Effects of γ-polyglutamic acid on grassland sandy soil properties and plant functional traits exposed to drought stress
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT The current study provides field experimental data that support the use of γ-polyglutamic acid (γ-PGA) in drought stress and proposes its application in grassland management. We
hypothesized that water treatment combined with PGA application to sandy soil would reduce drought stress in grasslands more effectively than watering alone. A randomized block design was
used, with three replicate watering blocks (no watering, weekly watering, and monthly watering) and PGA treatments at four different concentrations (0%, 0.3%, 1%, and 2% PGA). The results
showed that PGA acts as a biostimulant, alleviating the effects of stress in plants by: (1) increasing the availability of ions, especially K+, Zn2+, Mn2+, Fe2+/3+, Ca2+, and Mg2+, as well
as N-NH4+, and N-NO3−, (2) elongating plant roots, (3) increasing the aboveground biomass, (4) improving the resprouting capacity of the dominant grass _Nardus stricta_, and (5) improving
the regeneration of dicotyledons. In the case of meadows on sandy soils, the use of low PGA concentrations (0.3% or 1%) was the most beneficial for the availability of macro- and
microelements and improving the functional traits of plants. Irrigation had a greater effect than using PGA only for the dicotyledon to monocotyledon ratio. SIMILAR CONTENT BEING VIEWED BY
OTHERS EFFECTS OF Γ-POLYGLUTAMIC ACID SUPPLEMENTATION ON ALFALFA GROWTH AND RHIZOSPHERE SOIL MICROORGANISMS IN SANDY SOIL Article Open access 18 March 2024 BIOSTIMULANTS ALLEVIATE WATER
DEFICIT STRESS AND ENHANCE ESSENTIAL OIL PRODUCTIVITY: A CASE STUDY WITH SAVORY Article Open access 13 January 2023 ROOT BIOMASS AND CUMULATIVE YIELD INCREASE WITH MOWING HEIGHT IN _FESTUCA
PRATENSIS_ IRRESPECTIVE OF _EPICHLOË_ SYMBIOSIS Article Open access 13 December 2022 INTRODUCTION A changing climate has the potential to alter the composition and structure of plant and
soil communities and the interactions between them, so-called plant-soil feedbacks (PSFs)1. However, very little is known about the basic mechanisms and the consequences for PSF of climatic
events such as drought. In particular, most studies have examined the role of soil microbial communities in PSF, focusing on examining the effects of those microbes involved in positively or
negatively influencing plant performance2. Climate change creates shifts in microbial communities from bacterial on more humid soils to fungal on most dry soils and thus affects plant
inputs into the soil subsystem via litter and rhizodeposits. As a consequence, the litter quality changes from high to low3, while the number of metabolic products that play an important
role in the PSF relationship is also diminished. It is known that bacteria and their metabolites play key roles in soil functioning and interact with plants and trophic chains2. One of the
key metabolites is γ-polyglutamic acid (PGA), which is produced by _Lactobacillus_ strains and is concentrated in the extracellular component of biofilms. Biofilms are complex microbial
communities that are surrounded in an extracellular matrix containing many mucopolysaccharides, peptides, and anionic polyamides, whose function is related to the protection of
_Lactobacillus_ strains from the negative impacts of other bacteria, the maintenance of homeostasis, and the protection from external environmental conditions, such as drought stress,
temperature changes, salinity, or UV radiation. Plants growing in the proximity of these biofilms also benefit and can even become bacterially dependent4. In agrocenoses, where many natural
processes are broken5, crop plants are especially exposed to deficiencies in biofilm components. Therefore, in order to overcome this problem, modern agrostrategies include the treatment of
plants with bacterial strains6 or fermentation products such as humic acids. These applications, however, are ineffective in the long term because of unfavorable agricultural soil conditions
being magnified by chemical applications. One of the solutions to this problem is to produce bacterial metabolites in high amounts by biofermentation processes and apply them in the field7.
A promising example of such a strategy is the biotechnological production of PGA in very high concentrations due to high bioreactor intensity and the acceleration of metabolic processes8,9.
PGA is a natural biofilm component, an optically active anionic polymer, which exists in two isoforms: α-PGA and γ-PGA. This polymer is built with repeating units of d- or l-glutamate
connected via amide linkages that are formed between the α-amino and α/γ-carboxylic groups. Microorganisms produce three types of poly-glutamic acid: d-PGA, l-PGA (which are respectively
composed of d-glutamate or l-glutamate units), and dl-PGA (in which d- and l-glutamate units are randomly connected10). PGA is characterized by its molecular weight, the carboxyl-group (α or
γ) engaged in the amide bond, and by the ratio of d- to l-glutamic acid units8. γ-PGA can protect microorganisms against phagocytic attacks and high salt concentrations, while also
providing extracellular nutrient storage and promoting biofilm formation8. γ-PGA is water soluble, biodegradable, biocompatible, edible, and non-toxic to humans and the environment. It has
the ability to bind metals and absorb water, and its biotechnological production for agricultural applications is becoming increasingly profitable. This polymer has many potential
applications in the fields of pharmaceuticals, water and soil treatment, food, cosmetics, and agriculture11,12,13. One of the most important applications of PGA is in agriculture. Due to the
fact that γ-PGA and especially superabsorbent materials synthesized from PGA14 are able to hold a large amount of water, they can be used on crop fields as soil conditioners. Many studies
have reported that γ-PGA plays an important role in plant growth and its regulation; for example, it raises the dry weight of plants such as cucumber, Chinese cabbage, spinach, and
tomato15,16,17. γ-PGA can also be used to bind toxic metal ions and prevent plants from sequestering them. Due to its capacity to bind ions, γ-PGA can be used as a fertilizer, which delivers
to plant rhizospheres cations18 such as Ca2+, Fe2+, Fe3+, Zn2+, and Mn2+. PGA can also be used to reduce salt-induced inhibition of plant growth. Under salt stress, the accumulation of
toxic levels of cellular Na+ and restricted absorption of K+ were observed in plant cells. Interestingly, the foliar application of γ-PGA was shown to reduce ion-specific toxicity by
decreasing the cellular accumulation of Na+, while increased K+ accumulation was observed17. In addition, recent studies showed that this polymer has beneficial effects on the availability
of soil nitrogen, phosphorus, and potassium, as well as plant C and N metabolism13. Data from a limited number of single model plant species confirmed the acceleration of plant growth and
increase in crop yield following PGA application. Moreover, γ-PGA stimulated plant N uptake and improved N soil availability by enhancing microbial and urease activity15,16. It is also known
that γ-PGA can increase root biomass15,19 and that urease activity depends on PGA metabolites, such as glutamic acid or small homopeptides13. A recent experiment on the effect of PGA
acceleration on the drought tolerance of _Brassica napus_ seedlings confirmed that γ-PGA may induce a tendency towards plant tolerance to drought stress by promoting abscisic acid
accumulation20. In Europe, sandy soils are present mainly in Nordic countries and Baltic states, which reflects the glacial history of the continent21. In Poland, more than 60% of mineral
soils have developed from sandy parent material. Sandy soils naturally show low water retention capacity and are prone to drought22. Semi-natural grasslands on light soils that are managed
as meadows and pastures are especially endangered due to climate change23,24, which could result in reduced pastoral farming, crop loss25, and biodiversity loss26. _Nardus stricta_
grasslands play an important role in supporting the diversity of the agricultural landscape27 and vulnerable plant preservation28. The consequences of drought in _N. stricta_ grasslands can
be observed in adjacent fields, meadows, and forest (broken ecosystem connectivity and metapopulation changes of pollinators25 and predators29 in agrocenoses). Therefore, intervention
strategies aimed at mitigating the consequence of drought events in such ecosystems should be developed urgently. The current study provides field experimental data which supports the use of
γ-PGA in drought stress and proposes its application in grassland management. We hypothesize that water treatment combined with PGA application to light and sandy soil will reduce drought
stress in grasslands more effectively than watering alone, by one of following mechanisms: * 1. Increase in soil macro- and microelements’ availability to grasses and herbaceous plants, * 2.
Influence on plant root system development and aboveground biomass production, * 3. Influence on the resprouting capacity of grasses (_Nardus stricta_) undergoing drought stress, * 4.
Supporting the regeneration of dicotyledons in grasslands. MATERIALS AND METHODS STUDY SITE The survey was conducted in the vicinity of Staszów, Świętokrzyskie Voivodeship, Poland (50°35′N
21°11′E, 190 m a.s.l). The experimental site was a typical post-pasture grassland, mown irregularly only to sustain open areas. The site was surrounded by a mosaic of small arable areas,
where mainly potatoes and cereals are cultivated. The grassland was classified in the _Nardo-Callunetea_ class, with _Nardus stricta_ as the dominant species, with _Potentilla erecta_,
_Polygala vulgaris, Hieracium pilosella_, _Luzula campestris_, and _Rumex acetosella_ co-domination. Thanks to the owners, the 3600 m2 experimental site was fenced to avoid any animal
activity. The climate of the given region represents the transitional temperate zone, classified as Dfb (continental with warm summer) by the Köppen-Geiger system30 (adjacent to the
north-east boundary of a moderately warm climate with uniform moisture content, Cfb). The average annual temperature in Staszów is 8.0 °C (− 3.0 °C in January and 18.3 °C in July). The
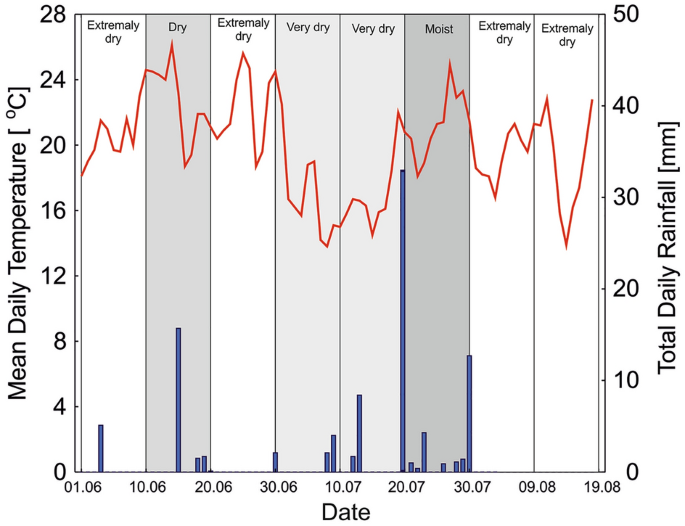
multiyear average annual rainfall in this area is 570 mm. Field experiments were conducted during the drought event of June–August 2019. A coincidental occurrence of drought and heavy
precipitation was observed during the spring and summer in the investigated region (Fig. 1). An assessment of the occurrence of meteorological drought conditions in consecutive 10-day
periods before and during the experiment was made on the basis of Selyaninov's Hydrothermal Coefficient, HTC1. During the experiment, four 10-day periods were classified as extremely
dry (HTC = 0.0–0.25), three periods as very dry or dry (HTC = 0.46–0.82), and only one period was moist (HTC = 2.38), due to days with heavy rainfall. SOIL PARAMETERS The soil in the study
area was loamy sand containing 81% sand, 16% silt, and 3% clay. The soil was highly acidic and generally nutrient poor (Table 1); especially low concentrations of Ca2+, Mg2+, K+, Zn2+, Mn2+,
Fe2+/3+ were observed. However, the content of available P and Nmin (N-NO3− + N-NH4+) may indicate the application of fertilizers in the past. STUDY DESIGN The experiment was carried out
between 1 June and 15 August 2019. A randomized block design was used in the experiment, with three watering blocks replicated eight times and four PGA treatments at various concentrations.
Eight blocks of 2.25 m2 (1.5 m × 1.5 m) were allocated to one of three different watering regimes (24 blocks in total). The spatial distance between blocks was greater than 10 m, while the
spatial distribution was random. In each watering block, four plots of 0.25 m2 (0.5 m × 0.5 m) were allocated to different PGA treatments (96 plots in total) (Fig. 2). The mode of watering
in each PGA treatment plot was the same. BLOCKS The amount of water applied to each watering block was calculated on the basis of the water deficit from the multiyear (1991–2018) average
monthly precipitation for the April–June period. The calculated water deficit (the difference between the average monthly precipitation and the April–June precipitation) since the beginning
of the vegetation season was estimated to be approximately 42 L per square meter. A certain percentage of the water deficit was covered for each block: (1) RW (reference watering): no
watering applied to establish very dry conditions, (2) WW (weekly watering): coverage of total (100%) calculated water deficit—2 L of water per treatment plot of 0.25 m2—applied weekly over
a 6-week time period, 3) MW (monthly watering): coverage of 30% of calculated water deficit—2 L of water per treatment plot of 0.25 m2, applied twice at the beginning of experiment and after
4 weeks. TREATMENTS In each watering block, four PGA treatments were employed: (1) PGA0: reference, without PGA application, (2) PGA2: PGA concentration 2% (40 mL of PGA per m2), (3) PGA1:
PGA concentration 1% (20 mL of PGA per m2), and (4) PGA0.3: PGA concentration 0.33% (6.6 mL of PGA per m2). Commercially available Ambiogel® PGA (Ambioteco, Sztombergi, Poland), which
contained 5% pure PGA, was applied once on the first day of the experiment. PLANT FUNCTIONAL TRAITS Plant species from abandoned fields were identified to species level by Prof. Elżbieta
Jędrszczyk (Department of Horticulture, University of Agriculture, Krakow, Poland). All collected specimens were not under of law restriction or any permission. Voucher specimens were
photographed and stored in the authors collection. On the last day of the experiment, plant samples (roots and shoots) were collected from each treatment plot from eight randomly distributed
area units using the ring method. Eight random measurements (ring throws) for each plot using a ring of a given size (mentioned below), which was adopted as the minimum area of the
estimated shoot variation, were carried out (768 samples in total). The plant's roots and stems were cut from 12.56 cm2 (measured with a loose-leaf ring, 4 cm in diameter) and rinsed
gently with distilled water to remove soil remnants. The root max length was determined to be the maximum length from the root base. The aboveground dry biomass of the dominant grass
species, _N. stricta_, was determined using the oven-dried biomass method31. The shoots were soaked in distilled water for 4 h and then dried at 80 °C for 24 h. In addition, the maximum
aboveground plant height (MH) per area unit (4 cm diameter = 12.56 cm2) was measured as the maximum point reached by the highest plant. Based on the measured plant parameters, two indices
were calculated: (1) DFR—the ratio of dry to fresh shoots of _N. stricta_. A ring of 1 cm diameter = 0.78 cm2 was randomly thrown eight times, and the fresh and dry stems were visually
calculated, (2) DMR—the ratio of dicotyledons to monocotyledons. The DMR was determined from the number of individual specimens per 78.5 cm2 ring size (10 cm diameter) to cover variations in
the plant abundance. To obtain a random effect, the ring was thrown eight times for each treatment plot. SOIL ANALYSIS Soil samples for laboratory analysis were taken from the 0–10 cm layer
of each treatment plot as pooled samples taken from triplicate samples (96 samples in all). The soil particle size distribution was determined by the hydrometer method according to the
PN-R-04032 standard (Polish Committee for Standardization: Warszawa), while soil pH and electrical conductivity (EC) were measured using the potentiometric method with a soil/distilled water
ratio of 1:5 (v/v). The levels of ammonium (N-NH4+) and nitrate (N-NO3−) nitrogen were determined by flow injection colorimetry analysis (FIAstar 5000, Foss), the levels of available
phosphorus (P) and potassium (K) by the Egner-Riehm method, the available magnesium (Mg) by the Schachtschabel method. Phosphorus content in the solution was determined colorimetrically
using UV–Vis spectrophotometer (Helios Beta UVB1002 E, Thermo Electron Corporation, Paisley, UK), while potassium and magnesium content by atomic absorption spectrometry (Varian
SpectrAA-20). The level of calcium (Ca) and available microelements (Fe, Mn, and Zn) was determined by atomic absorption spectrometry after extraction into 0.03 M CH3COOH. STATISTICAL
ANALYSES The influence of watering and PGA treatments on the soil and plant parameters were compared using two-way ANOVA. The normality of the data was tested using the Shapiro–Wilk test.
Post-hoc Duncan’s comparisons of the soil parameters were performed with a Bonferroni correction. All statistical analysis were performed using Statistica v.13 (TIBCO Software Inc., Palo
Alto, CA, USA). Multivariate redundancy analysis (RDA) was carried out using non-standardized plant parameters. For soil parameters, PGA concentration, and watering (as a dummy variable),
forward selection was employed to indicate the soil and treatment variables significantly describing the plant parameters’ variation. Multivariate statistical analyses were carried out using
CANOCO software version 4.56. Values were considered to be statistically significant at p ≤ 0.05. RESULTS Two-way analysis of variance indicated that watering, either as a single parameter
or in combination with PGA treatment, had no significant effect on soil parameters (Supplement 1). Only the PGA, as a single parameter, significantly influenced the soil macro- and
microelement content and soil pH. The available phosphorus (P) and mineral nitrogen (N-NH4+ + N-NO3−) content were significantly enhanced by the presence of PGA with the results varying at
different concentrations (Fig. 3). The available P content (Fig. 3a) in the soil was the highest when the lowest concentration of PGA was applied (PGA0.3). A similar pattern was observed for
N-NO3− (Fig. 3c). However, the highest N-NH4+ content was associated with the highest PGA concentration (PGA0.3) (Fig. 3b). Surprisingly, the reference (PGA0) content was always
approximately at the level of the medium PGA concentration (PGA1). The variation in macro- and microelements in the soil is presented in Fig. 4. The content of the two divalent base cations
in the soil, i.e., Ca2+ and Mg2+, significantly increased when the PGA was added (Fig. 4). The opposite effect was obtained for K+ and Mn2+, their distribution in the soil being decreased
when PGA was applied. The Zn2+ concentration in the soil was reduced by PGA2 and PGA0.3 relative to PGA0, while the Fe2+/3+ content was reduced only at the lowest concentration of PGA
(PGA0.3). High concentrations of PGA enhanced the soil pH values, while the lowest PGA concentration was responsible for the lowest pH values (Fig. 5a). Electrical conductivity (Fig. 5b),
which reflected the concentration of soluble salts, was also related to the PGA content. The EC increased significantly when the PGA content was lowest. Two-way ANOVA indicated that both
factors in combination, i.e., watering and PGA concentration, significantly influenced plant functional traits under drought stress (Supplement 2). PGA applied at low or medium levels
(PGA0.3, PGA1) significantly increased the mean root length (Fig. 6a) and aboveground biomass of _N. stricta_ (Fig. 6b), as well as the maximum shoot height of dicotyledons in the
experimental plots (Fig. 6c). The effect of watering the blocks, however important, only slightly influenced the _N. stricta_ parameters. The mean maximum height of all plants was also
significantly stimulated by low amounts of PGA, especially PGA1. PGA2 treatment decreased the three parameters to levels similar to those observed in the PGA0 plots. In this case, watering
had only a small effect (F-values more than 10-times lower for watering than for PGA treatment (Supplement 2) (Fig. 6c)). The regeneration of shoots of _N. stricta_ expressed by DFR depended
mostly on the PGA treatment (Fig. 6d). The watering had no effect on shoot regeneration only with the PGA0.3 concentration, where the lowest DFR values indicated the best regeneration of
shoots. In the case of PGA0 treatment, better shoot regeneration was observed in the WW blocks, but in general the watering, even at high amounts, only slightly influenced _N. stricta_
regeneration. The watering appeared much more important for dicotyledon density expressed as DMR (Fig. 6e). Both parameters, watering and PGA application at a low or medium concentration
highly enhanced the appearance of dicotyledons, whereas in water stress conditions (RW blocks) no beneficial effect on the dicotyledon plant density was observed. Redundancy analysis
revealed 96.6% of the variance of the experimental variables (watering, PGA concentration) for the first ordination axis (Fig. 7). The main gradient along the first axis was related to the
PGA2 treatment (r = 0.26) and watering (r = 0.13), while the second gradient, describing only 2.8% of the variance, was related to PGA0.3 (r = − 0.20) and PGA1 (r = − 0.18). The
concentration of PGA2 correlated positively with the content of most of the ions in the soil, as well as with pH, while N-NO3− and Zn2+ were related to lower concentrations of PGA (PGA0.3
and PGA1). Along the PGA0.3 and PGA1 gradient, some plant functional traits such as mean plant height, root max length, and aboveground dry biomass also increased, which indicated an
improvement in plant growth during drought stress. Another plant parameter, DRF, and the K+ content were positively related to the PGA0 gradient and negatively to the PGA0.3 and PGA1
gradient. This relationship indicated that the application of low doses of PGA (0.3–1%) stimulated regeneration of _N. stricta_ shoots and enhanced K+ uptake from the soil. DISCUSSION Our
studies have shown for the first time that drought stress effects in grasslands were positively mitigated by the application of specific doses of γ-PGA into light soil. We found a positive
significant impact of PGA, supporting both nutrient management and plant functional traits in grasslands affected by drought. In general, the results of the research indicated that for
reducing the effects of drought on meadow vegetation, the use of a certain concentration of PGA was more important than irrigation. We therefore demonstrated the biostimulatory effect of PGA
in combating drought stress in grassland vegetation. Our results confirmed previous hydroponic studies for _Brassica napus_20 and a pot trial of _Brassica rapa_ cultivars13. Drought-induced
stress disrupts the uptake and translocation of certain nutrients in the whole plant25. The more severe the drought, the more limited the flow of water and nutrients becomes, and the more
limited is the availability of nutrients that can be absorbed by the roots32. The use of PGA can reduce this stress, most probably because it behaves as a chelator and biostimulator13,33 and
facilitates the penetration of water and nutrients through the membranes of plant cells and the supply of nutrients in conditions of water deficit34,35,36. The uptake of most nutrients by
plants depends on the water content in the soil37. The nitrogen supply of plants is closely related to the availability of water, and in drought conditions nitrogen mineralization and
mobility are very limited38. The major source of plant nitrogen is mineral nitrogen, Nmin, which occurs in two main forms (Nmin = N-NH4+ + N-NO3−). Our results indicate that after the
application of PGA, the concentration of Nmin in the soil generally increased (Fig. 3). In the case of PGA2 and PGA1, the Nmin content was at a medium level, while in the case of PGA0.3 it
increased to a very high level (according to the proposed reference values for very light and light soils in Poland)39. N-NH4+ cations are retained in the soil by cation exchange, unlike
N-NO3−, which can be leached40,41. In the case of sandy soils (as in our experiment) characterized by a low cation exchange capacity, the addition of PGA played an essential role in the
binding of N-NH4+. At the highest concentration of PGA2, the N-NH4+ content was the highest. The binding of ions, including N-NH4+, is possibly due to the specific structure of this anionic
biopolymer, which has numerous carboxyl groups in the side chains9. Some part of the NH4+ probably resulted from PGA degradation, but we assume that it was a negligible amount. Otherwise,
the NH4+ content should be strongly dependent on the PGA concentration used and higher than in PGA0, but such an effect was not observed (Fig. 3b). The role of PGA in the “N-NH4+ turnover
pool” was described by Liu et al.42, highlighting the regulatory role of PGA, which enables the release of ammonium ions during its deficit in plants as well as the binding of any excess.
Consistent with our findings, the content of mineral forms of nitrogen, as well as the total nitrogen in the sandy clay loam soil, increased with an increase in the applied PGA dose. Such a
trend was also noted in a simplified system by Zhang et al.13. In soils, N-NH4+ is transformed into N-NO3− as a result of the nitrification process41. Our results following PGA2 treatment
demonstrated that the nitrification process was twice as slow relative to PGA0, as evidenced by the NH4+:NO3− ratio. The mean ratio for the reference treatment was 1.30, while for PGA2 it
was 2.95. The nitrogen nitrification process was most intense at the lowest PGA concentration. The NH4+:NO3− ratios were significantly lower at 0.98 and 0.65 for PGA1 and PGA0.3,
respectively. Similar results related to the retention and transitions over time of N-NH4+ and N-NO3− were reported by Zhang et al.43. This trend suggests that when higher doses of PGA are
applied on light soils, it may be possible to retain more N-NH4+ ions and reduce N-NO3− losses due to leaching, which is consistent with the results of the studies by Zhang et al.13. Due to
its chelating properties, PGA forms fully water-soluble salts with K+, Na+, Ca2+, and Mg2+ ions44. The present study showed an increase in the retention of alkaline cations by PGA, which is
a positive effect, especially in light soils with a low cation exchange capacity. The content of Ca2+ and Mg2+ ions was higher if higher concentrations of PGA were used. In conditions of
water deficit, Mg2+ is practically physiologically inaccessible to plants45, despite it being essential for the development of the root system and adequate root feeding46. Xu et al.47 showed
that the positive effect of PGA on nitrogen metabolism and thus plant growth is related to Ca2+ ions. In general, under drought stress, the availability of Ca2+ to plants decreases, but
only slightly compared to available P and K+45. The availability of P for plants and its accumulation in biomass decreases even under moderate drought conditions38. In the current study, a
similar tendency of available P accumulation in the soil was observed when using different PGA concentrations, as in the case of Ca2+ and Mg2+. In turn, Zhang et al.13 and Xue and Zhang48
found a decrease in the available P content in the soil after the application of PGA, which was associated with microbial immobilization. In the case of sandy soils (as in our experiment),
microbial biomass (C and N) is lower compared to silty or clayey soils49, hence a higher content of available P after using PGA compared to PGA0, especially when using lower concentrations.
The K+ content in soil, however, was different. The highest K+ content was found in PGA0, while in the case of PGA application, its content in the soil decreased with decreasing PGA
concentration, which proves its most intense uptake at lower PGA concentrations. K plays a very important role in the context of plant resistance to the effects of drought stress, as it is
responsible for regulating osmotic pressure and maintaining the turgor of plant cells50. Its mobility, and thus bioavailability, decreases as the water content of the soil decreases45. Zhang
et al.43 did not observe any effect of the application of the PGA additive on the available potassium content under watering conditions. In turn, the obtained results suggest that under
stress conditions, the addition of PGA (probably the higher the concentration of PGA, the more potassium ions were bound) increased the potassium uptake by plants. This proves the importance
of PGA in reducing the effects of drought stress in plants. The use of PGA also increased the uptake of Mn2+ and Zn2+ (apart from the PGA1 concentration), as evidenced by lower
concentrations of these ions compared to PGA0. Zn uptake is well illustrated by the RDA diagram, where its concentration is related to good N-NO3− supply51. Also, the availability of Fe and
its uptake by plants could be more closely related to the intensity of N-NO3− and H+ ion uptake, which leads to an increase in the reaction in the root zone and a reduction in iron
availability52. The increase in the pH value in relation to PGA0 at PGA2 (also PGA1) can be associated with: (1) increased content of calcium and magnesium ions, or (2) increased uptake of
N-NO3−, which leads to an increase in the reaction in the rhizosphere because H+ is transported along with NO3−. An increase in pH values by 0.1 to 0.2 units was also observed by Zhang et
al.43. On the other hand, at PGA0.3, the pH dropped slightly below the value for PGA0, which may have been the result of the most intensive oxidation of N-NH4+ to N-NO3− taking place at this
concentration, during which hydrogen ions are released, which promotes acidification41. The water content and its availability in the substrate determine the rate of nutrient supply to
plants during their life cycle. Drought stress is a key factor limiting root size growth, which in turn affects nutrient uptake38. Since the roots are the main system that allows water to be
taken from the soil, the rate of their growth, their degree of compaction, and the size they reach are of fundamental importance for plant tolerance of drought stress53. The architecture of
the root system is closely related to the distribution of moisture and nutrients in the soil profile. The development of long roots is related to the plant's adaptation to drought
conditions by its uptake of water available deeper in the profile54 and nutrients, such as nitrogen, that are leached to deeper layers55. The size of the root system is the main factor
limiting the uptake of P and Ca in particular, and to a lesser extent NO3− and K+32. The application of PGA has a positive effect on the length and activity of plant roots43,56. The results
of our experiments indicate that PGA stimulates the growth of the rhizosphere. The lowest concentration of PGA (PGA0.3) stimulated the growth of roots, as well as being responsible for a
significant concentration decrease for potassium in the soil. Potassium is essential for root cell proliferation in drought conditions, which accelerates root elongation50. Due to the better
development of the root system, the plant’s supply of nutrients and water also improved. This resulted in an increase in the yield of dry aboveground biomass and a smaller amount of dried
plants compared to fresh plants, the best results being obtained with the use of the lowest concentration of PGA (PGA0.3). In general, the better development of vegetation with the use of
PGA0.3 was associated with the lowest levels of the available forms of most macro- and micronutrients in the soil (except N-NO3−) (Fig. 7), which may be due to the more efficient uptake of
nutrients at the lowest PGA concentration used. At higher PGA concentrations, the effect of viscosity on the ion exchange between salt particles and soil solution, and thus on the
availability of nutrients, may be important. It was surprising that watering had no effect on soil parameters under conditions of drought stress and only a slight effect on plant parameters.
Sandy soils exhibit a high filtration index57, and even when watering was carried out with different PGA concentrations, no positive effect was noted. The only exception was the increase in
the share of dicotyledonous plants in the plots studied, their survival and germination on PGA plots being further enhanced by watering. Drought stress is also responsible for the reduction
in growth of perennials in agricultural and seminatural habitats58,59,60. Drought immobilizes the seeds in the soil as a survival adaptation to drought stress61. As a consequence, prolonged
drought events influence negatively on plant diversity, ecosystem functioning, and the available resources for pollinators25,26. In our studies, a low concentration of PGA increased the
DMR. This parameter was also accelerated by the watering conditions. These results indicate that PGA accelerates the dicotyledons’ growth, but it is necessary to water during the growing
season, and even giving 30% of the calculated water deficit for a given month in a multi-year period increased the DMR. PGA is a biopolymer that is produced by bacteria as a biofilm
component in artificial conditions, but should be treated as a natural element of ecosystems25,62. Its presence in agrosystems is reduced due to the elimination of many bacterial strains
during agricultural activities and soil changes63. Artificial supplementation of PGA into agroecosystems is beneficial for crops13,51, but it has not been previously applied to grasslands.
We showed that precise amounts of PGA enhance plant growth and the regeneration of shoots during drought periods. We do not believe that it directly holds water as a hydrogel, but it can be
regarded as an effective biostimulator64, helping the plant to grow and survive through dry periods. These data are preliminary and studies should be continued using different soil
conditions and also on intensively managed grasslands, as well as those of conservation value. CONCLUSIONS The results of our field experiment combining watering and the application of
different concentrations of γ-PGA to grassland during a period of drought clearly showed that PGA acts as biostimulant, alleviating the effects of stress in plants. The biostimulation effect
of PGA consisted in increasing the availability of ions, especially K+, Zn2+, Mn2+, Fe2+/3+, Ca2+, Mg2+, and mineral forms of nitrogen (N-NH4+ and N-NO3−), elongating plant roots, and
increasing the aboveground biomass, as well as improving the resprouting capacity of the dominant grass, _N. stricta_, and the regeneration of dicotyledons. In the case of meadows on sandy
soils, the use of low PGA concentrations (0.3% or 1%) was the most beneficial for both the availability of macro- and microelements and for plant functional traits. Irrigation had a greater
effect than using PGA only for the dicotyledon to monocotyledon ratio (DMR). Again, it was most advantageous to use the lowest concentration of PGA, but in combination with watering. On the
basis of the obtained results, we recommend using a PGA concentration in the range of 0.3–1% to manage grassland on light soils. PGA in the given doses is a promising treatment, which
protects grassland ecosystems from drought stress. DATA AVAILABILITY The data supporting the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES * Bever, J. D. Feedback between plants and their soil communities in an old field community. _Ecology_ 75, 1965–1977 (1994). Article Google Scholar * Wagg, C., Boller, B.,
Schneider, S., Widmer, F. & van der Heijden, M. G. A. Intraspecific and intergenerational differences in plant-soil feedbacks. _Oikos_ 124, 994–1004 (2015). Article ADS Google Scholar
* Pugnaire, F. I. _et al._ Climate change effects on plant-soil feedbacks and consequences for biodiversity and functioning of terrestrial ecosystems. _Sci. Adv._ 5, 1–12 (2019). Article
Google Scholar * Bennett, J. A. _et al._ Plant-soil feedbacks and mycorrhizal type influence temperate forest population dynamics. _Science_ 355, 181–184 (2016). Article ADS Google
Scholar * Pizano, C., Kitajima, K., Graham, J. H. & Mangan, S. A. Negative plant–soil feedbacks are stronger in agricultural habitats than in forest fragments in the tropical Andes.
_Ecology_ 100, 1–12 (2019). Article Google Scholar * Numan, M. _et al._ Plant growth promoting bacteria as an alternative strategy for salt tolerance in plants: A review. _Microbiol. Res._
209, 21–32 (2018). Article CAS PubMed Google Scholar * Bajaj, I. & Singhal, R. Poly(glutamic acid)—An emerging biopolymer of commercial interest. _Bioresour. Technol._ 102,
5551–5561 (2011). Article CAS PubMed Google Scholar * Buescher, J. M. & Margaritis, A. Microbial biosynthesis of polyglutamic acid biopolymer and applications in the
biopharmaceutical, biomedical and food industries. _Crit. Rev. Biotechnol._ 27, 1–19 (2007). Article CAS PubMed Google Scholar * Goto, A. & Kunioka, M. Biosynthesis and hydrolysis of
poly(γ-glutamic acid) from _Bacillus_ _subtilis_ IF03335. _Biosci. Biotechnol. Biochem._ 56, 1031–1035 (1992). Article CAS PubMed Google Scholar * Ogunleye, A. _et al._ Poly-γ-glutamic
acid: Production, properties and applications. _Microbiology (United Kingdom)_ 161, 1–17 (2015). CAS Google Scholar * Nair, P., Navale, G. R. & Dharne, M. S. Poly-gamma-glutamic acid
biopolymer: A sleeping giant with diverse applications and unique opportunities for commercialization. _Biomass Convers. Biorefinery_ https://doi.org/10.1007/s13399-021-01467-0 (2021).
Article Google Scholar * Sung, M. H. _et al._ Natural and edible biopolymer poly-γ-glutamic acid: Synthesis, production, and applications. _Chem. Rec._ 5, 352–366 (2005). Article CAS
PubMed Google Scholar * Zhang, L. _et al._ Effects of poly-γ-glutamic acid (γ-PGA) on plant growth and its distribution in a controlled plant-soil system. _Sci. Rep._ 7, 1–13 (2017). ADS
Google Scholar * Guo, J., Shi, W., Wang, P., Hao, Q. & Li, J. Performance characterization of γ-poly (glutamic acid) super absorbent polymer and its effect on soil water availability.
_Arch. Agron. Soil Sci._ 00, 1–14. https://doi.org/10.1080/03650340.2020.1871473 (2021). Article CAS Google Scholar * Xu, Z. _et al._ Effect of poly(γ-glutamic acid) on microbial
community and nitrogen pools of soil. _Acta Agric. Scand. Sect. B Soil Plant Sci._ 63, 657–668 (2013). CAS Google Scholar * Xu, Z. _et al._ Calcium involved in the poly(γ-glutamic
acid)-mediated promotion of Chinese cabbage nitrogen metabolism. _Plant Physiol. Biochem._ 80, 144–152 (2014). Article CAS PubMed Google Scholar * Guo, Z., Yang, N., Zhu, C. & Gan,
L. Exogenously applied poly-γ-glutamic acid alleviates salt stress in wheat seedlings by modulating ion balance and the antioxidant system. _Environ. Sci. Pollut. Res._ 24, 6592–6598 (2017).
Article CAS Google Scholar * Nambara, E. & Marion-Poll, A. Abscisic acid biosynthesis and catabolism. _Annu. Rev. Plant Biol._ 56, 165–185 (2005). Article CAS PubMed Google
Scholar * Wang, Q. _et al._ Co-producing lipopeptides and poly-γ-glutamic acid by solid-state fermentation of _Bacillus subtilis_ using soybean and sweet potato residues and its biocontrol
and fertilizer synergistic effects. _Bioresour. Technol._ 99, 3318–3323 (2008). Article CAS PubMed ADS Google Scholar * Xu, Z. _et al._ Poly-γ-glutamic acid induces system tolerance to
drought stress by promoting abscisic acid accumulation in _Brassica napus_ L. _Sci. Rep._ 10, 1–10 (2020). Google Scholar * Ballabio, C., Panagos, P. & Monatanarella, L. Mapping topsoil
physical properties at European scale using the LUCAS database. _Geoderma_ 261, 110–123 (2016). Article ADS Google Scholar * Jadczyszyn, J. & Bartosiewicz, B. Processes of soil
drying and degradation. _Studia i Raporty IUNG-PIB_ 64, 49–60 (2020) (IN POLISH). Google Scholar * Carlsson, M., Merten, M., Kayser, M., Isselstein, J. & Wrage-Mönnig, N. Drought stress
resistance and resilience of permanent grasslands are shaped by functional group composition and N fertilization. _Agric. Ecosyst. Environ._ 236, 52–60 (2017). Article CAS Google Scholar
* Bollig, C. & Feller, U. Impacts of drought stress on water relations and carbon assimilation in grassland species at different altitudes. _Agric. Ecosyst. Environ._ 188, 212–220
(2014). Article Google Scholar * Broadhurst, L. M. _et al._ Maximizing seed resources for restoration in an uncertain future. _Bioscience_ 66, 73–79 (2016). Article Google Scholar *
Nogueira, C., Bugalho, M. N., Pereira, J. S. & Caldeira, M. C. Extended autumn drought, but not nitrogen deposition, affects the diversity and productivity of a Mediterranean grassland.
_Environ. Exp. Bot._ 138, 99–108 (2017). Article Google Scholar * Ellison, A. M. & Degrassi, A. L. All species are important, but some species are more important than others. _J. Veg.
Sci._ 28, 669–671 (2017). Article Google Scholar * Korzeniak, J. Mountain _Nardus stricta_ grasslands as a relic of past farming—The effects of grazing abandonment in relation to elevation
and spatial scale. _Folia Geobot._ 51, 93–113 (2016). Article Google Scholar * Matos, I. S., Eller, C. B., Oliveras, I., Mantuano, D. & Rosado, B. H. P. Three eco-physiological
strategies of response to drought maintain the form and function of a tropical montane grassland. _J. Ecol._ 109, 327–341 (2021). Article Google Scholar * Chen, D. & Chen, H. W. Using
the Köppen classification to quantify climate variation and change: An example for 1901–2010. _Environ. Dev._ 6, 69–79 (2013). Article Google Scholar * Cornelissen, J. H. C. _et al._ A
handbook of protocols for standardised and easy measurement of plant functional traits worldwide. _Aust. J. Bot._ 51, 335–380 (2003). Article Google Scholar * Rouphael, Y., Cardarelli, M.,
Lucini, L., Rea, E. & Colla, G. Nutrient solution concentration affects growth, mineral composition, phenolic acids, and flavonoids in leaves of artichoke and cardoon. _HortScience_ 47,
1424–1429 (2012). Article CAS Google Scholar * Xu, J. _et al._ Properties of starch–polyglutamic acid (PGA) graft copolymer prepared by microwave irradiation—Fourier transform infrared
spectroscopy (FTIR) and rheology studies. _Starch/Staerke_ 69, 1–7 (2017). Article Google Scholar * Bai, N. _et al._ Effects of application rates of poly-γ-glutamic acid on vegetable
growth and soil bacterial community structure. _Appl. Soil Ecol._ 147, 103405 (2020). Article Google Scholar * Norton, M. R. _et al._ Plant drought survival under climate change and
strategies to improve perennial grasses. A review. _Agron. Sustain. Dev._ 35, 1–15 (2015). Google Scholar * Liang, J., Shi, W., He, Z., Pang, L. & Zhang, Y. Effects of poly-γ-glutamic
acid on water use efficiency, cotton yield, and fiber quality in the sandy soil of southern Xinjiang, China. _Agric. Water Manag._ 218, 48–59 (2019). Article Google Scholar *
Meyer-Grünefeldt, M., Calvo, L., Marcos, E., Von Oheimb, G. & Härdtle, W. Impacts of drought and nitrogen addition on _Calluna_ heathlands differ with plant life-history stage. _J.
Ecol._ 103, 1141–1152 (2015). Article Google Scholar * da Silva, E., Nogueira, R., da Silva, M. & de Albuquerque, M. Drought stress and plant nutrition. _Plant Stress_ 5, 32–41 (2011).
Google Scholar * Faber, A., Jarosz, Z., Rutkowska, A. & Jadczyszyn, T. Reduction of nitrogen losses in winter wheat grown on light soils. _Agronomy_ 11, 2337 (2021). Article CAS
Google Scholar * Nordin, A., Högberg, P. & Näsholm, T. Soil nitrogen form and plant nitrogen uptake along a boreal forest productivity gradient. _Oecologia_ 129, 125–132 (2001). Article
PubMed ADS Google Scholar * Haygarth, P. M., Bardgett, R. D. & Condron, L. M. Nitrogen and phosphorus cycles and their management. In _Soil Conditions and Plant Growth_ (eds
Gregory, P. J. & Northcliff, S.) 132–159 (Blackwell Publishing, 2013). https://doi.org/10.1002/9781118337295.ch5. Chapter Google Scholar * Chen, L. _et al._ The effects of ploy
(γ-glutamic acid) on spinach productivity and nitrogen use efficiency in north-west China. _Plant Soil Environ._ 64, 517–522 (2013). Article Google Scholar * Zhang, L. _et al._ Effects of
poly-γ-glutamic acid on soil nitrogen and carbon leaching and CO2 fluxes in a sandy clay loam soil. _Can. J. Soil Sci._ 97, 319–328 (2017). CAS Google Scholar * Ho, G. H. _et al._
γ-polyglutamic acid produced by _Bacillus subtilis_ (natto): Structural characteristics, chemical properties and biological functionalities. _J. Chin. Chem. Soc._ 53, 1363–1384 (2006).
Article CAS Google Scholar * Hu, Y. & Schmidhalter, U. Drought and salinity: A comparison of their effects on mineral nutrition of plants. _J. Plant Nutr. Soil Sci._ 168, 541–549
(2005). Article CAS Google Scholar * Verbruggen, N. & Hermans, C. Physiological and molecular responses to magnesium nutritional imbalance in plants. _Plant Soil_ 368, 87–99 (2013).
Article CAS Google Scholar * Xu, Z. _et al._ Calcium involved in the poly(γ-glutamic acid)-mediated promotion of Chinese cabbage nitrogen metabolism. _Plant Physiol. Biochem._ 80, 144–152
(2014). Article CAS PubMed Google Scholar * Xue, Y. & Zhang, W. Effect of γ-polyglutamic acid on available phosphorus in soil with phosphate rock powder. _IOP Conf. Ser. Earth
Environ. Sci._ 697, 012031 (2021). Article Google Scholar * Nannipieri, P. Soil is still an unknown biological system. _Appl. Sci._ 10, 3717 (2020). Article CAS Google Scholar * Sustr,
M., Soukup, A. & Tylova, E. Potassium in root growth and development. _Plants_ 8, 435 (2019). Article CAS PubMed PubMed Central Google Scholar * Erenoglu, E. B., Kutman, U. B.,
Ceylan, Y., Yildiz, B. & Cakmak, I. Improved nitrogen nutrition enhances root uptake, root-to-shoot translocation and remobilization of zinc (65Zn) in wheat. _New Phytol._ 189, 438–448
(2011). Article CAS PubMed Google Scholar * Barker, A. V. & Bryson, G. M. Nitrogen. In _Handbook of plant nutrition_ (eds Barker, A. V. & Pilbeam, D. J.) 21–51 (CRC Press, 2016).
Chapter Google Scholar * Ahmad, N., Malagoli, M., Wirtz, M. & Hell, R. Drought stress in maize causes differential acclimation responses of glutathione and sulfur metabolism in leaves
and roots. _BMC Plant Biol._ 16, 1–15 (2016). Article Google Scholar * Comas, L. H., Becker, S. R., Cruz, V. M. V., Byrne, P. F. & Dierig, D. A. Root traits contributing to plant
productivity under drought. _Front. Plant Sci._ 4, 1–16 (2013). Article Google Scholar * Khan, M. A., Gemenet, D. C. & Villordon, A. Root system architecture and abiotic stress
tolerance: Current knowledge in root and tuber crops. _Front. Plant Sci._ 7, 1–13 (2016). Article Google Scholar * Liu, J. _et al._ Root size and nitrogen-uptake activity in two maize
(_Zea_ _mays_) inbred lines differing in nitrogen-use efficiency. _J. Plant Nutr. Soil Sci._ 172, 230–236 (2009). Article CAS Google Scholar * Tolk, J. A., Evett, S. R., Xu, W. &
Schwartz, R. C. Constraints on water use efficiency of drought tolerant maize grown in a semi-arid environment. _Food Crops Res._ 186, 66–77 (2016). Google Scholar * Emami Bistgani, Z.,
Siadat, S. A., Bakhshandeh, A., Ghasemi Pirbalouti, A. & Hashemi, M. Interactive effects of drought stress and chitosan application on physiological characteristics and essential oil
yield of _Thymus daenensis_ Celak. _Crop J._ 5, 407–415 (2017). Article Google Scholar * Norton, M. R., Malinowski, D. P. & Volaire, F. Plant drought survival under climate change and
strategies to improve perennial grasses. A review. _Agron. Sustain. Dev._ 36, 29 (2016). Article Google Scholar * Siebert, F., Klem, J. & Van Coller, H. Forb community responses to an
extensive drought in two contrasting land-use types of a semi-arid Lowveld savanna. _Afr. J. Range Forage Sci._ 37, 53–64 (2020). Article Google Scholar * Morena-de las Heras, M.,
Turnbull, L. & Wainwright, J. Seed-bank structure and plant-recruitment conditions regulate the dynamics of a grassland-shrubland Chihuahuan ecotone. _Ecology_ 97, 2303–2318 (2016).
Article Google Scholar * Kunioka, M. Biodegradable water absorbent synthesized from bacterial poly(amino acids). _Macromol. Biosci._ 4, 324–329 (2004). Article CAS PubMed Google Scholar
* Singh, V. K., Singh, A. K., Singh, P. P. & Kumar, A. Interaction of plant growth promoting bacteria with tomato under abiotic stress: A review. _Agric. Ecosyst. Environ._ 267,
129–140 (2018). Article CAS Google Scholar * Ma, H. _et al._ Poly-γ-glutamic acid enhanced the drought resistance of maize by improving photosynthesis and affecting the rhizosphere
microbial community. _BMC Plant Biol._ 22(1), 11 (2022). Article CAS PubMed PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS The authors are grateful to the anonymous
landowners who provided the experimental site and agreed the application of experimental PGA concentrations. The project was funded by SUB/2021-050013-DO11. Thanks are due to eCORRECTOR Ltd
for the English proofreading. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Tunneling Group, Biotechnology Center, Silesian University of Technology, Gliwice, Poland Tomasz Skalski,
Katarzyna Papaj, Artur Góra & Divine Shytum * Department of Chemistry, Silesian University of Technology, Gliwice, Poland Anna Kasprzycka * Department of Land Reclamation and
Development, University of Agriculture, Krakow, Poland Ewelina Zając * Department of Horticulture, University of Agriculture, Krakow, Poland Elżbieta Jędrszczyk * Institute of Biology,
Biotechnology and Environmental Protection, University of Silesia, Katowice, Poland Joanna Kohyt * Department of Ecology, Climatology and Air Protection, University of Agriculture, Krakow,
Poland Barbara Skowera & Agnieszka Ziernicka-Wojtaszek Authors * Tomasz Skalski View author publications You can also search for this author inPubMed Google Scholar * Ewelina Zając View
author publications You can also search for this author inPubMed Google Scholar * Elżbieta Jędrszczyk View author publications You can also search for this author inPubMed Google Scholar *
Katarzyna Papaj View author publications You can also search for this author inPubMed Google Scholar * Joanna Kohyt View author publications You can also search for this author inPubMed
Google Scholar * Artur Góra View author publications You can also search for this author inPubMed Google Scholar * Anna Kasprzycka View author publications You can also search for this
author inPubMed Google Scholar * Divine Shytum View author publications You can also search for this author inPubMed Google Scholar * Barbara Skowera View author publications You can also
search for this author inPubMed Google Scholar * Agnieszka Ziernicka-Wojtaszek View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS T.S.:
Supervision, Conceptualization, Methodology, Validation, Formal analysis, Resources, Data Curation, Writing—Original Draft and Review and Editing, Visualization. E.Z: Methodology,
Writing—Review and Editing. E.J.: Methodology, Investigation, Resources, Data Curation. K.P.: Data Curation, Writing—Review and Editing, J.K.: Methodology, Investigation, Resources, Data
Curation, Writing—Original Draft and Review. A.G.: Writing—Original Draft and Review and Editing, Funding acquisition. A.K.: Investigation, Resources, Data Curation. D.S.: Resources, Data
Curation, Writing—Review and Editing. B.S.: Methodology, Investigation, Resources, Data Curation. A.Z.W: Methodology, Investigation, Resources, Data Curation. CORRESPONDING AUTHOR
Correspondence to Tomasz Skalski. ETHICS DECLARATIONS COMPETING INTERESTS TS, EJ, BS, AZW, and EZ declare their authorship of the patent application (P.446020) where some of the presented
results were included. ADDITIONAL INFORMATION PUBLISHER'S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION 1. SUPPLEMENTARY INFORMATION 2. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0
International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the
source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's
Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not
permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Skalski, T., Zając, E., Jędrszczyk, E. _et al._ Effects of γ-polyglutamic acid on
grassland sandy soil properties and plant functional traits exposed to drought stress. _Sci Rep_ 14, 3769 (2024). https://doi.org/10.1038/s41598-024-54459-1 Download citation * Received: 21
September 2023 * Accepted: 13 February 2024 * Published: 14 February 2024 * DOI: https://doi.org/10.1038/s41598-024-54459-1 SHARE THIS ARTICLE Anyone you share the following link with will
be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt
content-sharing initiative