as the temporal window of illusion),
the participant reports two flashes rather than one39,40. Appling tACS at three frequencies, PAF−2, PAF, and PAF+2, causally altered the time window of this cross-modal illusion
(visual-auditory), such that stimulation at a higher (PAF+2)/lower (PAF−2) frequency decreased/increased the size of this window, increasing/decreasing the temporal resolution of perception.
Battaglini et al.105 showed that the temporal binding window (TBW) with a non-alpha tACS stimulation (with 18 Hz) is no different from a sham-control condition. However, applying 10 Hz-tACS
reduces the size of the temporal window of segregation. Venskus et al.113 used the double-flash illusion task to measure the TBW and the filled-duration illusion task with auditory modality
to measure time perception. The authors’ initial assumption was that there would be a negative correlation between peak alpha frequency with time perception and the TBW. However, their
results showed that although PAF had a positive relationship with TBW, it had no relationship with time perception. In addition, they found no relationship between alpha power and TBW. Mioni
et al.6,34 used a time generalization task with a standard interval of 600 ms in the visual modality. They showed that applying tACS at these three frequencies (i.e., PAF−2, PAF, and PAF+2)
affected the perception of duration. The authors concluded that the application of tACS did not cause changes in the dispersion of the results (better or worse temporal resolution) but led
to a shift in the psychometric function, meaning that higher frequencies of tACS lead to shorter perceived intervals (and not less temporal resolution). Although these studies utilized
different tasks, all focused on the occipital area with or without parieto-occipital and/or parietal areas for alpha peak extraction and tACS delivery to investigate temporal phenomena.
However, due to the far distance between the period of PAF (around 100 ms) and the one-second interval (1000 ms), studies showed less tendency to investigate this relationship; the studies
tend to explore sub-second intervals, so the relationships between PAF/PAP and subjective one-second must be clarified. On the other hand, studies employ different tasks to tackle the
problem, but a pure temporal task was rarely used. Here, we directly investigated the relationship between alpha peak characteristics and the pre-existing one-second perception. MATERIALS
AND METHODS PARTICIPANTS One hundred twelve students (50 women, 62 men, mean age = 24.30, SD = 4.49, 18–36 years old) volunteered to participate in this study through a public invitation at
the University of Isfahan. We selected this age spectrum due to the consistency of the alpha peak in this period44,45. Experiment procedures were explained to the individuals over the phone.
Those who agreed to participate in the study completed a pre-stimulus screening questionnaire for transcranial electrical stimulation46, which includes ten questions such as existing metal
or electronic implants in the brain/skull or other parts of the body, surgical procedures involving the head or spinal cord, skin problems, epilepsy, and some other questions. Due to
considering healthy participants, they also completed an Iranian version of the 28-item general health questionnaire (GHQ), including four criteria of somatic symptoms, anxiety/insomnia,
social dysfunction, and severe depression47. Both questionnaires were filled out by telephone: Participants were instructed to set aside one hour to complete the questionnaires at their
convenience, ensuring a solitary environment during the response period. Special attention was given to framing the questions in a manner that facilitated participants' thorough
understanding. Those meeting the criteria for transcranial electrical stimulation were then directed to respond to the GHQ questions. Of those, 35 participants (mean age = 24.80, SD = 4.08,
18–36 years old) met the criteria required for tACS application and scored below 24 in the GHQ (screened as healthy, mean = 19.26, SD = 3.22, min = 12, max = 23). Twenty-four of these
volunteers were randomly selected, but two were randomly replaced by two of the remaining 11 eligible participants because they did not have a clear peak in the EEG frequency spectrum. The
final sample consisted of 24 participants (11 women, 13 men, mean age = 25.79, SD = 4.36, 18–36 years old). This number of participants was adopted due to 24(4!) counterbalanced order of
stimulation, which also aligns with the previous studies (Supplementary Table 1). All participants had normal or corrected-to-normal vision (with glasses) and signed a written and informed
consent form to participate in the study. Each participation’s attendance in the Isfahan Cognitive Laboratory was adjusted according to their own schedule. All phases of the experiment were
conducted between 8:00 a.m. and 2:00 p.m. The final sample had no dropouts (e.g., non-participation). The study was approved by the Research Ethics Committee of the University of Isfahan and
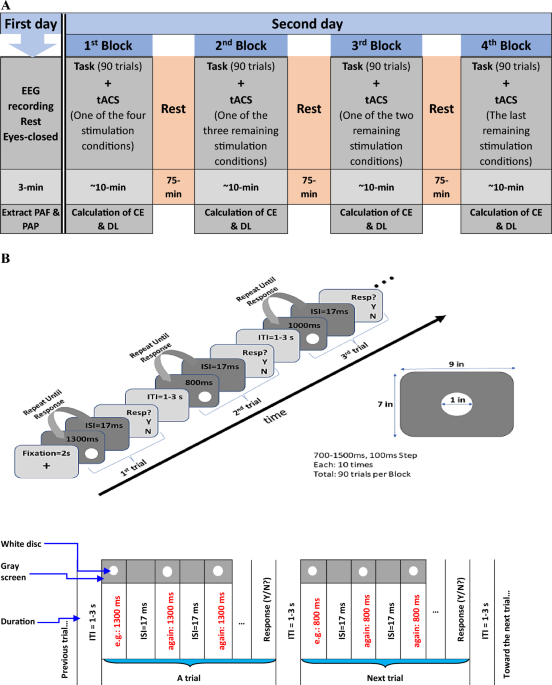
performed in accordance with the Declaration of Helsinki. PROCEDURE The participants attended the laboratory on two separate days. On the first day, a 3-min eyes-closed resting-state EEG
was recorded for each participant. It took approximately 30 min to place the EEG cap on each participant’s head and an additional 10 min to remove it and wash their hair afterward. Following
that, the PAF and PAP were extracted for each participant. It is essential to mention that there were no temporal tasks or tACS procedures conducted on the first day. On the second day,
concurrent with tACS stimulation, each participant performed a time generalization task, comprising four blocks, each lasting around 10 min. The blocks included sham stimulation, as well as
stimulation at PAF−2, PAF, and PAF+2 frequencies counterbalanced across 24 (4!) orders. These blocks were separated with a 75-min break (Fig. 1A). Staying in the laboratory during breaks,
participants were free to do what they wanted but were required to put aside their cellphones and watches to avoid looking at their second’s counter. They had access only to caffeine-free
biscuits and juice, not tea or coffee, and were also not allowed to smoke cigarettes48,49. To prevent participants from practicing counting real clock seconds, none of them knew the details
of the temporal task until the second day. The task was fully explained on the second day in the laboratory to ensure they understood it well. TEMPORAL TASK We selected a time generalization
task in which the standard time interval is initially presented to the participant multiple times to encode it into memory. The task aims to measure human accuracy and precision in
perceiving one second. Concerning the one-second interval, it is customary to divide brief intervals into sub- and supra-second categories50. Sub-second intervals involve more automatic
processes, while supra-second intervals are more associated with high-level cognitive processes. In between, the one-second interval, which is at the heart of our study, is a culturally
learned interval through clocks that remains arbitrary51. However, since the standard interval in this study is one second, already encoded into the memory in daily life, we did not present
this pre-existing interval to participants. In the next step, comparison intervals are presented with a duration equal to or close to the one-second as the standard interval. Participants
respond with yes/no, indicating whether the comparison interval is the same/different from the standard interval52. In the time generalization task, the responses (same/different) are
non-directional in nature. Therefore, they resist response bias compared with tasks that rely on directional responses (i.e., longer/shorter)53. The participants sat relaxed state in a
quiet, dimly light room, positioned 60 cm from a screen (LED, vertical refresh rate = 60 Hz, Resolution = 1024 × 768, 9 × 7 inch2). The visual stimulus was a one-inch diameter white disc
displayed at the center of the gray background. The “M” key, corresponding to the answer “same as one second”, was labeled “yes,” while the “N” key corresponding to the answer “different
from one second” was labeled “no.” Participants were tasked with reporting whether the stimulus on the screen was present for exactly one second (same/different). Participants were
explicitly told to report “no” if they recognized any difference, even if it was a slight deviation from one second. Participants were not shown the physical one-second interval before the
start of the experiment; instead, they were asked to recall one second from their long-term memory based on their life experiences (i.e., pre-existing one-second). In this context, this task
resembles the verbal estimation task, wherein participants must report the duration of the stimulation in conventional units (i.e., one second) but respond only by stating whether this is
the same or different (without providing the numerical value of the stimulus duration in seconds). As this task relies on conventional temporal units, it is more pertinent than other methods
(e.g., time reproduction) for probing the pacemaker component of the internal clock54. A significant challenge arises from the random fluctuations in discrimination performance from one
moment to another. For instance, there are occasions when a specific physical difference between two stimuli is perceived, while on other occasions, this difference is not perceived55.
Therefore, to enhance the results, the single stimulus (e.g., 800 ms) was presented multiple times (with the same duration, i.e., 800 ms) until the participant decided on a response (in this
case, “no”)56,57. In other words, in a trial, the participants were not exposed to the white disc just one times but repeatedly (i.e., they were shown the disc twinkling with a constant
period of circa 1000 ms) (Fig. 1B). Participants were informed that in a given trial, the white disc would repeatedly turn on and off at a stable rate and that this rate remains constant
during all repetitions in the trial until response but may change in the subsequent trial. Participants had the liberty to respond after any number of stimulus repetitions as they wanted,
but they were encouraged to provide answers promptly (e.g., after 3 or 4 representations). These measures were implemented to minimize the potential impact of distractions and fatigue on the
experiment. The inter-stimulus interval (ISI) was adopted to one screen frame (ISI = 1/60 s ~ 17 ms), as verified by an oscilloscope (Supplementary Fig. 1), and was perceptible to
participants58. This brief yet discernible ISI was chosen to mimic the temporal dynamics of the second hand of a real clock. After pressing the response key (yes/no), the twinkling stimulus
disappeared for a random period between 1 and 3 s (equivalent to the inter-trial interval; ITI) before initiating the next trial. The stimulus was then presented again with another
comparison interval (e.g., 1300 ms) and started twinkling until a response was received. This cyclic process continued similarly for other comparison intervals. Comparison intervals were
initially set at 500, 600, 700, 800, 900, 1000, 1100, 1200, 1300, 1400, and 1500 ms. These intervals were selected to create symmetry around the 1000 ms (i.e., standard interval). Interval
lengths are typically chosen in proximity to the standard interval to ensure that the number of “yes” responses is close to zero at both ends of the temporal spectrum55. A pilot study
involving 9 participants (five men, distinct from 24 participants of the study) revealed infrequent selections of 500 and 600 ms (Supplementary Table 2). Consequently, these two intervals
were excluded from the task to reduce the experiment time and fatigue, which could adversely impact the results. Trials were presented randomly, with each interval appearing in ten trials
per block. Thus, each block comprised 90 trials (nine intervals, each presented ten times). Participants were unaware of the exact number of one-second intervals within a block (i.e., 10/90
~ 11% of all trials). All phases of the temporal task were designed using PsychoPy v2022.2.5 software59. Regarding temporal perception, the two critical dependent variables are accuracy and
precision of time perception60. This means that the closer the perceived time (subjective) is to the physical time (objective), the higher the accuracy of the results, and the lower the
variability of perceived time, the higher the precision and temporal resolution. In psychophysics, the parameters suitable for studying time perception are constant error (CE) and difference
limen (DL)3,55,61. CE is the difference between perceived time (i.e., point of subjective equality; PSE) and actual time (i.e., point of objective equality; POE) (i.e., CE = PSE−POE). The
closer the time perceived by the subject (i.e., PSE) to the real-time of the phenomenon (i.e., POE), the higher the accuracy of the perceived duration. On the other hand, DL (= 0.6745SD,
where SD stands for the standard deviation) serves as a measure of the threshold or sensitivity to discriminate between two temporal intervals. A smaller DL value indicates less scattered
results, suggesting a higher precision in time perception. Put differently, a reduced DL signifies an enhanced ability to detect even subtle differences in time intervals, contributing to a
finer resolution in temporal discrimination. Therefore, in the context of studying time perception, a lower DL is indicative of heightened precision and a more refined capacity to discern
temporal distinctions55. The temporal task was developed in PsychoPy in such a way that the number of “yes” responses corresponding to each interval was stored in a separate text file. Using
these responses in the waveform moment analysis (WMA)62 in MATLAB R2022b (www.mathworks.com), CE and DL were extracted for each block of each participant55. EEG RECORDING AND ANALYSIS EEG
data were recorded from the participants using a 64-channel EEG system with passive electrodes (EEG5000Q, CMRR > 110 dB, 54 scalp channels, Negar Andishgan Co. Ltd.) at a sampling rate of
500 Hz. The electrodes were placed according to the international standard 10–20 system (Fig. 2A, Left). Each participant underwent a 3-min eyes-closed resting-state EEG recording while the
impedance was kept below 20 kΩ. The online filter was 0.5–70 Hz, and AFz and Cz electrodes were adopted as the ground and reference electrodes, respectively. For offline pre-processing
using EEGLAB version v2021.063, a high-pass filter of one Hz and a low-pass filter of 40 Hz were applied. Electrodes were then re-referenced to the common average64, and the ASR (spherical
splines) method was employed to check noisy parts and channels ass recommended by EEGLAB. Subsequently, some signal parts were removed manually, and noisy channels were interpolated (mean =
1.92, SD = 2.89). Then, with the help of ICA (Runica algorithm), components lacking a 1/f pattern (mean = 3.88, SD = 4.41) were manually removed65, followed by baseline correction was
performed (Supplementary Fig. 2). Following this, the power spectra of the posterior electrodes were plotted employing EEGLAB channel time–frequency defaults [sub-epoch: the length of the
signal, wavelet cycle: (min = 3, max/fact = 0.8), 200 time points, limits: Padding1, divisive baseline (DIV)] for better frequency resolution (Δf = 0.06 Hz) (Fig. 2A, Right). Finally, due to
the non-normal distribution of PAF and PAP across the six desired posterior electrodes (O1, O2, Oz, PO3, PO4, and POz), we opted for the weighted median (instead of the weighted average) in
the calculation of PAF and the median (instead of the average) for calculating PAP. TACS NeuroStim2 (Medina Teb) was utilized for tACS delivery. Sponge-covered rubber electrodes moistened
with saline were centrally placed on the Cz (11.5 cm × 8.5 cm ~ 98 cm2) as the reference and the Oz (4.5 cm × 4.5 cm ~ 20 cm2) as the target point, according to the international standard
10–20 system and fixed by elastic bands (Fig. 2A, Left). Due to the large size of electrodes, tACS is a non-focal device which result in contaminating expanded area (i.e., parietal and
posterior area) with the electric current. As the posterior area was the desired target, the smaller electrode was placed on this area. The peak-to-peak current intensity was chosen to be
1.6 mA. Thus, the posterior area was targeted with a current density of 79 μA/cm2, and the reference area was targeted at a current density of 16 μA/cm2. Given that the minimum required
current density for effective stimulation is 17 μA/cm2, the posterior area was stimulated with a current density above the threshold. In contrast, the reference region was targeted for
stimulation with a current density below the required threshold66. It is worth noting that this is the current direction, not the current density, that alternates between the two electrodes.
140 mM saline was consistently applied to the sponges to avoid skin irritation caused by electrical stimulation67. However, a small amount of saline was added during the task to avoid
potential interruption of electric current68. The stimulation duration in each block equaled to the duration of the 90 trials of the temporal task, approximately ten minutes, including a
20-s ramp-up and a 20-s ramp-down in both sham and real stimulations. Each participant engaged the temporal task across four random blocks, comprising three stimulation conditions at PAF−2,
PAF, and PAF+2 frequencies and the sham condition, resulting in a total real stimulation duration of 30 min. Adopting PAF−2 and PAF+2 was in accordance with the literature which usually
define these frequencies as the boundaries (off-peaks) of the alpha band. By using PAF as an anchor point, the tACS frequencies for each participant were adopted based on their individual
PAF.69,70,71,72. As the effects of tACS stimulation in the alpha band may last up to 70 min, blocks were separated by a 75-min break (Fig. 1A)73. Participants were asked to report
phosphenes74 at the beginning of each stimulation, but none reported observing any. After each block, participants were asked to report any side effects in the post-stimulation
questionnaire75. The SimNIBS-3.2 toolbox was applied to investigate the efficacy of the electric field produced by tACS through the posterior area under our protocol (I = 1.6 mA, Oz
electrode = 20 cm2, Cz electrode = 98 cm2)76. STATISTICAL ANALYSES The values of CE and DL were extracted for each participant across four blocks including real PAF−2, PAF, PAF+2
stimulations, and sham condition, resulting in a total of 96 CE and 96 DL measurements (i.e., 4 blocks × 24 participants). The waveform moment analyses (WMA) were used for this purpose. In
this method the \(f_{i}\) is considered to be the reported relative frequency of ‘yes’ responses associated with comparison interval \(c_{i}\)(i.e., number of ‘yes’ responses to \(c_{i}\)
divided by ten related trials of a block). Initially, these frequencies are transferred into a probability distribution by the transformation \(p_{i} = \frac{{f_{i} }}{{\sum f_{i} }}\),
where \(i\) = 700–1500 ms with 100 ms steps. Then, considering the standard interval of one second, the CE and the DL are calculated as \(CE = - 1 + \Sigma p_{i} c_{i}\) and \(DL =
0.6745\sqrt {\sum p_{i} \left( {c_{i} - CE - 1} \right)^{2} }\). Ready MATLAB scripts are available at77 in55. This technique has been applied in other studies investigating temporal
discrimination78,79. The data were processed and statistically analyzed hereafter using IBM SPSS Statistics (version 26). GraphPad Prism (GraphPad Software, Version 9.5.1 (733) for Windows,
San Diego, California, USA, www.graphpad.com) and Microsoft Excel (Microsoft Corporation (2018). Microsoft Excel. Retrieved from https://office.microsoft.com/excel) were utilized to generate
graphs for better visualization. In the initial step, we assessed outliers and the normality of data, including the Shapiro–Wilk test and Q-Q plot visual inspection. Then, the collected
data were divided into two parts for the primary analyses. The first part focused on examining the correlation between peak alpha frequency (PAF) and peak alpha power (PAP), extracted on the
first day, with CE and DL extracted on the second day. For this purpose, separate Pearson correlation analyses were performed, and a false discovery rate (FDR) analysis using the
Benjamini–Hochberg procedure was applied to control the multiple comparison error. These analyses aimed to understand the plausible correlation between the alpha oscillations and time
perception. Applying repeated-measures ANOVAs, the second part of the analysis aimed to compare the effects of tACS in four different stimulation conditions. These conditions comprised the
sham condition, where no stimulation was applied, as along with three real tACS conditions: PAF−2, PAF, and PAF+2. These were considered as within-subjects factors, assessing their impact on
time perception. RESULTS The average power spectra across all participants, captured under the six desired electrodes, are depicted in Fig. 2C (Left). Additionally, Fig. 2C (Right) portrays
the mean heat map of brain activity from PAFmin−2 (6.05 Hz) to PAFmax+2 (13.33 Hz) for all participants, revealing higher activity in the posterior region. Given the applied age range
(i.e., 18–36 years), age did not serve as a predictor of either PAF (r = 0.320, p = 0.127) and PAP (r = 0.247, p = 0.245), aligning with expectations80 (Supplementary Fig. 3, Top).
Concerning tACS, the SimNIBS-3.2 toolbox76 confirmed that 1.6 mA electric current is a sufficient flow passing through the posterior area while the centers of two electrodes were located at
Oz (20 cm2) and Cz (98 cm2) (Fig. 2B). The post-stimulation questionnaire75 showed that participants were unable to distinguish the sham stimulation from real stimulations above chance
(correct answers < 25%). Besides, participants tend to respond to comparison intervals closer to the standard interval (i.e., one second) after more repetition (4.15 times for 1000 ms)
and time (4.22 s for 1000 ms) (Supplementary Fig. 3, Bottom). This result was predictable because decreasing the difference between stimuli increases response time81. After employing WMA,
each participant’s CE and DL for four tACS conditions were calculated (Fig. 3), and an outlier analysis was performed, but no outliers were identified. Due to our relatively small sample
size, we assessed the distribution of variables to determine an appropriate statistical method. The Shapiro–Wilk test indicated no evidence of non-normality (W > 0.94, p-value > 0.19
for all ten variables, including PAF, PAP, and CE, DL in the sham, PAF-2, PAF, and PAF + 2 tACS conditions). Additionally, Q-Q plots were visually inspected to validate the normality
assumption (Supplementary Fig. 4). Based on these results and visual assessments, we deemed a parametric test to be appropriate for further analyses. The data of PAF, PAP, CE, and DL for all
four tACS conditions is gathered in Table 1A. We conducted Pearson’s correlation tests to examine potential associations between PAF and PAP with CE and DL. Considering the “sham”
condition, the correlation analysis revealed a non-significant relationship between CE and PAF (r = 0.082, p = 0.703) and between DL and PAP (r = − 0.185, p = 0.386). In contrast, the
correlation analysis between CE and PAP revealed a significant positive relationship (r = 0.480, p = 0.018, FDR-corrected p = 0.036). CE (ms) increased moderately with higher PAP (dB)
values, as indicated by the linear relationship in CE = 4.79PAP–44.88 (R2 = 0.231). Specifically, CE reached its root at PAP = 9.37 dB and increased by 4.79 ms for each dB increase in PAP
(Fig. 4A, Left). Additionally, the correlation analysis between DL and PAF revealed a significant negative relationship (r = − 0.466, p = 0.022, FDR-corrected p = 0.044). The linear
relationship between PAF (Hz) and DL (ms) was obtained as DL = − 18.39PAF + 279.71 (R2 = 0.217). The negative correlation between DL and PAF indicates that DL tends to decrease with higher
PAF values: DL decreases by 18.39 ms for each Hz increase in PAF (Fig. 4A, Right). Finally, Pearson correlation tests were also conducted to investigate the correlation between PAF and PAP
with CE and DL across the three “real” stimulation conditions. These analyses revealed non- or marginally significant associations, particularly after FDR-correction, as summarized in Table
1B. In addition, the correlation between each electrode’s PAF and PAP with each CE and DL is presented in the Supplementary Table. 3. In the final step, we conducted repeated-measures ANOVAs
to assess the impact of different stimulation conditions, including PAF−2, PAF, PAF+2, and sham, on CE and DL. The results revealed no significant changes in CE (F(3, 69) = 0.950, p =
0.421, partial η2 = 0.040) and DL (F(3, 69) = 0.741, p = 0.532, partial η2 = 0.031) across the four conditions. These findings in repeated-measures ANOVAs suggest that the different tACS
conditions applied in this study did not lead to significant alterations in participants’ CE and DL during the time generalization task (Fig. 4B). To ensure our sample size adequacy, post
hoc power analyses using G*Power (version 3.1.9.482) indicated 22 (CE-PAP) and 22 (DL-PAF) total sample size for correlation relationships and 4 (CE) and 6 (DL) total sample size for ANOVAs.
These results affirm the study’s statistical power to detect observed effects and it means our sample size (N = 24) was an appropriate number for this study. DISCUSSION Building on existing
evidence, we aimed to shed new light on the neural underpinnings of time perception. The primary objective of our study was to directly investigate the existence of a correlation between
the parameters of peak alpha and the accuracy and precision of one-second perception. Furthermore, we sought to explore a potential causal relationship by examining whether modulating this
peak through tACS could influence the accuracy and precision of perceived time. These inquiries were designed to provide valuable insights into the neural oscillations that may play a role
in human temporal discrimination ability. In the absence of brain stimulation (i.e., sham condition), our study revealed no correlation between DL and PAP, but a negative correlation was
observed between DL and PAF during the one-second generalization task. These results suggested that higher peak frequencies of neural oscillations in the alpha band corresponded to lower
variability in time perception, leading to greater precision and resolution in perceiving one second. In contrast, CE did not exhibit any significant correlation with PAF but positively
correlated with PAP. When PAP values were greater/less than 7.67 dB, individuals reported the subjective second as greater/less than the objective second. These findings suggest that PAF and
PAP play a crucial role in shaping the subjective perception of one-second time intervals. In comparison to previous studies, we obtained similar, different, and new results. Glicksohn et
al.118 (2009) observed a significant positive correlation between the left–right asymmetry index for PAF (but not the values of PAF and PAP) and time perception. Additionally, their study
employed a time production task, which might have been susceptible to higher errors33 with 4, 8, 16, and 32 s intervals. However, we employed a time generalization task with one second as
the standard interval. Finally, their PAF data were extracted from P3 and P4 regions rather than the posterior electrodes used in our study. Samaha et al. (2015) employed a two-flash fusion
task to measure the temporal resolution of visual perception. The blank intervals were 10–50 ms between a pair of 40 ms-visual stimuli. They found that individuals with higher alpha
frequencies have vision with finer temporal resolution concurrent with our study. Cecere et al.112 and Ronconi et al.111 investigated time perception with different tasks and time intervals,
finding that higher frequencies correspond to higher temporal resolution. Cecere et al.112 utilized a sound-induced double-flash illusion task, while Ronconi et al.111 employed a
segregation/integration task for time intervals close to the alpha-hand period (approximately 100 ms). Venskus et al.113 distinguished between the temporal binding window and time
perception, interpreting PAF as related to the temporal binding window but not time perception. In contrast, Battaglini et al.105 delivered tACS at 10 Hz (but not PAF) to the V5/MT area of
the right hemisphere (but not V1), resulting in a decline in temporal resolution compared to sham and 18 Hz tACS. However, they did not modulate PAF or other frequencies within the alpha
band. Mioni et al.6,34 employed a time generalization task with a standard interval of 600 ms, and they found that entrainment of the alpha peak did not affect temporal resolution (related
to DL) but did shift the psychometric function (related to CE). An important consideration is that using conventional intervals, such as the second in our task, can provide advantages as
these intervals are likely encoded in the brain due to long-term memory (i.e., pre-existing interval). Nonconventional intervals like 600 ms may not be as stably encoded or as easily
compared to conventional intervals, therefore the examiner should present the nonconventional standard interval to the participants before presenting comparison intervals. Consider that our
study showed that PAF correlated with DL, while PAP correlated with CE. This differs from Mioni et al.’s findings, where PAF modulation affected CE but not temporal resolution. All in all,
we urge that alpha oscillations’ crucial role in sub-second time perception (abovementioned studies) can be generalized to one-second time perception despite this interval being far from the
period of alpha frequency. In terms of models, our results can be explained by internal clock model mechanisms as the alpha peak characteristics can be considered as the vital parameters of
the clock stage of SET by playing role in estimation of pre-existing one second. Frequency (PAF) and power (PAP) of alpha peak may determine the underpinning mechanisms of clock stage in
time perception as these properties determine the precision and accuracy of one-second time perception, respectively. Alternatively, these results can be described by intrinsic time
perception models, which suggest the overall activity of the brain (probably in a specific phase of synchronization, here alpha band) can present an internal sense of time8. Particularly,
alterations in alpha waves may impact a broad range of brain regions responsible for constructing functional brain networks, such as the visual network, frontoparietal network, and default
mode network83,84,85,86. These network modifications can alter state-dependent networks potentially involved in time perception16,87, or influence neural information processing, thereby
changing our internal sense of time passage15,88. Despite the intriguing correlation findings, our attempts to establish causal relationships by modulating the alpha peak using tACS were
unsuccessful. The lack of effect of tACS on time perception in this experiment may have been due to using a conventional tACS device, which is a non-focal device. In this case, an HD-tES
device is recommended to achieve the effects of alpha peak modulation on time perception because these devices generate a stronger electric field in the cortex with higher focal power89,90.
It is also possible to use the same laboratory settings, but other tools (e.g., rhythmic sensory stimulation around PAF) can be used to modulate alpha peak frequency and its effect on
one-second time perception35. Further investigation into the factors influencing tACS modulation and potential limitations encountered during the tACS experiments would be valuable for
future studies. Although the administration of tACS did not yield a discernible impact on the observed outcomes (i.e., CE and DL, using ANOVAs, as indicated in the preceding paragraph), it
undermined the correlations’ significance between CE and PAP, as well as between DL and PAF, as illustrated in Table 1B. More precisely, the outcomes reveal a regular increase in p-values
and a regular decrease in Pearson correlation coefficients through the sham condition to PAF−2, PAF, and PAP+2, corresponding to an escalation in stimulation frequency. We conjecture that
this phenomenon may be attributed to the activation of additional brain regions prompted by the stimulation of the posterior area. Several investigations employing diverse methods such as
behavioral assessments, neuroimaging, and electrophysiology have suggested the involvement of various brain regions in temporal processing. These encompass the basal ganglia, cerebellum,
supplementary motor area, premotor area, parietal cortex, occipital cortex, and dorsolateral prefrontal cortex91,92,93,94,95,96,97,98. The dynamic contribution of these interconnected
structures to temporal information processing is not uniform; it is contingent not only upon the duration of the time interval under consideration but also on the cognitive framework
implicated in the specific task and the sensory modality utilized for time delineation6. Given the interconnected nature of the brain and its operation as a network of regions, stimulating
one area may instigate activity in other areas99. These additional regions may, in turn, contribute to the complex network dynamics involved in time perception and/or other pertinent
cognitive functions such as attention. Consequently, we propose exploring the stimulation of other brain areas to investigate their potential roles in shaping one-second time perception, as
elucidated in this study. Finally, functional connectivity and brain network analysis serve as essential tools for future studies. They offer a comprehensive approach to unraveling the
complexities of the brain’s functional organization and provide valuable information on the neuronal underpinning of time perception98,100. We employed resting state eyes-closed EEG since
the alpha oscillation is commonly more pronounced in this condition compared to the eyes-open condition and, notably, during task engagement101. However, as a suggestion for future studies,
one can assess the possible relationships between other EEG characteristics (such as power and functional connectivity) instead of the alpha peak, which can be assessed in both rest and task
performance EEG recording. Furthermore, one can assess alpha band (and also other EEG) characteristics during the critical interval (i.e., from stimulus presentation to the participant’s
response) by epoching the EEG signals based on this period. This targeted analysis may reveal significant correlations between ongoing alpha (and also other brain) oscillations and time
perception features. Attention plays a pivotal role in time perception41. In the attentional-gate model (AGM)42,43, attention directed towards temporal aspects influences judgments regarding
prospective duration. The AGM introduces an attentional gate positioned between the pacemaker and the counter, controlling the flow of pulses. More pulses are accumulated when heightened
attention is directed towards the passage of time, particularly in the absence of attentional resources allocated to a concurrent non-temporal secondary task (as in the case of a dual-task
paradigm)33. Given our exclusive focus on time in this study, without any concurrent task, participants dedicated all their attentional resources to temporal considerations. In terms of
memory, increasing working memory (WM) load consistently reduced subjective duration, with this effect scaling with durations102. However, our task imposed minimal memory demands.
Participants were simply asked to remember the duration of a regularly perceived standard one-second interval103. In contrast, many tasks required participants to encode various intervals
and subsequently retrieve them from memory (higher memory demands). Thus, our tasks involved lower memory demands. Regarding brain oscillations, given that delta-to-theta (∼ 2–8 Hz)
oscillations over frontal brain regions coordinate alpha oscillations over the visual cortex during both the initiation and transition of representational states in visual working memory
when executing multitask sequences104, we suggest that researchers explore the impact of this phenomenon on the perception of pre-existing visual one-second interval. Indeed, disentangling
the roles of attention and working memory from time perception remains a significant challenge102. Some critics may argue that using a periodic stimulus in our research could lead to the
entrainment of brain oscillations. However, we carefully considered the frequency of the periodic visual stimulus used in our study, which was approximately one hertz (ranging from 0.67 Hz
for 1500 ms to 1.43 Hz for 700 ms). In contrast, brain oscillations in the alpha region typically range from 8 to 12 Hz. The visual stimulus frequency (around one hertz) was far from the
alpha region (around 10 Hz) to minimize the likelihood of resonance and entrainment of brain oscillations in the alpha range. Similar studies have shown that sensory rhythms far from the
alpha region are unlikely to affect the alpha rhythm and time perception105. In fact, just some specific frequencies far from one hertz can affect brain oscillations106. For a more
comprehensive understanding of this topic, readers can refer to the work by Norcia et al. (2015), which provides valuable insights into the relationship between sensory stimulation and brain
oscillations. In conclusion, the distinct distance between the visual stimulus frequency and the alpha range used in our study provides confidence in the reliability of our results
concerning time perception and alpha oscillations. In the realm of time perception, heartbeats present an intriguing aspect to consider. While Schwarz et al.116 argue that heart rate may not
significantly impact time perception, Pollatos et al.115 propose that this physiological parameter plays a role in shaping our perception of time. Recent studies have further shed new light
on this connection, demonstrating that a slower pre-stimulus heart rate107 and stimulus presentation during the relaxation period of the heart108 result in longer duration estimates in the
milliseconds range. Given that the frequency of heartbeats approximates one hertz109, which is remarkably close to the time intervals examined in our study, it is plausible to consider heart
rate as a potentially influential factor due to its resonance with our perception of one-second intervals. Furthermore, the link between heart rate and arousal suggests an impact on the
speed of the internal clock’s pacemaker3. Interestingly, peak alpha frequency and heart rate might share a common foundation, as discussed comprehensively in Klimesch114. Consequently, we
recommend recording participants’ heartbeats as another relevant parameter in future studies focused on one-second tasks. Lastly, we highly recommend extending this temporal task to include
other sensory modalities, particularly the auditory domain. By doing so, we can investigate whether the observed correlation between alpha peak parameters and time perception holds across
different modalities. This exploration will help ascertain whether the alpha peak and time perception relationship are independent of the sensory modality involved. Understanding the
generalizability of these findings across modalities can provide valuable insights into the fundamental mechanisms governing time perception and its neural underpinnings. Additionally, such
an approach can contribute to a more comprehensive understanding of how the brain processes temporal information, allowing for a more holistic perspective on the intricate interplay between
alpha oscillations and time perception. Future studies incorporating multiple sensory modalities will undoubtedly enrich our knowledge in this field, bringing us closer to a more unified
understanding of temporal cognition. CONCLUSION Regarding one second, the precision and accuracy of time perception are correlated with peak alpha frequency and peak alpha power,
respectively. Our attempt to modulate the alpha peak by tACS to manipulate time perception failed. Employing HD-tACS and stimulating other regions of the cortex are recommended to overcome
the obstacle, resulting in a significant effect of tACS. DATA AVAILABILITY Correspondence and requests for materials should be addressed to Mahgol Tavakoli. REFERENCES * Marinho, V. _et al._
Impaired decision-making and time perception in individuals with stroke: Behavioral and neural correlates. _Rev. Neurol._ 175(6), 367–376 (2019). Article CAS PubMed Google Scholar *
Ogden, R. S. _et al._ The psychophysiological mechanisms of real-world time experience. _Sci. Rep._ 12(1), 12890 (2022). Article ADS CAS PubMed PubMed Central Google Scholar *
Wittmann, M. The inner experience of time. _Philos. Trans. R. Soc. Lond. B Biol. Sci._ 364(1525), 1955–1967 (2009). Article PubMed PubMed Central Google Scholar * Fontes, R. _et al._
Time perception mechanisms at central nervous system. _Neurol. Int._ 8(1), 5939 (2016). Article PubMed PubMed Central Google Scholar * Grondin, S. _Psychology of Time_ (Emerald Group
Publishing, 2008). Google Scholar * Mioni, G. _et al._ Understanding time perception through non-invasive brain stimulation techniques: A review of studies. _Behav. Brain Res._ 377, 112232
(2020). Article CAS PubMed Google Scholar * Bueti, D. The sensory representation of time. _Front. Integr. Neurosci._ 5, 34 (2011). Article PubMed PubMed Central Google Scholar *
Ivry, R. B. & Schlerf, J. E. Dedicated and intrinsic models of time perception. _Trends Cogn. Sci._ 12(7), 273–280 (2008). Article PubMed PubMed Central Google Scholar * Droit-Volet,
S., Meck, W. H. & Penney, T. B. Sensory modality and time perception in children and adults. _Behav. Process._ 74(2), 244–250 (2007). Article Google Scholar * Gibbon, J., Church, R.
M. & Meck, W. H. Scalar timing in memory. _Ann. N. Y. Acad. Sci._ 423(1), 52–77 (1984). Article ADS CAS PubMed Google Scholar * Maniadakis, M. & Trahanias, P. Time models and
cognitive processes: A review. _Front. Neurorobot._ 8, 7 (2014). Article PubMed PubMed Central Google Scholar * Woodrow, H. The reproduction of temporal intervals. _J. Exp. Psychol._
13(6), 473 (1930). Article Google Scholar * Golesorkhi, M. _et al._ The brain and its time: Intrinsic neural timescales are key for input processing. _Commun. Biol._ 4(1), 970 (2021).
Article PubMed PubMed Central Google Scholar * Kononowicz, T. W. & van Wassenhove, V. In search of oscillatory traces of the internal clock. _Front. Psychol._ 2016, 224 (2016).
Google Scholar * Ghaderi, A., Niemeier, M. & Crawford, J. D. Linear vector models of time perception account for saccade and stimulus novelty interactions. _Heliyon_ 8, 3 (2022).
Article Google Scholar * Ghaderi, A. H. _et al._ Time estimation and beta segregation: An EEG study and graph theoretical approach. _PLoS One_ 13(4), e0195380 (2018). Article PubMed
PubMed Central Google Scholar * Hinrichs, J. V. A two-process memory-strength theory for judgment of recency. _Psychol. Rev._ 77(3), 223 (1970). Article Google Scholar * Meck, W. H.
Selective adjustment of the speed of internal clock and memory processes. _J. Exp. Psychol. Anim. Behav. Process._ 9(2), 171–201 (1983). Article CAS PubMed Google Scholar * Noulhiane, M.
_et al._ How emotional auditory stimuli modulate time perception. _Emotion_ 7(4), 697 (2007). Article PubMed Google Scholar * Rutrecht, H. _et al._ Time speeds up during flow states: A
study in virtual reality with the video game thumper. _Timing Time Percept._ 9(4), 353–376 (2021). Article Google Scholar * Treisman, M. Temporal discrimination and the indifference
interval: Implications for a model of the “internal clock”. _Psychol. Monogr. Gener. Appl._ 77(13), 1 (1963). Article CAS Google Scholar * Treisman, M. _et al._ The internal clock:
Electroencephalographic evidence for oscillatory processes underlying time perception. _Q. J. Exp. Psychol. A_ 47(2), 241–289 (1994). Article CAS PubMed Google Scholar * Treisman, M. _et
al._ The internal clock: Evidence for a temporal oscillator underlying time perception with some estimates of its characteristic frequency. _Perception_ 19(6), 705–742 (1990). Article CAS
PubMed Google Scholar * Wittmann, M. & Schmidt, S. Mindfulness meditation and the experience of time. _Medit. Neurosci. Approach. Philos. Implic._ 2014, 199–209 (2014). Google
Scholar * Ramsay, I. S. _et al._ Individual alpha peak frequency is slower in schizophrenia and related to deficits in visual perception and cognition. _Sci. Rep._ 11(1), 17852 (2021).
Article ADS CAS PubMed PubMed Central Google Scholar * Rathee, S. _et al._ Peak alpha frequency in relation to cognitive performance. _J. Neurosci. Rural Practice_ 11(03), 416–419
(2020). Article Google Scholar * Bagherzadeh, Y. _et al._ Alpha synchrony and the neurofeedback control of spatial attention. _Neuron_ 105(3), 577–587 (2020). Article CAS PubMed Google
Scholar * de Vries, I. E., Marinato, G. & Baldauf, D. Decoding object-based auditory attention from source-reconstructed MEG alpha oscillations. _J. Neurosci._ 41(41), 8603–8617 (2021).
Article PubMed PubMed Central Google Scholar * Akiyama, M. _et al._ Theta-alpha EEG phase distributions in the frontal area for dissociation of visual and auditory working memory. _Sci.
Rep._ 7(1), 42776 (2017). Article ADS MathSciNet PubMed PubMed Central Google Scholar * Samaha, J. _et al._ Spontaneous brain oscillations and perceptual decision-making. _Trends
Cogn. Sci._ 24(8), 639–653 (2020). Article PubMed Google Scholar * Elliott, M. A. & Giersch, A. What happens in a moment. _Front. Psychol._ 6, 1905 (2016). Article PubMed PubMed
Central Google Scholar * Smith, M. L., Gosselin, F. & Schyns, P. G. Perceptual moments of conscious visual experience inferred from oscillatory brain activity. _Proc. Natl. Acad. Sci._
103(14), 5626–5631 (2006). Article ADS CAS PubMed PubMed Central Google Scholar * Block, R. A., Grondin, S. & Zakay, D. Prospective and retrospective timing processes: Theories,
methods, and findings. _Timing Time Percept. Proced. Meas. Appl._ 2018, 32–51 (2018). Google Scholar * Mioni, G. _et al._ Modulation of individual alpha frequency with tacs shifts time
perception. _Cerebral Cortex Commun._ 1(1), tgaa064 (2020). Article Google Scholar * Herrmann, C. S. _et al._ EEG oscillations: From correlation to causality. _Int. J. Psychophysiol._ 103,
12–21 (2016). Article PubMed Google Scholar * Pozdniakov, I. _et al._ Online and offline effects of transcranial alternating current stimulation of the primary motor cortex. _Sci. Rep._
11(1), 3854 (2021). Article ADS CAS PubMed PubMed Central Google Scholar * Frohlich, F. & McCormick, D. A. Endogenous electric fields may guide neocortical network activity.
_Neuron_ 67(1), 129–143 (2010). Article CAS PubMed PubMed Central Google Scholar * Helfrich, R. F. _et al._ Entrainment of brain oscillations by transcranial alternating current
stimulation. _Curr. Biol._ 24(3), 333–339 (2014). Article CAS PubMed Google Scholar * Keil, J. Double flash illusions: Current findings and future directions. _Front. Neurosci._ 14, 298
(2020). Article PubMed PubMed Central Google Scholar * Shams, L., Kamitani, Y. & Shimojo, S. What you see is what you hear. _Nature_ 408(6814), 788–788 (2000). Article ADS CAS
PubMed Google Scholar * Baldauf, D., Burgard, E. & Wittmann, M. Time perception as a workload measure in simulated car driving. _Appl. Ergon._ 40(5), 929–935 (2009). Article PubMed
Google Scholar * Block, R. A. & Zakay, D. Timing and remembering the past, the present, and the future. _Psychol. Time_ 1, 367–394 (2008). Google Scholar * Zakay, D. & Block, R. A.
Temporal cognition. _Curr. Direct. Psychol. Sci._ 6(1), 12–16 (1997). Article Google Scholar * Bazanova, O. & Aftanas, L. Individual measures of electroencephalogram alpha activity
and non-verbal creativity. _Neurosci. Behav. Physiol._ 38, 227–235 (2008). Article CAS PubMed Google Scholar * Berchicci, M. _et al._ Development of mu rhythm in infants and preschool
children. _Dev. Neurosci._ 33(2), 130–143 (2011). Article CAS PubMed PubMed Central Google Scholar * Screening questionnaire for TES.
http://www.neurologie.uni-goettingen.de/downloads.html (2023). * Ebrahimi, A. _et al._ Psychometric properties, factor structure, clinical cut-off point, sensitivity and specificity of
28-item General Health Questionnaire (GHQ-28) in Iranian patients with psychiatric disorders. _J. Res. Behav. Sci._ 5(1), 5–12 (2007). Google Scholar * Sayette, M. A. _et al._ Effects of
smoking urge on temporal cognition. _Psychol. Addict. Behav._ 19(1), 88–93 (2005). Article PubMed PubMed Central Google Scholar * Wickham, K. A. & Spriet, L. L. Administration of
caffeine in alternate forms. _Sports Med._ 48(Suppl 1), 79–91 (2018). Article PubMed PubMed Central Google Scholar * Penney, T. B. & Vaitilingam, L. Imaging time. _Psychol. Time_
2008, 261–294 (2008). Google Scholar * Mioni, G. Methodological issues in the study of prospective timing. _Timing Time Percept. Proced. Meas. Appl._ 2018, 79–97 (2018). Google Scholar *
Wearden, J. H. _et al._ Internal clock processes and the filled-duration illusion. _J. Exp. Psychol. Hum. Percept. Perform._ 33(3), 716–729 (2007). Article PubMed Google Scholar * Yates,
M. J., Loetscher, T. & Nicholls, M. E. A generalized magnitude system for space, time, and quantity? A cautionary note. _J. Vis._ 12(7), 9–9 (2012). Article PubMed Google Scholar *
Glicksohn, J. & Hadad, Y. Sex differences in time production revisited. _J. Individ. Differ._ 33, 35 (2011). Article Google Scholar * Bausenhart, K. M., Di-Luca, M. & Ulrich, R.
Assessing duration discrimination: Psychophysical methods and psychometric function analysis. _Timing Time Percept. Proced. Meas. Appl._ 2018, 52–78 (2018). Google Scholar * McAuley, J. D.
& Miller, N. S. Picking up the pace: Effects of global temporal context on sensitivity to the tempo of auditory sequences. _Percept. Psychophys._ 69(5), 709–718 (2007). Article PubMed
Google Scholar * Miller, N. S. & McAuley, J. D. Tempo sensitivity in isochronous tone sequences: The multiple-look model revisited. _Percept. Psychophys._ 67(7), 1150–1160 (2005).
Article PubMed Google Scholar * Sawides, L. _et al._ High speed visual stimuli generator to estimate the minimum presentation time required for an orientation discrimination task.
_Biomed. Opt. Express_ 9(6), 2640–2647 (2018). Article PubMed PubMed Central Google Scholar * Peirce, J. _et al._ PsychoPy2: Experiments in behavior made easy. _Behav. Res. Methods_ 51,
195–203 (2019). Article PubMed PubMed Central Google Scholar * Thoenes, S. & Oberfeld, D. Meta-analysis of time perception and temporal processing in schizophrenia: Differential
effects on precision and accuracy. _Clin. Psychol. Rev._ 54, 44–64 (2017). Article PubMed Google Scholar * Grondin, S. Timing and time perception: A review of recent behavioral and
neuroscience findings and theoretical directions. _Atten. Percept. Psychophys._ 72(3), 561–582 (2010). Article PubMed Google Scholar * Cacioppo, J. T. & Dorfman, D. D. Waveform moment
analysis in psychophysiological research. _Psychol. Bull._ 102(3), 421–438 (1987). Article CAS PubMed Google Scholar * Delorme, A. & Makeig, S. EEGLAB: An open source toolbox for
analysis of single-trial EEG dynamics including independent component analysis. _J. Neurosci. Methods_ 134(1), 9–21 (2004). Article PubMed Google Scholar * Hagemann, D. Individual
differences in anterior EEG asymmetry: Methodological problems and solutions. _Biol. Psychol._ 67(1–2), 157–182 (2004). Article PubMed Google Scholar * Delorme, A., Sejnowski, T. &
Makeig, S. Enhanced detection of artifacts in EEG data using higher-order statistics and independent component analysis. _Neuroimage_ 34(4), 1443–1449 (2007). Article PubMed Google Scholar
* Nitsche, M. A. & Paulus, W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. _J. Physiol._ 527(3), 633–639 (2000). Article CAS
PubMed PubMed Central Google Scholar * Dundas, J. E., Thickbroom, G. W. & Mastaglia, F. L. Perception of comfort during transcranial DC stimulation: Effect of NaCl solution
concentration applied to sponge electrodes. _Clin. Neurophysiol._ 118(5), 1166–1170 (2007). Article CAS PubMed Google Scholar * Loo, C. K. _et al._ Avoiding skin burns with transcranial
direct current stimulation: Preliminary considerations. _Int. J. Neuropsychopharmacol._ 14(3), 425–426 (2011). Article CAS PubMed Google Scholar * Babiloni, C. _et al._ Sensorimotor
interaction between somatosensory painful stimuli and motor sequences affects both anticipatory alpha rhythms and behavior as a function of the event side. _Brain Res. Bull._ 81(4–5),
398–405 (2010). Article PubMed Google Scholar * Borghini, G. _et al._ Correlation and similarity between cerebral and non-cerebral electrical activity for user’s states assessment.
_Sensors_ 19(3), 704 (2019). Article ADS PubMed PubMed Central Google Scholar * Goljahani, A. _et al._ A novel method for the determination of the EEG individual alpha frequency.
_NeuroImage_ 60(1), 774–786 (2012). Article CAS PubMed Google Scholar * Klimesch, W. _et al._ Phase-locked alpha and theta oscillations generate the P1–N1 complex and are related to
memory performance. _Cogn. Brain Res._ 19(3), 302–316 (2004). Article Google Scholar * Kasten, F. H., Dowsett, J. & Herrmann, C. S. Sustained aftereffect of alpha-tACS lasts up to 70
min after stimulation. _Front. Hum. Neurosci._ 10, 245 (2016). Article PubMed PubMed Central Google Scholar * Evans, I., Palmisano, S. & Croft, R. J. Effect of ambient lighting on
frequency dependence in transcranial electrical stimulation-induced phosphenes. _Sci. Rep._ 12(1), 7775 (2022). Article ADS CAS PubMed PubMed Central Google Scholar * Fertonani, A.,
Ferrari, C. & Miniussi, C. What do you feel if I apply transcranial electric stimulation? Safety, sensations and secondary induced effects. _Clin. Neurophysiol._ 126(11), 2181–2188
(2015). Article PubMed Google Scholar * Thielscher, A., Antunes, A. & Saturnino, G. B. Field modeling for transcranial magnetic stimulation: A useful tool to understand the
physiological effects of TMS? In _2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), IEEE_ (2015). * Vatakis, A.
_Timing-and-Time-Perception-Book/Chapter3.zip_. https://github.com/ArgiroVat/Timing-and-Time-Perception-Book/blob/master/Chapter3.zip (2017). * Birngruber, T., Schröter, H. & Ulrich, R.
Duration perception of visual and auditory oddball stimuli: Does judgment task modulate the temporal oddball effect?. _Attent. Percept. Psychophys._ 76(3), 814–828 (2014). Article Google
Scholar * Dyjas, O. & Ulrich, R. Effects of stimulus order on discrimination processes in comparative and equality judgements: Data and models. _Q. J. Exp. Psychol. (Hove)_ 67(6),
1121–1150 (2014). Article PubMed Google Scholar * Christie, S. _et al._ Individual alpha peak frequency in ice hockey shooting performance. _Front. Psychol._ 8, 762 (2017). Article
PubMed PubMed Central Google Scholar * Link, S. W. _The Wave Theory of Difference and Similarity_ (Routledge, 2020). Book Google Scholar * Faul, F. _et al._ G* Power 3: A flexible
statistical power analysis program for the social, behavioral, and biomedical sciences. _Behav. Res. Methods_ 39(2), 175–191 (2007). Article PubMed Google Scholar * Bowman, A. D. _et al._
Relationship between alpha rhythm and the default mode network: An EEG-fMRI study. _J. Clin. Neurophysiol._ 34(6), 527 (2017). Article PubMed PubMed Central Google Scholar * Knyazev, G.
G. _et al._ The default mode network and EEG alpha oscillations: An independent component analysis. _Brain Res._ 1402, 67–79 (2011). Article CAS PubMed Google Scholar * Mo, J. _et al._
Coupling between visual alpha oscillations and default mode activity. _Neuroimage_ 68, 112–118 (2013). Article PubMed Google Scholar * Wiesman, A. I., Groff, B. R. & Wilson, T. W.
Frontoparietal networks mediate the behavioral impact of alpha inhibition in visual cortex. _Cerebral Cortex_ 29(8), 3505–3513 (2019). Article PubMed Google Scholar * Karmarkar, U. R.
& Buonomano, D. V. Timing in the absence of clocks: Encoding time in neural network states. _Neuron_ 53(3), 427–438 (2007). Article CAS PubMed PubMed Central Google Scholar *
Ghaderi, A. H. Heat transfer, entropy and time perception: Toward finding a possible relation between subjective and objective time. _Med. Hypotheses_ 122, 172–175 (2019). Article PubMed
Google Scholar * Johari, K., Tabari, F. & Desai, R. H. _Right Frontal HD-tDCS Reveals Causal Involvement of Time perception Networks in Temporal Processing of Concepts_.
https://doi.org/10.21203/rs.3.rs-2909328/v1 (2023). * Masina, F. _et al._ Neurophysiological and behavioural effects of conventional and high definition tDCS. _Sci. Rep._ 11(1), 7659 (2021).
Article ADS CAS PubMed PubMed Central Google Scholar * Kononowicz, T. W., Van Rijn, H. & Meck, W. H. Timing and time perception. _Stevens’ Handb. Exp. Psychol. Cogn. Neurosci.
Learn. Mem._ 1, 453 (2018). Google Scholar * Meck, W. H. Neuroanatomical localization of an internal clock: A functional link between mesolimbic, nigrostriatal, and mesocortical
dopaminergic systems. _Brain Res._ 1109(1), 93–107 (2006). Article CAS PubMed Google Scholar * Merchant, H., Harrington, D. L. & Meck, W. H. Neural basis of the perception and
estimation of time. _Annu. Rev. Neurosci._ 36, 313–336 (2013). Article CAS PubMed Google Scholar * Muller, T. & Nobre, A. C. Perceiving the passage of time: Neural possibilities.
_Ann. N. Y. Acad. Sci._ 1326(1), 60–71 (2014). Article ADS PubMed PubMed Central Google Scholar * Nani, A. _et al._ The neural correlates of time: A meta-analysis of neuroimaging
studies. _J. Cogn. Neurosci._ 31(12), 1796–1826 (2019). Article MathSciNet PubMed Google Scholar * Paton, J. J. & Buonomano, D. V. The neural basis of timing: Distributed mechanisms
for diverse functions. _Neuron_ 98(4), 687–705 (2018). Article CAS PubMed PubMed Central Google Scholar * Wiener, M., Turkeltaub, P. & Coslett, H. B. The image of time: A voxel-wise
meta-analysis. _Neuroimage_ 49(2), 1728–1740 (2010). Article PubMed Google Scholar * Protopapa, F. _et al._ Effective connectivity in a duration selective cortico-cerebellar network.
_Sci. Rep._ 13(1), 20674 (2023). Article ADS CAS PubMed PubMed Central Google Scholar * Fischer, D. B. _et al._ Multifocal tDCS targeting the resting state motor network increases
cortical excitability beyond traditional tDCS targeting unilateral motor cortex. _Neuroimage_ 157, 34–44 (2017). Article CAS PubMed Google Scholar * Chiarion, G. _et al._ Connectivity
analysis in EEG data: A tutorial review of the state of the art and emerging trends. _Bioengineering_ 10(3), 372 (2023). Article PubMed PubMed Central Google Scholar * Woodman, G. F. _et
al._ Alpha suppression indexes a spotlight of visual-spatial attention that can shine on both perceptual and memory representations. _Psychon. Bull. Rev._ 29(3), 681–698 (2022). Article
PubMed Google Scholar * Polti, I., Martin, B. & van Wassenhove, V. The effect of attention and working memory on the estimation of elapsed time. _Sci. Rep._ 8(1), 6690 (2018). Article
ADS PubMed PubMed Central Google Scholar * Grondin, S., Laflamme, V. & Tétreault, E. One psychological second does not necessarily last 1000 ms. _PsyCh J._ 9(3), 414–416 (2020).
Article PubMed Google Scholar * de Vries, I. E., Slagter, H. A. & Olivers, C. N. Oscillatory control over representational states in working memory. _Trends Cogn. Sci._ 24(2), 150–162
(2020). Article PubMed Google Scholar * Battaglini, L. _et al._ The effect of alpha tACS on the temporal resolution of visual perception. _Front. Psychol._ 11, 1765 (2020). Article
PubMed PubMed Central Google Scholar * Herrmann, C. S. Human EEG responses to 1–100 Hz flicker: Resonance phenomena in visual cortex and their potential correlation to cognitive
phenomena. _Exp. Brain Res._ 137, 346–353 (2001). Article ADS CAS PubMed Google Scholar * Sadeghi, S. _et al._ Wrinkles in subsecond time perception are synchronized to the heart.
_Psychophysiology_ 2022, e14270 (2022). Google Scholar * Arslanova, I., Kotsaris, V. & Tsakiris, M. Perceived time expands and contracts within each heartbeat. _Curr. Biol._ 33(7),
1389–1395 (2023). Article CAS PubMed Google Scholar * Avram, R. _et al._ Real-world heart rate norms in the Health eHeart study. _NPJ Digit. Med._ 2(1), 58 (2019). Article MathSciNet
PubMed PubMed Central Google Scholar * Horr, N. K., Wimber, M. & Di Luca, M. Perceived time and temporal structure: Neural entrainment to isochronous stimulation increases duration
estimates. _Neuroimage_ 132, 148–156 (2016). Article PubMed Google Scholar * Ronconi, L., Busch, N. A. & Melcher, D. Alpha-band sensory entrainment alters the duration of temporal
windows in visual perception. _Sci. Rep._ 8(1), 11810 (2018). Article ADS PubMed PubMed Central Google Scholar * Cecere, R., Rees, G. & Romei, V. Individual differences in alpha
frequency drive crossmodal illusory perception. _Curr. Biol._ 25(2), 231–235 (2015). Article CAS PubMed PubMed Central Google Scholar * Venskus, A. & Hughes, G. Individual
differences in alpha frequency are associated with the time window of multisensory integration, but not time perception. _Neuropsychologia_ 159, 107919 (2021). Article PubMed Google
Scholar * Klimesch, W. The frequency architecture of brain and brain body oscillations: An analysis. _Eur. J. Neurosci._ 48(7), 2431–2453 (2018). Article PubMed PubMed Central Google
Scholar * Pollatos, O. _et al._ How much time has passed? Ask your heart. _Front. Neurorobot._ 8, 15 (2014). Article PubMed PubMed Central Google Scholar * Schwarz, M. A., Winkler, I.
& Sedlmeier, P. The heart beat does not make us tick: The impacts of heart rate and arousal on time perception. _Attent. Percept. Psychophys._ 75, 182–193 (2013). Article Google Scholar
* Samaha, J. & Postle, B. R. The speed of alpha-band oscillations predicts the temporal resolution of visual perception. _Curr. Biol._ 25(22), 2985–2990 (2015). Article CAS PubMed
PubMed Central Google Scholar * Glicksohn, J., _et al._, Time production and EEG alpha revisited. _NeuroQuantology_ 7(1) (2009). Download references ACKNOWLEDGEMENTS In gratitude, we
acknowledge Dr. Mohammad Ali Nazari for his profitable comments, Drs. Marc Wittmann, Farhad Farkhondeh Tale Navi, and Jason Samaha for their valuable feedback, and Drs. Argiro Vatakis and
Rolf Ulrich for their guidance in experimental design and all students who participated in the study. Your contributions have greatly enhanced our study's quality and success. AUTHOR
INFORMATION AUTHORS AND AFFILIATIONS * Department of Psychology, Faculty of Education and Psychology, University of Isfahan, Isfahan, Iran Ehsan Mokhtarinejad, Mahgol Tavakoli & Amir
Hossein Ghaderi * Center for Affective Neuroscience, Development, Learning and Education, University of Southern California (USC), Los Angeles, USA Amir Hossein Ghaderi Authors * Ehsan
Mokhtarinejad View author publications You can also search for this author inPubMed Google Scholar * Mahgol Tavakoli View author publications You can also search for this author inPubMed
Google Scholar * Amir Hossein Ghaderi View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS E.M. (designed the study, implemented experiments,
developed the computerized temporal task, collected the data, analyzed the data, interpreted the results, and wrote the paper), M.T. (supervised the study, designed the study, reviewed and
edited the manuscript), A.G. (interpreted the results, reviewed and edited the manuscript), and all authors approved the final version of the paper. CORRESPONDING AUTHOR Correspondence to
Mahgol Tavakoli. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE Springer Nature remains neutral with regard
to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed
under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate
credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article
are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons
licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of
this licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Mokhtarinejad, E., Tavakoli, M. & Ghaderi, A.H. Exploring
the correlation and causation between alpha oscillations and one-second time perception through EEG and tACS. _Sci Rep_ 14, 8035 (2024). https://doi.org/10.1038/s41598-024-57715-6 Download
citation * Received: 15 October 2023 * Accepted: 21 March 2024 * Published: 05 April 2024 * DOI: https://doi.org/10.1038/s41598-024-57715-6 SHARE THIS ARTICLE Anyone you share the following
link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature
SharedIt content-sharing initiative