A systematic review and network meta-analysis of pharmaceutical interventions used to manage chronic pain
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT It is estimated 1.5 billion of the global population suffer from chronic pain with prevalence increasing with demographics including age. It is suggested long-term exposure to
chronic could cause further health challenges reducing people’s quality of life. Therefore, it is imperative to use effective treatment options. We explored the current pharmaceutical
treatments available for chronic pain management to better understand drug efficacy and pain reduction. A systematic methodology was developed and published in PROSPERO (CRD42021235384).
Keywords of opioids, _acute pain_, _pain management_, _chronic pain_, _opiods_, _NSAIDs_, and_ analgesics_ were used across PubMed, Science direct, ProQuest, Web of science, Ovid Psych INFO,
PROSPERO, EBSCOhost, MEDLINE, ClinicalTrials.gov and EMBASE. All randomised controlled clinical trials (RCTs), epidemiology and mixed-methods studies published in English between the 1st of
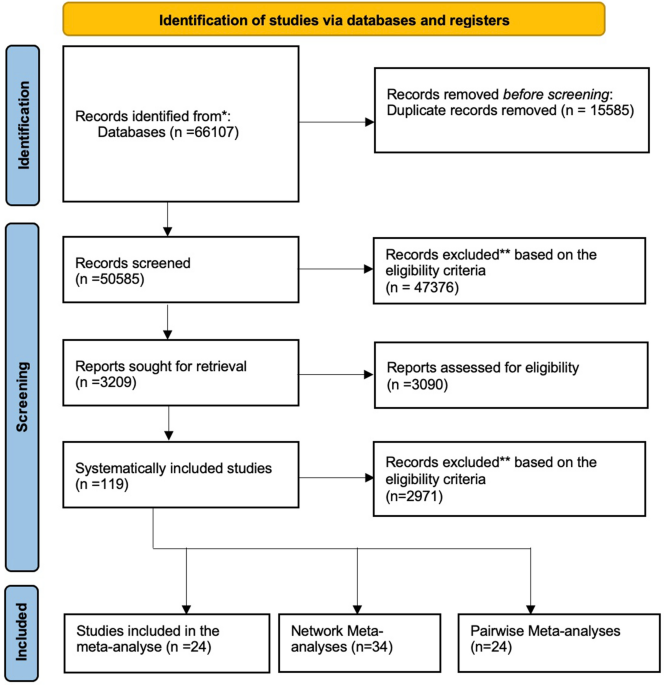
January 1990 and 30th of April 2022 were included. A total of 119 studies were included. The data was synthesised using a tri-partied statistical methodology of a meta-analysis (24),
pairwise meta-analysis (24) and network meta-analysis (34). Mean, median, standard deviation and confidence intervals for various pain assessments were used as the main outcomes for
pre-treatment pain scores at baseline, post-treatment pain scores and pain score changes of each group. Our meta-analysis revealed the significant reduction in chronic pain scores of
patients taking NSAID versus non-steroidal opioid drugs was comparative to patients given placebo under a random effects model. Pooled evidence also indicated significant drug efficiency
with Botulinum Toxin Type-A (BTX-A) and Ketamine. Chronic pain is a public health problem that requires far more effective pharmaceutical interventions with minimal better side-effect
profiles which will aid to develop better clinical guidelines. The importance of understanding ubiquity of pain by clinicians, policy makers, researchers and academic scholars is vital to
prevent social determinant which aggravates issue. SIMILAR CONTENT BEING VIEWED BY OTHERS A SYSTEMATIC REVIEW AND BAYESIAN META-ANALYSIS OF MEDICAL DEVICES USED IN CHRONIC PAIN MANAGEMENT
Article Open access 12 June 2024 PARACETAMOL VERSUS IBUPROFEN IN TREATING EPISODIC TENSION-TYPE HEADACHE: A SYSTEMATIC REVIEW AND NETWORK META-ANALYSIS Article Open access 06 December 2023
TOLERABILITY OF DIFFERENT DOSES OF OLICERIDINE VERSUS TRADITIONAL OPIOIDS IN ACUTE PAIN MANAGEMENT: A SYSTEMATIC REVIEW AND META-ANALYSIS Article Open access 03 April 2025 INTRODUCTION
Chronic non-cancer pain conditions are prevalent, highly debilitating and have high cost implications to health and social care. These conditions affect patients, their families and society
at large, impacting 20% of the global population1. The prevalence of pain conditions among females of all ages appears to be increasing2. Complexities around diagnosis and treatment of
chronic pain conditions have meant that there is a paucity of standardised clinical guidelines that could potentially improve the clinical practice landscape, globally. Convalescent periods
for many chronically ill patients can be protracted and daunting. This may be especially true where pain medication has been used in the long term3. Long-term exposures to chronic pain
coincide with mental health and wellbeing, exacerbating patient-reported outcomes such as sleep disturbances, depression, dependence and morbidities such as myalgia and fatigue4. Better
understanding of long-term implications requires consideration of “life-course approaches” and at present, this could evolve further within pain medicine epidemiology5. Increases in chronic
pain conditions contributes to higher healthcare costs towards clinical management of patients and also reduced levels of productivity for employers6. This may be partly due to increases in
opioid use within this population of patients, often reducing their capacity to conduct normal working hours. Current clinical guidelines recommend non-invasive pain management options as a
first-line treatment among non-cancer patients in particular, although overdose, dependency and mortality due to opioid use has consistently increased over time7,8. It was reported that
global opioid use has doubled between 2001 and 2003 to 2011 and 2013 to 7.35 billion daily doses per year9,10. It is particularly important to develop evidence-based guidelines specific to
each condition, with flexible pain medication use as a single regimen or a combination of treatments that could improve the overall quality of life of these patients11,12. The premise to
increase the strength and frequency of pain medications is in general based on disease burden i.e., progression of symptoms and patients reported symptoms4. We have designed the POP project
as the initial step to conduct exploratory work on pharmaceutical management of chronic pain. With the rising need for comparative effectiveness research, increasingly more systematic
reviews focus on evaluating the relative efficacy and acceptability of drugs and therapeutic interventions3,13. However, some of the interventions for long-term conditions are not available
for clinical practice and there are several options with varying efficacy even within a specific class of interventions14. METHODS We developed a wide systematic methodology and published
this as a protocol with multiple research questions in the first instance in PROSPERO (CRD42021235384). Data from studies meeting the inclusion criteria were extracted and Pairwise
Meta-Analysis with random and fixed effects models was carried out. Pooled mean difference (MD) together with 95% confidence intervals (CIs) are reported overall and for sub-groups. By
combining the direct and indirect comparisons between different interventions, Network Meta-Analysis was conducted to explore the relative treatment effects among all the drugs included in
our analysis. AIMS The aims of the study was to explore the prevalence of treatments of effects in chronic pain based on pharmaceutical treatments. SEARCH STRATEGY The search strategy used
key words of _chronic pain_, _opioids_, _acute pain_, _pain management_, _opiods_, _NSAIDs, analgesics_ across multiple databases (PubMed, Science direct, ProQuest, Web of science, Ovid
Psych INFO, PROSPERO, EBSCOhost, MEDLINE, ClinicalTrials.gov and EMBASE). ELIGIBILITY CRITERIA All randomised controlled clinical trials (RCTs), epidemiology and mixed-methods studies
reporting the use of pain medication for non-cancer chronic pain conditions published in English between the 1st January 1990 and 30st April 2022 were included. Opinions, commentaries and
editorials were excluded (Fig. 1). DATA EXTRACTION Participants included in the study populations had chronic non-cancer pain conditions. All studies reporting drug efficacy were extracted
by way of the interventions, measures of tool and numeric results. An extraction template specific to the objectives of the study was developed. Sub-studies were extracted from the same
clinical trials with different duration periods. Data was extracted by two investigators and any disputes for eligibility was discussed and agreed with the Chief Investigator of the study.
All studies included within the analyses were independently reviewed. OUTCOME MEASURES Outcomes were reported as mean, median, standard deviation and confidence intervals. Mean and Standard
deviation (SD) were extracted as the main outcomes including pre-treatment pain scores at baseline, post-treatment pain scores and pain score changes of each group. Multiple pain assessments
for confirming a clinical diagnosis, severity and progression of chronic pain were identified. These include VAS (visual analogue scale, 0–10 or 0–100), NRS (11-point numeric rating scale,
0–10), BPI (Brief Pain Inventory interference scale, 0–10), MPQS (McGill Pain Questionnaire-Short Form (Sensory and Affective subscales, VAS intensity measure, 0–10), VRS (verbal rating
scale, 0–10), NIH-CPSI (National Institutes of Health Chronic Prostatitis Symptom Index, pain scores, 0–21), PI (pain intensity on a 20-point scale, 0–20). As most widely used tools for
assessing pain such as VAS, NRS, VRS, use a 11-point numeric rating scale from 0 to 10, the following standardisation formula was used to unify all pain scores into the same scale:
$$\mathrm{Scaled \, Pain \, Score }=\mathrm{ Original \, Pain \, Score }* \frac{10}{\mathrm{Scale \, Range}}$$ As all outcomes of interest were continuous, the calculation based on pain
scores was performed by using mean differences (MD) with a 95% confidence interval (CI) to report the effects between the group comparisons. EXPOSURES The exposures of interest were selected
based on the key features of pharmacological management used to treat non-cancer chronic pain, including and not limited to a pain condition being the primary or the secondary condition.
Neurological and psychological symptoms leading up to the use of pharmaceutical use within the included population were also considered. STATISTICAL ANALYSIS PLAN A meta-analysis, pairwise
meta-analysis (PMA) and Network meta-analysis (NMA) were used to compare all treatments used in managing non-cancer chronic pain. The fundamental difference between them is that PMA produced
only one estimate of pooling effects from the selected pair of interventions, while the NMA produced multiple comparative estimates of pooling effects by connecting all alternative
interventions16. We incorporated direct and indirect treatment comparisons within the NMA providing greater statistical precision compared to a PMA. Rankings of a set of drugs or combined
interventions for assessing chronic pain with respect to their efficacy was calculated based on the network models. Homogeneity and Consistency were tested to see if the assumptions in NMA
were violated. The overall pharmaceutical efficacy of extracted studies was produced by pooling all treatment effects. PMA was also used on studies with the same drug as the treatment group
to see the specific drug efficacy. \({{\text{I}}}^{2}\) and p-value were commonly used to detect statistical heterogeneity. A value of \({{\text{I}}}^{2}\) larger than 50% with a much
smaller p-value indicates strong heterogeneity. Correspondingly, \({{\text{I}}}^{2}\) less than 50% with a large p-value indicates fairly weak heterogeneity17. A random effects model was
chosen when there was high heterogeneity, whereas a fixed effects model was used if weak or no heterogeneity was detected18. Due to the presence of high heterogeneity, subgroup analyses were
carried out to identify the sources. To assess the robustness of the pooled results within the PMA, a sensitivity analysis was completed. Publication bias was evaluated with funnel plots
and Egger tests. The statistical analyses were produced by R and packages were used to provide outputs in compliance with best practice and reporting guidelines19. RESULTS Of the 119
systematically included studies (Table 1) with 17,708 participants, 24 studies were used in the meta-analysis and 34 within the NMA to build a connected network. Opioids (Table 2) were
tested in 32 (26.89%) studies with 5518 (31.16%) participants, where _Morphine_, _Oxycodone_ and _Fentanyl_ were common. _Lidocaine_, _Naloxone_ and _Gabapentin_ were the most frequently
tested non-opioid drugs for chronic pain. The most common pain among chronic pain patients were lower back pain, which was explored in 26 (21.85%) studies with a pooled sample of 4626
(26.12%) while 13 studies reported chronic back pain among 1068 (6.03%) participants. The following pain types are post-surgical pain and neuropathic pain with 19 (15.97%) and 10 (8.4%)
studies involved to test the efficiency of NSAID drugs on patients. Meta-analysis of mean difference of pain scores were applied to 24 studies with a sample of 2546 participants, producing a
pooled mean difference (MD) of – 0.89 (95% CI [− 1·31, − 0·47]). There was a significant difference between chronic pain scores of patients taking NSAIDs compared to a placebo. Averagely,
0.89 point (0–10 scale) of pain reduction was observed based on the random effects model. A significant statistical drug efficiency was observed with BTX-A and Ketamine. A negative pooled
mean difference was determined between BTX-A and Ketamine versus a placebo with a pain reduction of 0.98–1.26 based on a − 10 scale, respectively. Similar statistical results were not
observed with other drugs in comparison to a placebo. Within the common comparator as a “_placebo_”, the connected network included 34 studies, 52 pairwise comparisons, 32 interventions and
29 study designs. Gabapentin had a significant mean difference equalling to – 1.49 (95% CI [− 2⋅76, − 0⋅23], p-value < 0.05). Most interventions had a negative mean difference compared to
a _placebo_, but a 95% CI covering 0 indicated insignificant effects for reducing pain. The results within the network were more conservative with the combination of direct and indirect
evidence indicating most pharmaceutical interventions selected might have benefited from the “_placebo effect_”. PAIRWISE META-ANALYSIS (PMA) The PMA included 24 studies with pairwise
comparisons between drugs and a placebo. The experimental and control group comprised of "Amitriptyline", "BTX-A”, “Gabapentin", "Ketamine",
"Lidocaine", "Morphine", "Naloxone" and a placebo, respectively. A single study reported "Fentanyl", "Ningmitai", "THC", and
"Oxycodone". PMA FOR BASELINE PAIN SCORE The PMA was used to test baseline pain score differences between the experimental and control group in 18 studies which comprised of a
total sample of 1691 participants. The experimental and control groups comprised of 837 and 854 participants, respectively, with a pooled mean difference (MD) of – 0.02 (95% CI [− 0.13,
0.08]). The 95% CI was 0 and therefore, no statistically significant difference between baseline pain scores of two groups (Fig. 2). A weak statistical heterogeneity of 15% of \({I}^{2}\) (p
= 0.26) was determined. This combined with the statistical insignificance indicates the randomisation of was completed accurately and that it is scientifically justifiable to use the
post-treatment pain scores directly as the outcomes to evaluate treatment effects. PMA FOR DRUG EFFICACY BETWEEN NSAID COMPARED TO A PLACEBO This PMA included 24 studies (Fig. 3) with 2418
participants, with a MD of − 0.89 (95% CI [− 1.31, − 0.47]). The experimental and control group comprised of 1219 and 1199, respectively. A significant statistical heterogeneity of 92% of
\({I}^{2}\) (p-value < 0.01) was identified. Mean difference (MD) was calculated to assess if there is statistically significant difference of post-treatment pain scores between
experimental group and control group. The 95% CI was less than 0 which indicated a significant treatment effect with a reduction in pain by 0.89-point (0–10 scale) compared to those who were
given a placebo. META-ANALYSES A statistically low heterogeneity of 0% of \({I}^{2}\) (p-value > 0.5) was identified among studies with _BTX-A, Ketamine_ and _Naloxone_ (Fig. 4b,d).
_BTX-A_ (Fig. 4b) and _Ketamine _(Fig. 4d) indicated statistically significant drug efficacy of – 1.07 [−1.51, − 0.64] and − 1.26 [− 1.85, − 0.68], respectively. The treatment efficiency
compared to the placebo had a 1 point pain reduction within a 0–10 evaluation scale. Ketamine demonstrated optimal efficacy with a 1·26 point pain reduction on average. The PMA for _BTX-A_
(Fig. 4b) and _Naloxone_ (Fig. 4g) showed a low heterogeneity as the data was pooled from a single study. Studies on _Amitriptyline, Gabapentin, Lidocaine and Morphine_ had a high
heterogeneity and a statistically insignificant drug efficacy (Fig. 4a,c,e,f). The mean difference of 95% CI was 0 indicating an insignificant treatment difference between the drugs and
placebo based on the random effects model. OPIOIDS DRUGS A meta-analysis was conducted with 4 studies (Fig. 5). A pooled MD of – 0.65 and a 95% CI [− 1.67, 0.37] was determined indicating an
insignificant treatment effect of opioids drugs compared to a placebo. A statistically significant heterogeneity of 92% of \({I}^{2}\) (p-value < 0·01) was identified. NETWORK
META-ANALYSIS (NMA) A NMA (Fig. 6) was completed for 34 studies. The nodes correspond to each intervention included within the network where the interventions with direct comparisons are
linked with a line. The thickness of lines corresponds to the number of trials evaluating the comparison. A connected network was built based on the _placebo_ which was mostly _Tolterodine_
based on the original studies. The evaluations between interventions were supported by direct comparison and indirect comparison. In the network with the placebo as the reference group,
_Gabapentin_ (Fig. 7) comprised of a MD equaling to – 1.49 (95% CI [− 2.76, − 0.23], p-value < 0.05) indicating a significant effect on reducing chronic pain and direct comparisons were
made using 4 studies (Fig. 8a). The pooled MD of _Botulinum_ and _Ketamine_ were −1.06 and – 1.24, respectively. These were similar to the results in the PWA, but their 95% CI was 0
therefore showed insignificant effect on pain reduction compared to a placebo. Most combined interventions had a negative MD compared to a placebo with a 95% CI of 0 indicated statistically
insignificant results for reducing pain. _Imipramine_, _Diosimin_, _Desipramine_, _Clobazam_, _Piroxicam_ and _Tiagabine_ had not been directly compared to a placebo based on the identified
data therefore the comparative treatment effected between them and a placebo was not possible to complete. SUBGROUP ANALYSIS A subgroup analyses was conducted for 24 studies within the
meta-analysis to explore the sources of heterogeneity and unbiased estimation based on age, pain type, period and geographical location (Fig. 9). The sub-group analysis for pain type, time
period and geographical location can be found in the Supplementary file whilst average age is shown below. SUBGROUP ANALYSIS FOR PAIN CORE DIFFERENCE BASED ON DIFFERENT AGE GROUPS It showed
that the heterogeneity among studies with participants who were older than 50 years old had changed with decreased _I_2 (_I_2 = 48% for “51–60”, _I_2 = 68% for “61–71”). A common effects
model was chosen for subgroup “51–60”, which produced a higher estimation of pain reduction with a mean difference of – 1.46 (95% CI [− 1.74, − 1.18]). Based on the high heterogeneity (_I_2
> 50%), random effects models were built for other subgroups. The group with participants younger than 40 years older obtained a significant drug efficiency (MD − 1·05, 95% CI [− 1.85, −
0.24]). The pooled drug effects (Fig. 9) in the 41–50 and 61–71 years of age groups were much lower than the overall treatment effect of NSAID drugs identified in the PMA. The 95% CI of 0
indicated statistically ineffective compared to the placebo. The random effects models showed the decrease of heterogeneity indicating that age may be a source of heterogeneity. SENSITIVITY
ANALYSIS The sensitivity analysis was conducted (Fig. 10) for the PMA where some studies influenced the pooled results compared to the overall estimation (− 0.89). To test this theory, study
number 71 and 100 were omitted and the pooled results were much lower, − 0.82 and – 0.79, respectively. Studies with _Amitriptyline_ and _Gabapentin_ produced unstable treatment results,
and the absence of these showed an overestimation (study 81, 45) or underestimates (study 71, 100). Collectively, the high heterogeneity (_I_2 = 92% p-value < 0.01) was stable and a
robust treatment effect with negative mean differences and a significant 95% CI remained. Therefore, the pooled treatment effects identified was credible. PUBLICATION BIAS The funnel plots
(Fig. 11) within the PMA indicated symmetry. Although several studies were not within the remit of the funnel, the Egger’s test showed a p value (0.22) larger than 0.05 which indicated the
lack of small-study effects (Table 3). DISCUSSION We identified opioids and non-opioids were the two primary classes of pharmacological interventions in chronic pain management. Opioids are
widely used in the management of cancer pain and non-cancer associated pain20,21. The long-term use of opioids in the management of chronic non-malignant pain has come under scrutiny more
recently and is now recommended only if benefits of initiating treatment would significantly outweigh the potential risks, and possibly as an adjunct to the primary intervention22,23. Our
study has shown that judicious use of non-opioid medications along with other treatment modalities could provide better outcomes in managing chronic pain thereby removing long-term
side-effects observed during opioid therapy. With cancer patients increasingly being cured or achieving long term remission, prolonged use of opioids could result in aberrant behaviour and
dependence. Awareness of an opioid crisis globally has prompted clinicians to exercise caution in their prescription habits, but the WHO supports the use of opioids including Fentanyl and
Methadone as an essential class of medication for the management of cancer pain24,25. The meta-analysis of baseline pain scores lacked statistical significance between experimental and
control groups. The significant reduction in chronic pain scores of patients taking NSAID versus non-steroidal opioid drugs compared to patients given placebo under a random effects model.
The presence of a significant drug efficiency with _BTX-A_ and _Ketamine_ is interesting although the pooled results of other drugs and interventions had statistically insignificant results
with a 95% CI of 0. The pooled evidence indicated Ketamine showed the highest pain reduction (1.26) followed by BTX-A (0.98). Studies testing on other drugs including Amitriptyline,
Gabapentin, Morphine and Lidocaine had a high heterogeneity and insignificant drug efficiency. Overall, evidence from the PMA showed a strong efficacy within the NSAIDs group with managing
pain which were remarkably narrowed when exclusive trials with low risk of bias were included26,27,28. In this study, a pairwise meta-analysis and NMA consolidating the evidence of 46
studies was carried out, with the former comparing several different opioids. Morphine has traditionally been used for the management of moderate to severe chronic pain29. Despite morphine
being a potent analgesic [MD 0.01 (95% CI [− 1.18, 1.21], newer opioids are now being employed owing to their superior safety profile. Oxycodone and Fentanyl appear to be popular due to
better availability and vast clinical experience including the well accepted effectiveness demonstrated, as per patient and clinically reported outcomes. Our results are aligned to these
trends where the effectiveness is shown to include a MD 1.77 (95% CI [− 2.11, − 1.43]) for Oxycodone and a MD of − 0.90 (95% CI [− 2.03, 0.23])] for Fentanyl (32). However, untoward
gastrointestinal effects (constipation, nausea, and vomiting) still remain a major concern with opioid use and are often responsible for discontinuation of treatment30,31. Recent evidence
favours the use of a combination of oxycodone and naloxone in patients with chronic pain (after ensuring that there is no cause for porto-systemic anastomosis), to offer an improved bowel
function without any effective change in analgesia32. The concerns of developing tolerance, opioid-induced hyperalgesia, aberrant behaviour and dependence with opioids is a pragmatic reason
to develop effective alternative treatment modalities especially for vulnerable individuals. In pairwise comparison, we observed Ketamine to be superior to other pharmacological
interventions with a mean difference MD − 1.26 (95% CI [− 1.85, − 0.68]). There are several guidelines recommending the use of Pregabalin, Gabapentin, Duloxetine, and Amitriptyline as first
line drugs in the management of neuropathic pain33,34,35. However, the use of gabapentinoids is being challenged as it lacks favourable robust evidence for efficacy against pain syndromes
other than fibromyalgia, post herpetic neuralgia and diabetic neuropathy, and many clinicians have also highlighted the potential for misuse and developing dependence36,37,38. The use of
BTX-A, Ketamine, Ningmitai and THC for the management of various chronic pain conditions is popular and well established39,40,41,42,43 and our study shows the effective use of these as
analgesics when compared to placebo. There is evidence to support the efficacy of BTX-A for the management of neuropathic pain although the sample sizes used in the studies were small and
therefore the real-world applicability remains limited29. BTX-A is also used in management of myofascial pains44,45 although further evidence on the efficacy and tolerability within all
populations, especially those with existing co-morbidities needs to be evaluated. Ketamine was found to be beneficial in managing some neuropathic pains46 and as an infusion the rates of
serious adverse effects were found to be similar to placebo47,48. Further studies are required to gather evidence to better understand its psychedelic effects and its role in the management
of PTSD, anxiety and depression. A renewed use of magnesium in managing chronic pain has been demonstrated in some literature49. Our results indicate similar evidence in the use of
magnesium, but will require further research to determine the efficacy, safety and effectiveness in managing short, medium and long-term pain. The NMA provided more reliable results with
direct and indirect comparisons between different drugs under different study designs. However, only a small number of multi-arm trials were eligible and the distribution of trials studying
different drugs was uneven. It resulted in the lack of direct evidence of certain drugs and their relative efficacy in the network was unstable due to excessive reliance on indirect
comparisons. Therefore, well designed and robust clinical trials should be conducted to verify the efficacy of pharmaceutical interventions used in chronic pain management. CONCLUSION To the
best of our knowledge, this is the first pairwise MA and NMA reporting the synthesis of the prevalence of the efficacy of pharmacological treatments used in the management of chronic pain
with a large sample size of 17,708 participants. Management of long-term chronic pain needs to be prioritised for several reasons including humanitarian, the strain on the healthcare systems
and the impact on the economy due to loss of productivity. The use of pharmaceutical agents in the long-term management of chronic pain has been debated for several decades, yet there has
not been a consensus on this matter. This study supports the importance of generating better evidence by way of robust clinical trials, the need for drafting clinical guidelines that is
pragmatic, practical as well as clinically significant and the use of better data-connectivity methods to improve clinical practice in the real-world. DATA AVAILABILITY The authors will
consider sharing the dataset gathered upon receipt of reasonable requests. CODE AVAILABILITY The authors will consider sharing the novel code created upon receipt of reasonable requests.
REFERENCES * Dahlhamer, J. _et al._ Prevalence of chronic pain and high-impact chronic pain among adults: United States, 2016. _MMWR Morb. Mortal. Wkly. Rep._ 67, 1001–1006.
https://doi.org/10.15585/mmwr.mm6736a2 (2018). Article PubMed PubMed Central Google Scholar * Zimmer, Z., Fraser, K., Grol-Prokopczyk, H. & Zajacova, A. A global study of pain
prevalence across 52 countries: examining the role of country-level contextual factors: Examining the role of country-level contextual factors. _Pain_ 163, 1740–1750 (2022). Article PubMed
Google Scholar * Brown, C. A. & Lilford, R. J. The stepped wedge trial design: A systematic review. _BMC Med. Res. Methodol._ 6, 54. https://doi.org/10.1186/1471-2288-6-54 (2006).
Article PubMed PubMed Central Google Scholar * Turk, D. C. & Monarch, E. S. _Biopsychosocial Perspective on Chronic Pain. Psychological Approaches to Pain Management: A
Practitioner’s Handbook_ 2nd edn. (Guilford, 2002). Google Scholar * Li, T. _et al._ Network meta-analysis-highly attractive but more methodological research is needed. _BMC Med._ 9, 79.
https://doi.org/10.1186/1741-7015-9-79 (2011). Article PubMed PubMed Central Google Scholar * Caldwell, D., Ades, A. & Higgins, J. Simultaneous comparison of multiple treatments:
Combining direct and indirect evidence. _BMJ_ 331, 897–900. https://doi.org/10.1136/bmj.331.7521.897 (2005). Article PubMed PubMed Central Google Scholar * Opioid Overdose. Who.int.
www.who.int/news-room/fact-sheets/detail/opioid-overdose. * Opioid. Opioid overdose crisis: Time for a radical rethink. _Lancet Public Health_ 7, e195 (2022). Article Google Scholar *
Berterame, S. _et al._ Use of and barriers to access to opioid analgesics: A worldwide, regional, and national study. _Lancet._ 387(10028), 1644–1656.
https://doi.org/10.1016/S0140-6736(16)00161-6 (2016). Article PubMed Google Scholar * Jansen, J. P. _et al._ Interpreting indirect treatment comparisons and network meta-analysis for
health-care decision making: Report of the ISPOR task force on indirect treatment comparisons good research practices: Part 1. _Value Health._ 14(4), 417–428 (2011). Article PubMed Google
Scholar * Efthimiou, O. _et al._ GetReal in network meta-analysis: A review of the methodology. _Res. Synth. Methods._ 7(3), 236–263 (2016). Article PubMed Google Scholar * Dallenbach,
K. M. Pain: History and present status. _Am. J. Psychol._ 52, 331 (1939). Article Google Scholar * Levene, J. L. _et al._ Local anesthetics and regional anesthesia versus conventional
analgesia for preventing persistent postoperative pain in adults and children: A Cochrane systematic review and meta-analysis update. _J. Clin. Anesth._ 55, 116–127 (2019). Article PubMed
PubMed Central Google Scholar * Campbell, J. (1996, November 11). Presidential Address. Speech given at the American Pain Society, Washington, DC. * Page, M. J. _et al_. The PRISMA 2020
statement: an updated guideline for reporting systematic reviews. _BMJ_ 372, n71. https://doi.org/10.1136/bmj.n71 (2021). Article PubMed PubMed Central Google Scholar * _Relieving Pain
in America: A Blueprint for Transforming Prevention, Care, Education, and Research_. (National Academies Press, 2011). * Pain Management Best Practices Inter-Agency Task Force Report:
Updates, Gaps, Inconsistencies, and Recommendations. (U. S. Department of Health and Human Services, 2019). https://www.hhs.gov/ash/advisory-committees/pain/reports/index.html. * Borenstein,
M., Hedges, L. V., Higgins, J. P. & Rothstein, H. R. A basic introduction to fixed-effect and random-effects models for meta-analysis. _Res. Synth. Methods_ 1, 97–111 (2010). Article
PubMed Google Scholar * Rothstein, H. R., Sutton, A. J. & Borenstein, M. _Publication Bias in Meta Analysis: Prevention, Assessment and Adjustments_ (Wiley, 2005). Book Google Scholar
* Busse, J. W. _et al._ Opioids for chronic noncancer pain: A systematic review and meta-analysis: A systematic review and meta-analysis. _JAMA_ 320, 2448–2460 (2018). Article PubMed
PubMed Central Google Scholar * Dowell, D., Ragan, K. R., Jones, C. M., Baldwin, G. T. & Chou, R. CDC clinical practice guideline for prescribing opioids for pain: United States, 2022.
_MMWR Recomm. Rep._ 71, 1–95 (2022). Article PubMed PubMed Central Google Scholar * Boudreau, D. _et al._ Trends in long-term opioid therapy for chronic non-cancer pain.
_Pharmacoepidemiol. Drug Saf._ 18, 1166–1175 (2009). Article PubMed PubMed Central Google Scholar * Noori, A. _et al._ Comparative benefits and harms of individual opioids for chronic
non-cancer pain: A systematic review and network meta-analysis of randomised trials. _Br. J. Anaesth._ 129, 394–406 (2022). Article CAS PubMed Google Scholar * World Health Organization
Model List of Essential Medicines: 22nd List, 2021. (WHO/MHP/HPS/EML/2021.02). (World Health Organization, 2021). * World Health Organization. _Cancer Pain Relief, Second Edition, With a
Guide to Opioid Availability_ (World Health Organization, 1996). Google Scholar * Trelle, S. _et al._ Cardiovascular safety of non-steroidal anti-inflammatory drugs: Network meta-analysis.
_BMJ_ 342, c7086 (2011). Article PubMed PubMed Central Google Scholar * Da Costa, B. R. _et al._ Effectiveness and safety of non-steroidal anti-inflammatory drugs and opioid treatment
for knee and hip osteoarthritis: Network meta-analysis. _BMJ_ 375, n2321 (2021). Article PubMed PubMed Central Google Scholar * Enthoven, W. T. M., Roelofs, P. D. D. M., Deyo, R. A., van
Tulder, M. W. & Koes, B. W. Non-steroidal anti-inflammatory drugs for chronic low back pain. _Cochrane Database Syst. Rev._ 2, 012087 (2016). Google Scholar * Hary, V., Schitter, S.
& Martinez, V. Efficacy and safety of botulinum A toxin for the treatment of chronic peripheral neuropathic pain: A systematic review of randomized controlled trials and meta-analysis.
_Eur. J. Pain_ 26, 980–990 (2022). Article CAS PubMed Google Scholar * Meng, Z. _et al._ Tolerability of opioid analgesia for chronic pain: A network meta-analysis. _Sci. Rep._ 7, 1995
(2017). Article ADS PubMed PubMed Central Google Scholar * Caraceni, A. _et al._ Use of opioid analgesics in the treatment of cancer pain: Evidence-based recommendations from the EAPC.
_Lancet Oncol._ 13, e58-68 (2012). Article CAS PubMed Google Scholar * Huang, L. _et al._ Opioid-induced constipation relief from fixed-ratio combination prolonged-release
oxycodone/naloxone compared with oxycodone and morphine for chronic nonmalignant pain: A systematic review and meta-analysis of randomized controlled trials. _J. Pain Symp. Manage_ 54,
737-748.e3 (2017). Article Google Scholar * Alberti, F. F. _et al._ Comparative efficacy of amitriptyline, duloxetine and pregabalin for treating fibromyalgia in adults: An overview with
network meta-analysis. _Clin. Rheumatol._ 41, 18965–18978 (2022). Article Google Scholar * Urquhart, D. M. _et al._ Efficacy of low-dose amitriptyline for chronic low back pain: A
randomized clinical trial: A randomized clinical trial. _JAMA Intern. Med._ 178, 1474–1481 (2018). Article PubMed PubMed Central Google Scholar * Sankar, V., Oommen, A. E., Thomas, A.,
Nair, J. V. & James, J. S. Efficacy, safety and cost effectiveness of amitriptyline and pregabalin in patients with diabetic peripheral neuropathy. _Indian J. Pharm. Sci._
https://doi.org/10.4172/pharmaceutical-sciences.1000274 (2017). Article Google Scholar * Wiffen, P. J. _et al._ Gabapentin for chronic neuropathic pain in adults. _Cochrane Database Syst.
Rev._ 6, 007938 (2017). Google Scholar * Shanthanna, H. _et al._ Benefits and safety of gabapentinoids in chronic low back pain: A systematic review and meta-analysis of randomized
controlled trials. _PLoS Med._ 14, e1002369 (2017). Article PubMed PubMed Central Google Scholar * Evoy, K. E., Morrison, M. D. & Saklad, S. R. Abuse and misuse of pregabalin and
gabapentin. _Drugs_ 77, 403–426 (2017). Article CAS PubMed Google Scholar * Jin, C., Chen, Z. & Zhang, J. Meta-analysis of the efficacy of Ningmitai capsule on the treatment of
chronic prostatitis in China. _Medicine_ 97, e11840 (2018). Article PubMed PubMed Central Google Scholar * Whiting, P. F. _et al._ Cannabinoids for medical use: A systematic review and
meta-analysis: A systematic review and meta-analysis. _JAMA_ 313, 2456–2473 (2015). Article CAS PubMed Google Scholar * Wei, J. _et al._ The efficacy and safety of botulinum toxin type A
in treatment of trigeminal neuralgia and peripheral neuropathic pain: A meta-analysis of randomized controlled trials. _Brain Behav._ 9, e01409 (2019). Article PubMed PubMed Central
Google Scholar * Zhang, K. _et al._ Efficacy and safety of Ningmitai capsule in patients with chronic prostatitis/chronic pelvic pain syndrome: A multicenter, randomized, double-blind,
placebo-controlled trial. _Urology_ 153, 264–269 (2021). Article PubMed Google Scholar * Jing, Z. _et al._ Efficacy and safety of Ningmitai capsules in patients with chronic epididymitis:
A prospective, parallel randomized controlled clinical trial. _Evid Based Complement. Altern. Med._ 2021, 9752592 (2021). Article Google Scholar * Khalifeh, M., Mehta, K., Varguise, N.,
Suarez-Durall, P. & Enciso, R. Botulinum toxin type A for the treatment of head and neck chronic myofascial pain syndrome. _J. Am. Dent. Assoc._ 147, 959-973.e1 (2016). Article PubMed
Google Scholar * Meister, M. R., Brubaker, A., Sutcliffe, S. & Lowder, J. L. Effectiveness of botulinum toxin for treatment of symptomatic pelvic floor myofascial pain in women: A
systematic review and meta-analysis: A systematic review and meta-analysis. _Female Pelvic. Med. Reconstr. Surg._ 27, e152–e160 (2021). Article PubMed PubMed Central Google Scholar *
Guimarães Pereira, J. E. _et al._ Efficacy and safety of ketamine in the treatment of neuropathic pain: A systematic review and meta-analysis of randomized controlled trials. _J. Pain Res_
15, 1011–1037 (2022). Article PubMed PubMed Central Google Scholar * Cohen, S. P. _et al._ Consensus guidelines on the use of intravenous ketamine infusions for chronic pain from the
American society of regional anesthesia and pain medicine, the American academy of pain medicine, and the American society of anesthesiologists. _Reg. Anesth. Pain Med._ 1, 47–50 (2018).
Google Scholar * Orhurhu, V., Orhurhu, M. S., Bhatia, A. & Cohen, S. P. Ketamine infusions for chronic pain: A systematic review and meta-analysis of randomized controlled trials.
_Anesth. Analg._ 129, 241–254 (2019). Article CAS PubMed Google Scholar * Park, R. _et al._ Efficacy and safety of magnesium for the management of chronic pain in adults: A systematic
review. _Anesth. Analg._ 131, 764 (2020). Article PubMed Google Scholar Download references ACKNOWLEDGEMENTS Dr Anish Thillainathan involved in formatting process. FUNDING University
College London Hospitals NHS Foundation Trust. AUTHOR INFORMATION Author notes * These authors contributed equally: Ash Shetty, Gayathri Delanerolle, Heitor Cavalini and Chunli Deng. AUTHORS
AND AFFILIATIONS * University College London Hospitals NHS Foundation Trust, London, UK Ashish Shetty * University College London, 235, Euston Road, London, NW1 2BU, UK Ashish Shetty &
Tacson Fernandez * Pain Medicine, Cleveland Clinic London, London, United Kingdom Ashish Shetty * Nuffield Department of Primary Care Health Sciences, University of Oxford, Oxford, OX3 7JX,
UK Gayathri Delanerolle * Southern Health NHS Foundation Trust, Southampton, SO40 2RZ, UK Heitor Cavalini, Peter Phiri & Jian Qing Shi * Southern University of Science and Technology,
Shenzhen, 518055, China Chunli Deng & Jian Qing Shi * School of Statistics and Mathematics, Yunnan University of Finance and Economics, Kunming, China Xiaojie Yang & Jian Qing Shi *
National Centre for Applied Mathematics Shenzhen, Shenzhen, China Xiaojie Yang * University of Oxford, Oxford, UK Amy Boyd * Psychology Department, Faculty of Environmental and Life
Sciences, University of Southampton, Southampton, SO17 1BJ, UK Peter Phiri * Imperial College Healthcare NHS Trust, London, UK Arun Bhaskar Authors * Ashish Shetty View author publications
You can also search for this author inPubMed Google Scholar * Gayathri Delanerolle View author publications You can also search for this author inPubMed Google Scholar * Heitor Cavalini View
author publications You can also search for this author inPubMed Google Scholar * Chunli Deng View author publications You can also search for this author inPubMed Google Scholar * Xiaojie
Yang View author publications You can also search for this author inPubMed Google Scholar * Amy Boyd View author publications You can also search for this author inPubMed Google Scholar *
Tacson Fernandez View author publications You can also search for this author inPubMed Google Scholar * Peter Phiri View author publications You can also search for this author inPubMed
Google Scholar * Arun Bhaskar View author publications You can also search for this author inPubMed Google Scholar * Jian Qing Shi View author publications You can also search for this
author inPubMed Google Scholar CONTRIBUTIONS A.S. and G.D. developed the study protocol and embedded this within the POP project. G.D. and J.Q.S. designed and completed the study analysis.
The data extraction was completed by H.C. and C.D. All authors critically appraised and commented on previous versions of the manuscript. All authors read and approved the final manuscript.
All authors consented to publish this manuscript. CORRESPONDING AUTHOR Correspondence to Ashish Shetty. ETHICS DECLARATIONS COMPETING INTERESTS AS has received funding from Medtronic and
Nevro Corp USA. PP has received research grants from Novo Nordisk, Queen Mary University of London, John Wiley & Sons, Otsuka, outside the submitted work. AB has received speaker fees
and has been an advisory board member from Pfizer, Vectura-Fertin and Reckitt. All other authors report no conflict of interest. The views expressed are those of the authors and not
necessarily those of the NHS, the National Institute for Health Research, the Department of Health and Social Care or the Academic institutions. ADDITIONAL INFORMATION PUBLISHER'S NOTE
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION 1. APPENDIX
APPENDIX See Table 4. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation,
distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and
indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit
line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use,
you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS
ARTICLE CITE THIS ARTICLE Shetty, A., Delanerolle, G., Cavalini, H. _et al._ A systematic review and network meta-analysis of pharmaceutical interventions used to manage chronic pain. _Sci
Rep_ 14, 1621 (2024). https://doi.org/10.1038/s41598-023-49761-3 Download citation * Received: 19 September 2023 * Accepted: 12 December 2023 * Published: 18 January 2024 * DOI:
https://doi.org/10.1038/s41598-023-49761-3 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative