Evaluation of in vitro biocompatibility of human pulp stem cells with allogeneic, alloplastic, and xenogeneic grafts under the influence of extracellular vesicles
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Therapies using dental pulp stem cells (DPSCs) or stem cell-derived extracellular vesicles (EVs) have shown promising applications for bone tissue engineering. This in vitro
experiment evaluated the joint osteogenic capability of DPSCs and EVs on alloplastic (maxresorp), allogeneic (maxgraft), and xenogeneic (cerabone) bone grafts. We hypothesize that osteogenic
differentiation and the proliferation of human DPSCs vary between bone grafts and are favorable under the influence of EVs. DPSCs were obtained from human wisdom teeth, and EVs derived from
DPSCs were isolated from cell culture medium. DPSCs were seeded on alloplastic, allogeneic, and xenogeneic bone graft substitutes for control, and the same scaffolds were administered with
EVs in further groups. The cellular uptake of EVs into DPSC cells was assessed by confocal laser scanning microscopy. Cell vitality staining and calcein acetoxymethyl ester staining were
used to evaluate cell attachment and proliferation. Cell morphology was determined using scanning electron microscopy, and osteogenic differentiation was explored by alkaline phosphatase and
Alizarin red staining. Within the limitations of an in vitro study without pathologies, the results suggest that especially the use of xenogeneic bone graft substitutes with DPSCS and EVs
may represent a promising treatment approach for alveolar bone defects. SIMILAR CONTENT BEING VIEWED BY OTHERS A PARTIALLY DEMINERALIZED ALLOGENEIC BONE GRAFT: IN VITRO OSTEOGENIC POTENTIAL
AND PRECLINICAL EVALUATION IN TWO DIFFERENT INTRAMEMBRANOUS BONE HEALING MODELS Article Open access 01 March 2021 THE HEALING CAPACITY AND OSTEOGENESIS PATTERN OF DEMINERALIZED DENTIN MATRIX
(DDM)-FIBRIN GLUE (FG) COMPOUND Article Open access 12 August 2023 BONE MORPHOGENETIC PROTEIN 7 MEDIATES STEM CELLS MIGRATION AND ANGIOGENESIS: THERAPEUTIC POTENTIAL FOR ENDOGENOUS PULP
REGENERATION Article Open access 20 July 2022 INTRODUCTION Bone defects of the alveolar ridge usually occur as a result of tooth loss, inflammation, trauma, or pathology and pose major
problems during dental or implant rehabilitation. Augmentation procedures thus aim to generate sufficient bone volume for implant placement1. Different bone grafting materials can be used
for the augmentation of bone defects. Because of its osteogenetic, osteoinductive, and osteoconductive properties, bone grafts of autologous origin represent the gold standard for the
therapy of bony defects in the human jaw, although they are associated with major drawbacks such as donor-side morbidity2, multiple required surgeries, and increased operation times3. In
addition to autologous bone, allogeneic, xenogeneic, and alloplastic bone graft substitutes (BGSs) can be used for the augmentation of bone defects. Bone substitutes offer the advantage of
eliminating harvest morbidity, but they are currently inferior to autologous bone in terms of osteogenesis, osteoinduction, and osteoconduction. Much research has thus been conducted to
improve the properties of BGSs. Multipotent mesenchymal stem cells (MSCs) are adult stem cells that can be isolated from a wide variety of tissues4,5,6,7. In 2000, MSCs were isolated for the
first time from the healthy pulp tissue of extracted third molars8. In addition to their rapid proliferation and colony-forming abilities9, MSCs derived from DPSCs have indicated strong
differentiation potential to osteoblasts, adipocytes, chondrocytes, odontoblasts, and neurons10. Compared to MSCs derived from bone marrow, DPSCs have a higher proliferation rate10, are
clonogenic cells, and have promising regenerative potential to treat severe tissue damage, all of which make DPSCs a frequently studied stem cell type among MSCs in research. The ability to
differentiate into bone-forming cells make DPSCs a promising therapeutic option in the regenerative medicine of bone deficiency3,11. Recent evidence suggests that the beneficial regenerative
effects of stem cell-based therapy are most likely orchestrated by their paracrine processes9,12,13,14,15,16,17. EVs are secreted by cells in a paracrine manner, are surrounded by a lipid
bilayer structure, and have a diameter of about 150–180 nm. They contain genetic substances such as microRNAs (miRNAs) and EVs are part of cell-to-cell communication in the form of exosomes
or microvesicles18,19,20,21. MicroRNAs are non-coding RNA components that inhibit the translation of mRNA. Thus, they are an elementary component of cell proliferation and cell
differentiation22. The target specificity and modulatory genes of miRNAS are related to osteogenesis, and consequently promote osteogenic differentiation of MSCs and thus induce bone tissue
regeneration23. Impairment by degradative enzymes has often limited the use of miRNAs in bone remodeling studies in the past24. These limitations could be overcome by the production of
naturally derived EVs, since these vesicles can be used to transport miRNAs to target cells25. EVs have the advantage of being easily obtained from body or cell culture fluids26,27, and they
are characterized by stability with low susceptibility, elicit little immune response if any, and have an intrinsic homing effect, which favors the uptake of EVs with osteogenic miRNAs by
target cells28. New approaches to stem cell therapy are thus being expanded to include the use of EVs9,13. Considering that EVs and DPSCs play a major role in cell-based therapies16, and
that EVs can potentially modulate the proliferation and differentiation of DPSCs in osteogenesis, an investigation of DPSCs on different scaffolds and EV interactions seems essential to the
development of new therapies for bone defects. Currently, an insufficient number of studies have investigated the osteogenic cell response of DPSC under the influence of EVs on different
conventional BGSs in vitro. The aim of this study was thus to investigate the internalization of EVs into DPSCs and to determine the influence on the attachment, growth, and osteogenic
differentiation of DPSCs on alloplastic, xenogeneic, and allogeneic bone scaffolds under the influence of EVs. MATERIAL AND METHODS DPSC EXTRACTION AND CELL CULTURE As previously
described29, DPSCs were generated from 3 impacted third molars extracted in healthy adult males in the Oral and Maxillofacial Surgery Department of RWTH Aachen University. All methods were
carried out in accordance with relevant guidelines and regulations of the ethical approval by the Ethics Committee of the Medical Faculty of RWTH Aachen University (EK 374/19). Written
informed consent was obtained from all participants. The teeth were placed in chlorhexidine before being opened with sterile dental fissure drills at the cementoenamel junction. Sterile
barbed broaches were used to dissect pulp tissue from the crown and root. Prior to the isolation of DPSC, a mixed enzymatic solution was prepared using 1 ml of collagenase type I (12 mg/ml),
1 ml of dispase (16 mg/ml) in 2 ml of sterile phosphate-buffered saline (PBS) containing 100 mg/ml of penicillin, and 100 mg/ml of streptomycin. The minced pulp tissue was transferred into
the prepared enzyme solution for digestion for 60 min at 37 °C and vortexed every 30 min. Using a 70 μM cell sieve, any large-cell aggregates were removed to obtain a single-cell suspension.
Digestion was terminated by the addition of 3 ml of minimal essential medium (MEM) containing 10% (v/v) fetal bovine serum (FBS). The single-cell suspensions were then centrifuged at 1,000
rpm for 5 min at room temperature. After removal of the supernatant, the pellets were resuspended in 1 ml of cell medium and cultured in a 25 cm2 cell culture dish at 37 °C in an incubator
at 5% CO2 atmosphere and 100% relative humidity. After approximately 3–5 days, the adherent DPSCs migrated outward from the pulp-tissue pieces. Briefly, the isolated DPSCs were cultured in
EV-free basic MEM medium (growth medium) containing 10% (v/v) FBS and 1% (v/v) antibiotic–antimycotic solution at 37 °C in an incubator with 5% CO2 atmosphere and 100% relative humidity.
Refreshing of the medium was performed every two days. DPSCs in stages 3 to 5 were used in the subsequent trials. Induction of osteogenic differentiation was conducted by culturing DPSCs in
osteogenic differentiation medium supplemented with 0.1 mM ascorbic acid, 100 nM dexamethasone, and 10 mM β-glycerophosphate. DPSC-DERIVED EV ISOLATION AND EXAMINATION After reaching roughly
80% confluence, DPSCs were rinsed twice with PBS and cultivated for another 24 h in medium without FBS. To remove cellular debris, and any unattached cells, the supernatants were collected,
differentially centrifuged (300 g, 2.000 g, and 5.000 g) for 15 min at 4 °C, and filtered with a 0.22 μm filter; the sediment was then discarded. The supernatant was then transferred to an
ultracentrifuge tube and subjected twice to ultracentrifugation at 20,000 g for 90 min at 4 °C to pellet DPSC-derived EVs. The pellets were re-suspended in 100 μL of sterile PBS to obtain
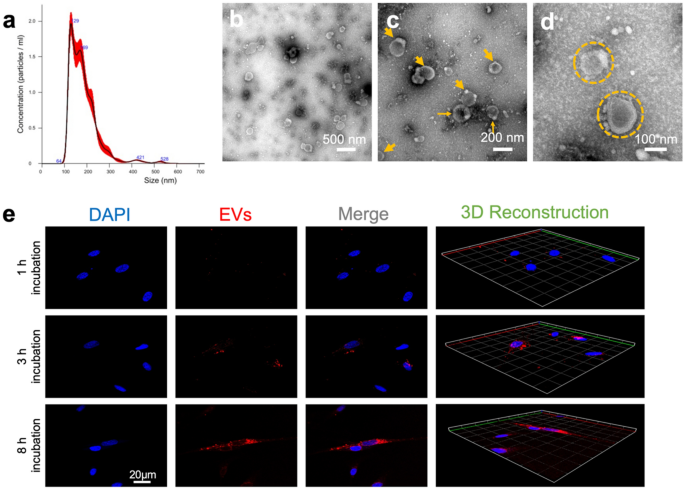
homogeneous EV suspension and then stored at − 80 °C for further application. Nanoparticle tracking analysis (NTA) was then used to test quantitative concentration and the particle size
distribution of DPSC-derived EVs. All samples were diluted to a final volume of 1 mL using PBS. A pre-test was performed to evaluate the ideal measurement concentration of 20–100 particles
per frame; see Fig. 1a. Settings were implemented according to the manufacturer’s software manual (NanoSight NS300). In addition, the morphology and size of the isolated DPSC-EVs were
assessed by transmission electron microscopy (TEM) (Fig. 1b–d). A total of 10 μL of DPSC-EVs was pelleted, resuspended in 10 μL of HEPES buffer, and dropped onto a carbon-coated copper grid
for TEM observations. BONE GRAFTS Commercially available particulate bone grafts (particle size of 0.5–2 mm) were used in this in vitro study. We used maxresorb (botiss biomaterials GmbH,
Germany) as a biphasic calcium phosphate (BCP) alloplastic scaffold. For the allogeneic scaffold, we used maxgraft (botiss biomaterials GmbH, Germany), a freeze-dried bone allograft. For the
xenogeneic bone graft, we used cerabone (botiss biomaterials GmbH, Germany), a deproteinized bovine bone mineral. INTERNALIZATION OF DPSC-DERIVED EVS First, the isolated DPSC-derived EVs
were fluorescently labeled with a molecular lectin probe, Wheat Germ Agglutinin Conjugates (WGA, Alexa Fluor 488 conjugate), for 30 min at room temperature before being terminated by
centrifugation at 20,000 g for 90 min and resuspended in PBS. These WGA-labeled EVs were co-cultured with DPSC (5 × 104/confocal dish) for different times (1 h, 3 h, and 8 h); the cells were
then rinsed with PBS and fixed in 4% paraformaldehyde. The cell nucleus was then stained with 6-diamidino-2-phenylindole (DAPI) (cat. no. 2381700, Invitrogen, USA) for another 15 min. The
cellular uptake of WGA-labeled EVs by DPSCs after different incubation times was observed using confocal laser scanning microscopy (CLSM). Additionally, 3D reconstruction images from WGA and
DAPI fluorescence were automatically formed using ZEN lite software at different time points (Fig. 1e). CYTOSKELETON AND MORPHOLOGY ASSESSMENT UNDER DPSC-EVS INTERVENTION After culturing
for 7 days, the cellular morphologies on various bone scaffolds under EV intervention at an initial density of 20,000 cells/dish were evaluated through cytoskeleton staining and scanning
electron microscopy (SEM). For the assessment of cytoskeletal organization, the cell-scaffold samples were pre-treated with EVs and afterward rinsed twice with PBS and fixed in 4%
paraformaldehyde for 20 min, followed by a pre-treatment with Triton X-100 (0.1% v/v) for 5 min. The samples were then co-stained with rhodamine-phalloidin (cat. no. R415, Invitrogen,
Germany) to visualize F-actin and DAPI (cat. no. 2381700, Invitrogen, Germany) to visualize the nuclei for 45 min and 5 min, respectively. Sample visualization was conducted after 1 h, 3 h,
and 8 h via CLSM, and Z-stack images for 3D construction were obtained. For SEM observations, the cell-scaffold constructs in various groups were gently washed twice with PBS buffer, fixed
with 2.5% glutaraldehyde for 4 h, and then dehydrated with a series of different concentrations of ethanol (30%, 50%, 70%, 90%, and 100%) twice. Next, cell/scaffold constructs were dried
overnight according to an established protocol30 and then sputtered with Au/Pd and observed using SEM (XL30 FEG; FEI, Eindhoven, the Netherlands) with an acceleration voltage of 10 kV. CELL
PROLIFERATION AND ATTACHMENT ON BONE GRAFTS Afterward, the cell-seeded BGSs were cultivated in confocal dishes at an initial density of 20,000 cells/dish for 3 or 7 days. Half the samples
underwent EV stimulation (5 × 109 particles/mL). At each predetermined time point, the medium was discarded and the cells were washed three times. Two different stainings were conducted, per
the manufacturer’s instructions: (1) live cell calcein AM staining (cat. no. PK-CA707-30002, PromoKine, Germany) and (2) DAPI staining (cat. no. 2381700, Invitrogen, USU). DPSC cells
growing on the bone scaffolds were rinsed three times and observed via CLSM to determine the proliferative effect. CELL VIABILITY ASSESSMENT A Live/Dead Cell Assay Kit (PromoKine, PromoCell
GmbH, Germany) was used to detect cell viability from intracellular esterase activity and the plasma membrane integrity of DPSCs. DPSCs were seeded on to the three different bone graft
scaffolds (20,000 cells/dish) in the osteogenic medium for 1, 3, 5, and 7 days. The scaffolds were then rinsed twice with warm PBS, and the cells were labeled with a mixed fluorescence dye
containing 2 μM calcein AM and 4 μM ethidium homodimer-III (EthD-III) for 45 min, per the manufacturer’s instructions. Finally, the ratio of green fluorescence to red fluorescence was
analyzed using ImageJ software. (Free Java software was provided by the National Institutes of Health, Bethesda, MD, USA.) OSTEOGENIC DIFFERENTIATION DETERMINATION Alkaline phosphatase (ALP)
staining was used to evaluate the early- and late-phase osteogenic differentiation in all groups after 7 and 14 days of cultivation. In brief, the culture medium was aspirated, and DPSCs
were gently washed with PBS buffer twice. A fixing solution was added and then incubated at room temperature. After 5 min, the fixing solution was aspirated, and ALP staining solution (cat.
no. GR3358974-1, Abcam Co., UK) was added in darkness and incubated at room temperature for a further 15 min. Observation of ALP-positive (ALP+) cells was conducted via optical microscopy
and analyzed using ImageJ software. Alizarin red staining (ARS) (cat. no. 3512879, Merck, Germany) was used to determine the mineralized nodule formation of late-phase osteogenic
differentiation by calcium deposition. After osteogenic incubation for 14 days, the cells in various groups were rinsed twice with PBS, fixed in 4% paraformaldehyde for 10 min, and then
stained with 0.1% ARS solution (40 mM, pH 4.2) in darkness for 25 min at room temperature. These mineralized precipitates were observed and photographed via an optical microscope and
analyzed using ImageJ software. The mineralized nodules appeared as a dark-red center and a light-red peripheral area. STATISTICAL ANALYSIS Analyses were performed by using Prism 7.0
(GraphPad Software, Inc., San Diego, CA, USA). Before analysis, the values were tested using D’Agostino–Pearson or Shapiro–Wilk test criteria for normal distribution and passed the
Brown–Forsythe test for equal variances. A two-way ANOVA was used to analyze the parametric data of the groups with respect to two different categorical variables: Variable one includes the
different bone material and variable two the presence or absence of EVs. A post hoc Tukey’s multiple comparison test, with individual variances computed for each comparison, was conducted.
The data in this paper represent the means ± standard deviation (SD). Differences between the groups were considered significant when _p_ < 0.05. RESULTS The yield of EVs obtained from
DPSCs had a measured concentration of 2 × 109 nanoparticles/ml. Confirming the vesicle size and morphology of EVs obtained in the study presented here showed similarities with values in the
literature, NTA via NanoSight NS300 and TEM were used to characterize the EVs. The SEM images illustrated that the EVs had a typical cup-shaped morphology with different hydrodynamic
diameters (Fig. 1b–d). NTA revealed EVs in the size range of about 184.9 ± 67.0 nm. The SEM images also showed that the isolated EVs had similar shapes, which together proved that the
isolation methods had resulted in homogenous EV batches. INTERNALIZATION OF DPSC-DERIVED EVS Figure 1e shows the interaction of the WGA-labeled EVs and the DPSCs with DAPI-stained nuclei.
After an incubation period of 1 h, few EVs were initially taken up into the cytoplasm of the DPSCs. Few WSA-positive EVs were evident near the DAPI-stained nuclei. Over the consecutive
duration of 3–8 h, we noted a steady uptake and an increasing accumulation of EVs into the cytoplasmic space. CYTOSKELETON AND MORPHOLOGY ASSESSMENT UNDER DPSC-EV INTERVENTION In
monocultured DPSC with EVs, an abundance of F-actin fibers were observed in all the bone graft materials under the influence of EVs. The fluorescent images shown in Fig. 2a–c indicate a
homogeneous distribution of actin fibers and filament elongation of DPSCs on alloplastic, allogeneic, and xenogeneic BGSs. Morphologically, no difference was evident between the groups in
the staining of the actin of the cytoskeleton of DPSCs under the influence of EVs. SEM images from day 7 (Fig. 2) of the DPSC cell cultures showed normal shapes of MSCs on the BGSs. The
cells on the alloplastic (d–g), allogeneic (h–k), and xenogeneic (l–o) BGSs showed normal expansion and a spindle-shaped morphology, first with EVs and second in the absence of EVs. The
cells were oriented along the surface of the grafts and grew in random regions. CELL PROLIFERATION AND ATTACHMENT ON BONE GRAFTS DPSC viability was evaluated with and without stimulation by
EV nanoparticles on different BGSs using a calcein AM assay. Table 1 and Fig. 3 show the measurement of cell proliferation and attachment by the fluorescence of calcein at days 3 and 7. The
number of calcein AM–positive (henceforth CAM+) cells increased from day 3 to day 7 in all groups. In addition, for both time points, DPSCs with the presence of EVs resulted in significantly
more CAM+ cells (_p_ < 0.001). At approximately 773.25 ± 5.95 CAM+ cells/mm2, the most significantly abundant (_p_ < 0.001) CAM+ cells were detected at 7 days in the DPSCs and EVs in
the xenogeneic BGSs, compared to the alloplastic BGSs (with 501.38 ± 6.86 CAM+ cells/mm2) and allogeneic BGSs (with 512.88 ± 15.45 CAM+ cells/mm2). CELL VIABILITY ASSESSMENT We next tested
the viability of DPSC under the influence of EVs cultured for 1, 3, 5, and 7 days in osteogenic medium on alloplastic, allogeneic, and xenogeneic BGSs (Fig. 4). At all observation periods,
very few dead cells were noted in the live/dead assay, which did not increase analogously with the growing cell number of live cells. For the significantly highest number of 959 ± 11.81 live
cells per mm2, the xenogeneic group had 18.00 ± 1.69 dead cells per mm2 after 7 days. At all four time points, no significant difference was noted in the number of dead cells per mm2
between all groups. OSTEOGENIC DIFFERENTIATION DETERMINATION In DPSCs, ALP activity was determined after 7- and 14-day treatment with EV and without EV stimulation as an early indicator of
cell differentiation toward the osteogenic lineage. Compared to DPSC without EVs, EVs significantly (_p_ < 0.001) increased ALP activity in all BGSs after 7 days of culture (Table 2 and
Fig. 5a,b). The highest proportion of the 40.85 ± 5.63% of ALP+ area was found in DPSC-EVs cultured on xenogeneic BGS. After 14 days, all EV groups showed a significant increase in ALP
activity compared to DPSCs without EVs. Again, DPSCs on the xenogeneic scaffold showed the highest ALP activity in the group without EVs, with a 39.81 ± 2.88% ALP+ area (_p_ < 0.001), and
also in the group of DPSCs + EVs, with a 44.85 ± 1.25% ALP+ area, which was significantly higher (_p_ < 0.001) than the alloplastic graft DPSC + EVs, with a 31.83 ± 2.50% ALP+ area
(Table 2 and Fig. 6a,b). Alizarin red staining (ARS) and Von Kossa staining were performed to evaluate the late phase of osteogenic differentiation after 14 days. Analogously to the ALP
staining, a significantly increased ARS+ area was also found under the influence of EVs in all BGS materials. This effect was particularly evident in xenogeneic BGS material. Without EVs,
the ARS+ area was 13.04 ± 1.02%, and with EVs the percentage significantly increased (_p_ < 0.001) to an ARS+ area of 34.53 ± 1.22% (Table 2 and Fig. 7a,b). DISCUSSION The therapeutic
potential of MSC-derived EVs has already been extensively reported9,12,15,23,25,28,31. Both in vivo and in vitro studies have sufficiently demonstrated that many key factors of bone
remodeling are controlled under the regulatory action of MSC-derived EVs9,19,23,25. In this study, we focused on MSCs isolated from dental pulp tissue. Human DPSC EVs were extracted, and we
compared the interaction as well as osteogenic differentiation of human DPSCs under the influence of EVs in different bone scaffolds to better understand the role of EVs derived from DPSCs
on the one hand and the influence of different bone substitutes on the therapeutic effects of DPSCs on the other. All scaffolds were able to carry DPSC, and all DPSCs we tested were able to
incorporate the added EVs in vitro. During TEM examination, the DPSCs in alloplastic, allogeneic, and xenogeneic scaffolds with and without EVs showed a normal shape, and no morphological
differences were observed. Likewise, the monocultured DPSCs with EVs showed a homogeneous distribution of actin fibers and filament extension on all bone substitute materials used in the
study. The general ability of DPSCs to internalize EVs was an important aspect of this study, suggesting that the uptake of EVs is related to our observed effects of DPSCs. Many studies have
already adequately explored miRNAs25, surface proteins, and glycoproteins, as well as the cell-specific pathways of EV internalization32. It was beyond the scope of this study to determine
the efficiency of internalization of our EVs or to identify and experimentally confirm the individual miRNAs, surface proteins, and glycoproteins of the cell-specific uptake pathways on the
EV surface. Our study focused primarily on the qualitative uptake of the extracted EVs into the DPSCs. Qualitative investigations of whether EVs can be internalized in target cells have
already been performed by other researchers25,32. Over the duration of 8 h, we observed a steady internalization and accumulation of fluorescently WGA-labeled EVs into the DPSCs with
DAPI-stained nuclei via CLSM in our experiments. Considering the results of the proliferation and differentiation experiments, we can assume that the EVs were internalized efficiently enough
into the DPSCs. According to Mondal et al.32, the isolation of EVs is a significant step for research on these vesicles. Although previous research has described disadvantages such as the
increased time and potential protein contamination required for the purification of EVs by ultracentrifugation32, we did not observe strong contamination by non-vesicular proteins by our
nano-tracking studies or by SEM examination. The heterogeneity of EV sizes is a natural phenomenon based on various intercellular signaling mechanisms of cells33. Previous studies have
described the heterogeneous sizes of EVs between 30–200 nm9,25,32. In line with this earlier work, EVs in the size range of about 184.9 ± 67.0 nm were determined in our study by
nano-tracking analysis. The morphological evaluation of EVs by SEM examination also showed that the isolated EVs were identical in their morphological shape, despite their heterogeneous
sizes. The treatment of craniofacial bone defects34 and an insufficient bone supply of the jaw pose great challenges to therapists3,35. Although pure bone substitutes are superior to
autologous bone in terms of harvest morbidity36, they have significant deficiencies in terms of osteogenesis, osteoinduction, and osteoconduction3. A rigorous search for alternative methods
is thus underway to improve the osteogenic properties of BGSs3,7,35,37. Re et al.13 described improved bone regeneration of bone substitutes when MSCs and derived EVs were added to the
scaffolds. Our investigations of DPSCs were performed on alloplastic, allogeneic, and xenogeneic bone grafts, and according to Re et al.’s findings13, all scaffolds examined in the present
study were capable of bearing DPSCs, and the influence of EVs resulted in an increased induction of osteoblastic differentiation. Nevertheless, significant differences in the amount of cell
proliferation, cell attachment, and osteogenic differentiation were observed under the influence of EVs as well as in the dependence of the BGSs. Calcein AM staining, which indicates
intracellular esterase activity, is an established method for assessing the viability of DPSCs31,35. In our experimentation, we showed that DPSCs under the influence of EVs grown on
xenogeneic scaffolds for 3 and 7 days, respectively, exhibited significantly higher numbers of attached and proliferated DPSCs, with up to 773.25 ± 5.95 CAM+ cells/mm2 (_p_ < 0.001)
compared to all other groups. This observation is consistent with the work of Zhang et al.31, who described in an in vivo study an increased number of calcein-positive MSCs in the presence
of EVs used with xenogeneic bone material with an EV concentration of 1 × 1012 nanoparticles/ml versus significantly decreased calcein-positive bone in the absence of EVs. We used a lower
amount of EVs in our study (2 × 109 nanoparticles/ml), but despite this lower concentration of EVs, we observed a highly significant (_p_ < 0.001) effect on the cell viability of DPSCs
under the stimulation of EVs. While in the live/death assay the number of live DPSCs with EVs on the xenogeneic scaffold (959 ± 11.81 live cells/mm2) was significantly higher (_p_ <
0.001) compared to all other groups, no difference was noted with a 1.88% of dead cells compared to the percentage of dead cells with the alloplastic scaffold (1.74%) and the allogeneic
scaffolds (2.28%), respectively. Considered together, these findings indicate that EVs and xenogeneic BGSs favor the attachment and proliferation of DPSCs, but that no difference in
biocompatibility exists between each material and the influence of EVs. Motamedian et al.29 reported a significant effect of different BGSs on the in vitro cell differentiation of DPSC. By
the evaluation of osteogenic differentiation by APL staining, this effect was confirmed in our studies. Compared to the alloplastic scaffold, the DPSC culture on the xenogeneic BGSs showed
significantly higher ALP activity at 7 days and at 14 days, with a 9.96 ± 0.92 ALP+ area (_p_ < 0.05) and a 39.81 ± 2.88% ALP+ area (_p_ < 0.001), respectively. In contrast to our
findings, a favorable effect on the differentiation of DPSCs by freeze-dried bone allograft has been described in the literature29, whereas the data of our study have shown that the
xenogeneic bone material was superior to the alloplastic and autogenous scaffolds in terms of osteogenic differentiation of DPSCs. This phenomenon can possibly be explained by the use of
different BGSs between the studies, which were obtained differently and varied in chemical composition. This process should be standardized for the future selection of an appropriate BGS in
further in vivo clinical investigations. In a comparative study, significantly increased ALP activity was determined after 14 days in MSCs under the influence of EVs compared to treatment by
an osteogenic medium in vitro25. Analogously, a 5.04% increase in ALP activity was observed in the DPSC group on the xenogeneic BGS under the influence of EVs, showing the strongest
osteogenic differentiation among all scaffold groups after an observation period of 14 days. In the context of bone defects and osteoporotic diseases, because researchers have focused
primarily on the utilization of EVs from MSCs from bone marrow and their biologically active content31,38, the field lacks knowledge of the use of DPSCs as alternative cell sources to obtain
EVs that have the osteogenic potential to enhance bone mineral formation. But EVs also have the potential to enhance stem cell differentiation, since they can accelerate bone mineral
deposition, among other applications9,31,39. Zhang et al.31 have described a higher percentage of ARS+ cells in xenogeneic bone in the presence of EVs after 8 weeks of bone healing.
Consistent with their findings, our data have demonstrated a significant increase in ARS+ cells by the presence of EVs in all groups after 21 days. Compared with all scaffolds with and
without EV intervention, the DPSC-EVs grown on the xenogeneic scaffold demonstrated significantly increased osteogenic differentiation in the late phase, with an increase of 21.45% ARS+
cells. CONCLUSION Within the limitations of an in vitro study, the data from the present work show that the effect of extracellular vesicles (EVs) on cell proliferation, cell attachment, and
osteogenic differentiation of dental pulp stem cells (DPSCs) cultivated on xenogeneic scaffolding is significantly superior to that of other bone grafts (BGSs). The ease of obtaining DPSCs
and the reliable isolation method of EVs, combined with the synergistic osteogenic differentiation rates, demonstrate their suitability for future bone tissue engineering studies. Further
investigation of the findings of this study on the corresponding bone substitutes, as well as the osteogenic properties of DPSCs under the influence of EVs on bone regeneration, should be
performed in future studies in living organisms in the form of animal experiments. DATA AVAILABILITY All data generated for this study are available from the corresponding authors upon
reasonable request. REFERENCES * Zakrzewski, W., Dobrzynski, M., Rybak, Z., Szymonowicz, M. & Wiglusz, R. J. Selected nanomaterials’ application enhanced with the use of stem cells in
acceleration of alveolar bone regeneration during augmentation process. _Nanomaterials (Basel)_ https://doi.org/10.3390/nano10061216 (2020). Article PubMed Google Scholar * Liu, T. _et
al._ Advances of adipose-derived mesenchymal stem cells-based biomaterial scaffolds for oral and maxillofacial tissue engineering. _Bioact. Mater._ 6, 2467–2478.
https://doi.org/10.1016/j.bioactmat.2021.01.015 (2021). Article CAS PubMed PubMed Central Google Scholar * Adamzyk, C. _et al._ Bone tissue engineering using polyetherketoneketone
scaffolds combined with autologous mesenchymal stem cells in a sheep calvarial defect model. _J. Craniomaxillofac. Surg._ 44, 985–994. https://doi.org/10.1016/j.jcms.2016.04.012 (2016).
Article PubMed Google Scholar * Zakrzewski, W., Dobrzynski, M., Szymonowicz, M. & Rybak, Z. Stem cells: Past, present, and future. _Stem Cell Res. Ther._ 10, 68.
https://doi.org/10.1186/s13287-019-1165-5 (2019). Article CAS PubMed PubMed Central Google Scholar * Wu, T. _et al._ Wet adhesive hydrogel cardiac patch loaded with anti-oxidative,
autophagy-regulating molecule capsules and MSCs for restoring infarcted myocardium. _Bioact. Mater._ 21, 20–31. https://doi.org/10.1016/j.bioactmat.2022.07.029 (2023). Article CAS PubMed
Google Scholar * Zheng, C., Chen, J., Liu, S. & Jin, Y. Stem cell-based bone and dental regeneration: A view of microenvironmental modulation. _Int. J. Oral Sci._ 11, 23.
https://doi.org/10.1038/s41368-019-0060-3 (2019). Article CAS PubMed PubMed Central Google Scholar * Probst, F. A. _et al._ Bone regeneration of minipig mandibular defect by adipose
derived mesenchymal stem cells seeded tri-calcium phosphate-poly(D, L-lactide-co-glycolide) scaffolds. _Sci. Rep._ 10, 2062. https://doi.org/10.1038/s41598-020-59038-8 (2020). Article CAS
PubMed PubMed Central ADS Google Scholar * Gronthos, S., Mankani, M., Brahim, J., Robey, P. G. & Shi, S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. _Proc.
Natl. Acad. Sci. U. S. A._ 97, 13625–13630. https://doi.org/10.1073/pnas.240309797 (2000). Article CAS PubMed PubMed Central ADS Google Scholar * Imanishi, Y. _et al._ Efficacy of
extracellular vesicles from dental pulp stem cells for bone regeneration in rat calvarial bone defects. _Inflamm. Regen._ 41, 12. https://doi.org/10.1186/s41232-021-00163-w (2021). Article
CAS PubMed PubMed Central Google Scholar * Kriegstein, A. & Alvarez-Buylla, A. The glial nature of embryonic and adult neural stem cells. _Annu. Rev. Neurosci._ 32, 149–184.
https://doi.org/10.1146/annurev.neuro.051508.135600 (2009). Article CAS PubMed PubMed Central Google Scholar * Noda, S. _et al._ Effect of cell culture density on dental pulp-derived
mesenchymal stem cells with reference to osteogenic differentiation. _Sci. Rep._ 9, 5430. https://doi.org/10.1038/s41598-019-41741-w (2019). Article CAS PubMed PubMed Central ADS Google
Scholar * Zhou, H. _et al._ The proangiogenic effects of extracellular vesicles secreted by dental pulp stem cells derived from periodontally compromised teeth. _Stem Cell Res. Ther._ 11,
110. https://doi.org/10.1186/s13287-020-01614-w (2020). Article CAS PubMed PubMed Central Google Scholar * Re, F., Gabusi, E., Manferdini, C., Russo, D. & Lisignoli, G. Bone
regeneration improves with mesenchymal stem cell derived extracellular vesicles (EVs) combined with scaffolds: A systematic review. _Biology (Basel)._ https://doi.org/10.3390/biology10070579
(2021). Article PubMed PubMed Central Google Scholar * Ye, T. _et al._ Large extracellular vesicles secreted by human iPSC-derived MSCs ameliorate tendinopathy via regulating macrophage
heterogeneity. _Bioact. Mater._ 21, 194–208. https://doi.org/10.1016/j.bioactmat.2022.08.007 (2023). Article CAS PubMed Google Scholar * Zheng, D. _et al._ Advances in extracellular
vesicle functionalization strategies for tissue regeneration. _Bioact. Mater._ https://doi.org/10.1016/j.bioactmat.2022.07.022 (2022). Article PubMed PubMed Central Google Scholar * Li,
Y., Duan, X., Chen, Y., Liu, B. & Chen, G. Dental stem cell-derived extracellular vesicles as promising therapeutic agents in the treatment of diseases. _Int. J. Oral Sci._ 14, 2.
https://doi.org/10.1038/s41368-021-00152-2 (2022). Article CAS PubMed PubMed Central Google Scholar * Zhang, Y. _et al._ Exosomes derived from 3D-cultured MSCs improve therapeutic
effects in periodontitis and experimental colitis and restore the Th17 cell/Treg balance in inflamed periodontium. _Int. J. Oral Sci._ 13, 43. https://doi.org/10.1038/s41368-021-00150-4
(2021). Article CAS PubMed PubMed Central Google Scholar * Witwer, K. W. _et al._ Defining mesenchymal stromal cell (MSC)-derived small extracellular vesicles for therapeutic
applications. _J. Extracell. Vesicles_ 8, 1609206. https://doi.org/10.1080/20013078.2019.1609206 (2019). Article CAS PubMed PubMed Central Google Scholar * Sonoda, S. _et al._
Extracellular vesicles from deciduous pulp stem cells recover bone loss by regulating telomerase activity in an osteoporosis mouse model. _Stem Cell Res. Ther._ 11, 296.
https://doi.org/10.1186/s13287-020-01818-0 (2020). Article CAS PubMed PubMed Central Google Scholar * Qiao, Z. _et al._ Fracture healing and the underexposed role of extracellular
vesicle-based cross talk. _Shock_ 49, 486–496. https://doi.org/10.1097/SHK.0000000000001002 (2018). Article PubMed Google Scholar * Qiao, Z. _et al._ The impact of plasma-derived
microvesicles from a femoral fracture animal model on osteoblast function. _Shock_ 53, 78–87. https://doi.org/10.1097/SHK.0000000000001336 (2020). Article CAS PubMed Google Scholar *
Wang, J., Liu, S., Li, J., Zhao, S. & Yi, Z. Roles for miRNAs in osteogenic differentiation of bone marrow mesenchymal stem cells. _Stem Cell Res. Ther._ 10, 197.
https://doi.org/10.1186/s13287-019-1309-7 (2019). Article CAS PubMed PubMed Central Google Scholar * Qin, Y., Wang, L., Gao, Z., Chen, G. & Zhang, C. Bone marrow stromal/stem
cell-derived extracellular vesicles regulate osteoblast activity and differentiation in vitro and promote bone regeneration in vivo. _Sci. Rep._ 6, 21961. https://doi.org/10.1038/srep21961
(2016). Article CAS PubMed PubMed Central ADS Google Scholar * Jing, D. _et al._ The role of microRNAs in bone remodeling. _Int. J. Oral Sci._ 7, 131–143.
https://doi.org/10.1038/ijos.2015.22 (2015). Article CAS PubMed PubMed Central Google Scholar * Pishavar, E., Copus, J. S., Atala, A. & Lee, S. J. Comparison study of stem
cell-derived extracellular vesicles for enhanced osteogenic differentiation. _Tissue Eng. Part A_ 27, 1044–1054. https://doi.org/10.1089/ten.TEA.2020.0194 (2021). Article CAS PubMed
PubMed Central Google Scholar * Garcia-Contreras, M. _et al._ Plasma-derived exosome characterization reveals a distinct microRNA signature in long duration Type 1 diabetes. _Sci. Rep._ 7,
5998. https://doi.org/10.1038/s41598-017-05787-y (2017). Article CAS PubMed PubMed Central ADS Google Scholar * Liu, A. _et al._ Macrophage-derived small extracellular vesicles
promote biomimetic mineralized collagen-mediated endogenous bone regeneration. _Int. J. Oral Sci._ 12, 33. https://doi.org/10.1038/s41368-020-00100-6 (2020). Article CAS PubMed PubMed
Central Google Scholar * Li, W. _et al._ Tissue-engineered bone immobilized with human adipose stem cells-derived exosomes promotes bone regeneration. _ACS Appl. Mater. Interfaces_ 10,
5240–5254. https://doi.org/10.1021/acsami.7b17620 (2018). Article CAS PubMed Google Scholar * Motamedian, S. R. _et al._ Response of dental pulp stem cells to synthetic, allograft, and
xenograft bone scaffolds. _Int. J. Periodontics Restor. Dent._ 37, 49–59. https://doi.org/10.11607/prd.2121 (2017). Article Google Scholar * Kniha, K. _et al._ Implant removal using
thermal necrosis-an in vitro pilot study. _Clin. Oral Investig._ 25, 265–273. https://doi.org/10.1007/s00784-020-03361-x (2021). Article PubMed Google Scholar * Zhang, J. _et al._
Exosomes/tricalcium phosphate combination scaffolds can enhance bone regeneration by activating the PI3K/Akt signaling pathway. _Stem Cell Res. Ther._ 7, 136.
https://doi.org/10.1186/s13287-016-0391-3 (2016). Article CAS PubMed PubMed Central Google Scholar * Mondal, A., Ashiq, K. A., Phulpagar, P., Singh, D. K. & Shiras, A. Effective
visualization and easy tracking of extracellular vesicles in glioma cells. _Biol. Proced. Online_ 21, 4. https://doi.org/10.1186/s12575-019-0092-2 (2019). Article CAS PubMed PubMed
Central Google Scholar * Willms, E., Cabanas, C., Mager, I., Wood, M. J. A. & Vader, P. Extracellular vesicle heterogeneity: Subpopulations, isolation techniques, and diverse functions
in cancer progression. _Front. Immunol._ 9, 738. https://doi.org/10.3389/fimmu.2018.00738 (2018). Article CAS PubMed PubMed Central Google Scholar * Mohlhenrich, S. C. _et al._
Establishing a new alveolar cleft model in rats to investigate the influence of jaw reconstructions on orthodontic tooth movement. _Ann. Anat._ 236, 151713.
https://doi.org/10.1016/j.aanat.2021.151713 (2021). Article PubMed Google Scholar * Gutierrez-Quintero, J. G. _et al._ Critical-sized mandibular defect reconstruction using human dental
pulp stem cells in a xenograft model-clinical, radiological, and histological evaluation. _Oral Maxillofac. Surg._ 24, 485–493. https://doi.org/10.1007/s10006-020-00862-7 (2020). Article
PubMed Google Scholar * Miron, R. J. _et al._ Osteoinductive potential of 4 commonly employed bone grafts. _Clin. Oral Investig._ 20, 2259–2265. https://doi.org/10.1007/s00784-016-1724-4
(2016). Article PubMed Google Scholar * Heitzer, M. _et al._ In vitro comparison of the osteogenic capability of human pulp stem cells on alloplastic, allogeneic, and xenogeneic bone
scaffolds. _BMC Oral Health_ 23, 1–13 (2023). Article Google Scholar * Murali, V. P. & Holmes, C. A. Mesenchymal stromal cell-derived extracellular vesicles for bone regeneration
therapy. _Bone Rep._ 14, 101093. https://doi.org/10.1016/j.bonr.2021.101093 (2021). Article CAS PubMed PubMed Central Google Scholar * Cui, Y., Luan, J., Li, H., Zhou, X. & Han, J.
Exosomes derived from mineralizing osteoblasts promote ST2 cell osteogenic differentiation by alteration of microRNA expression. _FEBS Lett._ 590, 185–192.
https://doi.org/10.1002/1873-3468.12024 (2016). Article CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS The authors gratefully acknowledge the use of the equipment and
facilities of the interdisciplinary. Center for Clinical Research of the Medical Faculty of RWTH Aachen University. We would like to thank botiss biomaterials (botiss biomaterials GmbH,
Germany) for providing the bone grafts free of charge. FUNDING Open Access funding enabled and organized by Projekt DEAL. Supported by a Grant from the Interdisciplinary Centre for Clinical
Research within the faculty of Medicine at the RWTH Aachen University (OC1-8). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Oral and Maxillofacial Surgery, University Hospital
of RWTH Aachen, Pauwelsstraße 30, 52074, Aachen, Germany Marius Heitzer, Philipp Winnand, Frank Hölzle & Ali Modabber * Department of Orthopedics, Trauma and Reconstructive Surgery,
University Hospital of RWTH Aachen, Pauwelsstraße 30, 52074, Aachen, NRW, Germany Qun Zhao, Johannes Greven, Xing Zhang, Felix Marius Bläsius & Frank Hildebrand * Institute of Pathology,
University Hospital of RWTH Aachen, Pauwelsstraße 30, 52074, Aachen, NRW, Germany Eva Miriam Buhl & Sabine Neuss * Department of Orthodontics, University Hospital of RWTH Aachen,
Pauwelsstraße 30, 52074, Aachen, NRW, Germany Michael Wolf * BioInterface Group, Helmholtz Institute for Biomedical Engineering, RWTH Aachen University, Pauwelsstraße 20, 52074, Aachen, NRW,
Germany Sabine Neuss Authors * Marius Heitzer View author publications You can also search for this author inPubMed Google Scholar * Qun Zhao View author publications You can also search
for this author inPubMed Google Scholar * Johannes Greven View author publications You can also search for this author inPubMed Google Scholar * Philipp Winnand View author publications You
can also search for this author inPubMed Google Scholar * Xing Zhang View author publications You can also search for this author inPubMed Google Scholar * Felix Marius Bläsius View author
publications You can also search for this author inPubMed Google Scholar * Eva Miriam Buhl View author publications You can also search for this author inPubMed Google Scholar * Michael Wolf
View author publications You can also search for this author inPubMed Google Scholar * Sabine Neuss View author publications You can also search for this author inPubMed Google Scholar *
Frank Hildebrand View author publications You can also search for this author inPubMed Google Scholar * Frank Hölzle View author publications You can also search for this author inPubMed
Google Scholar * Ali Modabber View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS M.H.: conception and design, performed the experiments,
acquisition of data, analysis and interpretation, drafting the work, final approval; Q.Z.: performed in vitro experiments, acquisition of data, revising the work, final approval; J.G.:
conception and design, analysis and interpretation, final approval; P.W.: acquisition of data, revising the work, final approval; X.Z.: performed in vitro experiments, acquisition of data,
revising the work, final approval; F.M.B.: conception and design, revising the work, final approval; E.M.B.: analysis and interpretation, revising the work, final approval; M.W.: conception
and design, analysis and interpretation, drafting the work, final approval; S.N.: analysis and interpretation, revising the work, final approval; F.Hi.: conception and design, analysis and
interpretation, revising the work, final approval; F.Ho.: conception and design, revising the work, final approval; A.M.: conception and design, analysis and interpretation, revising the
work, final approval; and all authors read and approved the final manuscript. CORRESPONDING AUTHOR Correspondence to Qun Zhao. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no
competing interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and
reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes
were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material.
If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to
obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS
ARTICLE Heitzer, M., Zhao, Q., Greven, J. _et al._ Evaluation of in vitro biocompatibility of human pulp stem cells with allogeneic, alloplastic, and xenogeneic grafts under the influence
of extracellular vesicles. _Sci Rep_ 13, 12475 (2023). https://doi.org/10.1038/s41598-023-39410-0 Download citation * Received: 26 May 2023 * Accepted: 25 July 2023 * Published: 01 August
2023 * DOI: https://doi.org/10.1038/s41598-023-39410-0 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable
link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative