Three-dimensional visualization of dentine occlusion based on fib-sem tomography
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT The occlusion of dentinal tubules has become a rapid and effective method for treating dentin hypersensitivity. Accurate evaluation of dentin occlusion is critical to illustrate the
efficacy of oral care products and to optimize dental therapy in the clinics, which is limited by the conventional two-dimensional (2-D) characterization methods. Here, we demonstrate the
visualization of the dentin occlusion via three-dimensional (3-D) characterization using a focused ion beam-scanning electron microscopy (FIB-SEM) tomography. Using the “Slice and View”
approach, the material used for occluding dentin tubules is imaged with a very high-resolution voxel (10 nm × 10 nm × 20 nm) from 2-D SEM images and then reconstructed into a 3-D volume,
which presents the mode of action of toothpaste for treating dentin hypersensitivity. Meanwhile, quantitative analysis of the depth of occlusion is successfully obtained. This work validates
the feasibility of FIB-SEM tomography in the analysis of dentin occlusion within the complicated networks of dentine tubules at the nanoscale, and provides a novel approach to facilitate
the research and development of oral care products. SIMILAR CONTENT BEING VIEWED BY OTHERS NANO-CT CHARACTERIZATION OF DENTINAL TUBULE OCCLUSION IN SDF-TREATED DENTIN Article Open access 23
September 2023 SUPER HIGH-QUALITY SEM/FIB IMAGING OF DENTINE STRUCTURES WITHOUT COLLAGEN FIBER LOSS THROUGH A METAL STAINING PROCESS Article Open access 11 February 2022 X-RAY DARK-FIELD
TOMOGRAPHY REVEALS TOOTH CRACKS Article Open access 07 July 2021 INTRODUCTION Dentin hypersensitivity has become a growing oral health concern globally1. As reported, adults between the ages
of 20 to 50 are frequently diagnosed with dentine hypersensitivity at a prevalence rate of up to 98%2,3. In addition, the prevalence distribution of patients with chronic periodontal
diseases has been recognized to be above 72%2,4. Dentin hypersensitivity is defined as a sharp and localized pain for a short period, typically associated with a stimulus of thermal,
osmotic, or chemical source to the exposed dentin tubules5, but not responsible for any other tooth defects or diseases6. Dentin hypersensitivity is caused by the exposed and open dentin
tubules, owing to enamel erosion and gingival recession7. The available treatment strategies for dentin hypersensitivity, either at home or in office, can be classified into two groups by
the mechanism of actions, which are the obstruction of the neural response to pain stimulus and the occlusion of dentinal tubules8. Occluding the open dentin tubules with specific materials
could be one of the most principal methods, which can immediately and efficiently reduce dentin hypersensitivity due to the direct interruption of the sensitive mechanisms9. Many models and
simulations related to dentin hypersensitivity have been reported previously to simplify the matrix of dentin tubules, such as considering it as a linear non-branched structure10. However,
the real tubule network features micro- or nano-branching and interconnection11. If the tubules sample has been deposited by dentin hypersensitivity treatment, the real sample are much more
complicated than the simplified non-branched model10. Therefore, it is necessary to apply microscopic approaches to probe the details of occluded dentin tubules in 2-D, and 3-D, to enable
more comprehensive evaluation of impacted treatment on the nanoscale components. Various 2-D characterization techniques, such as confocal microscopy12,13 and scanning electron microscopy
(SEM)14,15, have been used to determine the efficacy of targeted material (e.g., desensitized dentifrice for treating dentin hypersensitivity). However, these methods have a relatively low
resolution and/or only provide 2-D information, thus a comprehensive image of dentine occlusion is not attainable by these methods. X-ray microtomography is a developed tomographic technique
which displayed potential for 3D characterization of dentin tubules16,17, but the spatial resolution is in the order of several hundred nanometers. Such spatial resolution would not be able
to provide much more details on the nano-branching portion of the tubule network. A comprehensive visualization of dentinal tubule occlusion in 3-D domain could significantly promote the
development for a more effective therapy of dentin hypersensitivity by enabling a better understanding of the relationship between functional materials used for occlusion and the spatial
structure of dentin. Currently, focused ion beam-scanning electron microscopy (FIB-SEM) tomography has been well developed and used as a promising analytical approach based on
high-resolution 3-D reconstruction techniques18,19. This technique allows one to quantitatively explore the ultrastructural properties of colloidal nanoparticles20,21 and biological
samples22,23. FIB-SEM is a very robust technique, featuring the capability of 3-D real-space imaging of up to thousands of cubic microns while offering a very high resolution of down to
several nanometers24. The precise control of both the focused ions and the electron beam are usually based on an independent operation system, simultaneously allowing the accurate milling
process from FIB scanning and the high-resolution images captured from SEM recording25. In this study, we developed a desirable method dependent on a 3-D FIB-SEM tomography to quantitatively
visualize the spatial distribution of particle-occluded dentin tubules by a programmed reconstruction of image stacking. The method presented here can generate novel insights into the
evaluation of tubule-occluding efficacy. This FIB-SEM collaboration process can eventually provide a high-quality 3-D reconstruction of the specific specimen with a high-resolution voxel of
5 nm × 5 nm × 5 nm, thus extending to more delicate exploration of even a single dentinal tubule with nanostructures. This imaging technique could offer guidance for dentists to determine
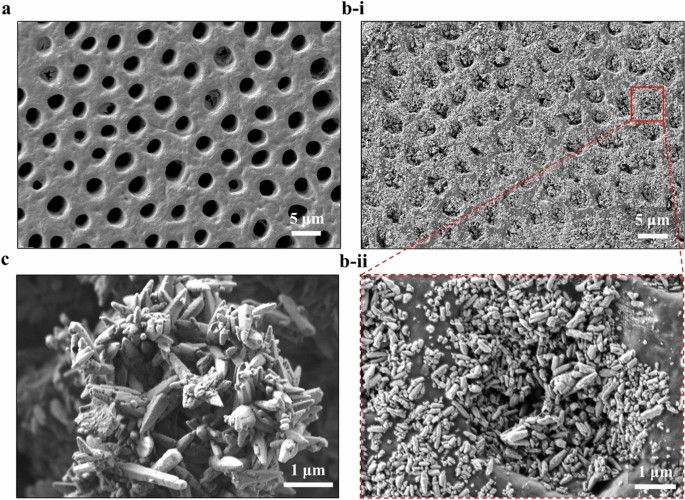
the performance of applied treatments and it can be utilized in the development of new treatment products for dentin hypersensitivity. RESULTS Figure 1 shows the representative SEM images of
the dentinal specimens before and after applying the testing toothpaste, and the comparison of the raw material and the testing toothpaste within the tubules through the morphology of the
involved calcium carbonate (CaCO3). Figure 1a presents a typical untreated specimen with the cleaned and smoothed surface, where the dentinal tubules are completely open without any smear
layer or smear plugs. In Fig. 1b-i, the treated dentin surface exhibits that almost all open tubules are blocked or plugged by the test toothpaste. An individual occluded tubule is enlarged
at a higher magnification (Fig. 1b-ii), which is compared to the raw material clearly shown in Fig. 1c. Both materials consisting of CaCO3 nanoparticles present a similar morphology,
confirming that the test toothpaste successfully attaches to the dentinal specimen surface and occluded the dentinal tubule. The occlusion depth will then be analyzed through cross-sectional
images of the tubules. To further characterize the dentin tubule occlusion caused by the raw material in the toothpaste, the dentinal specimen was mounted in the FIB-SEM chamber as shown in
Fig. 2a-i. A protective layer of Pt was deposited (thickness: 200 nm). The longitudinal FIB slicing, milling the sample along the length direction of the dentin tubules, was applied (Fig.
2a-ii). This was performed to expose the tubules’ cross-section (Fig. 2a-iii) to analyze the components of the test toothpaste occluded within the tubules as presented in Fig. 2b-i. The
high-magnification SEM image displays a well-occluded tubule (Fig. 2b-ii). The EDS spectrum was used to present the typical composition of the occluded testing toothpaste. As shown in Fig.
2c, the particles occluded in the dentin tubules consist of the same elements as the raw material, which are carbon (C), oxygen (O), and calcium (Ca). The EDS spectrum confirms that the
material occluded in the dentin tubule is from the major component of the test toothpaste, which is CaCO3, and designed for treating dentin hypersensitivity by occlusion. To further
investigate the efficacy of the test toothpaste for dentin occlusion, specifically, to illustrate dentin occlusion from 3-D domain, FIB-SEM tomography was performed. Figure 3 illustrates the
procedure for acquiring the 2-D SEM image stacks used for 3-D reconstruction. As shown in Fig. 3a, the dentin disc treated by the test toothpaste was mounted and tilted at an angle of 54°
for the process of FIB milling and SEM imaging (Fig. 3a). The region of interest was trimmed as an island and in situ coated with Pt as a protective layer before the sliced milling by FIB
for the SEM image stack acquisition. In the process for acquiring the SEM image stacks, a 20 nm thick slice was milled and then an SEM image was taken of the new exposed cross-section. This
process was repeated automatically until the desired distance (the length of the tubules) was reached, producing an SEM image stack as shown in Fig. 3b. The image stacks were then
reconstructed to a 3-D volume for the visualization of occluded dentinal tubules. The 3-D reconstruction of the scanned image stacks was accomplished by Dragonfly@ software, and a
comprehensive visualization of dentin occlusion is illustrated by the video in the Supplementary data. The visualized structure of interest, including the dentinal tubules and the material
used for occluding the dentin tubules, is displayed in Fig. 4. Figure 4a and b show a combination of the exposed structure of the material occluded in the tubules and the dentin structure by
a digital sectioning function of the software. The material occluded inside the dentin tubules, which may be called a “plug”, can be completely separated from the dentin. As shown in Fig.
4c, the material designed for occluding the dentin tubules, the plugs, occlude almost all of the dentin tubules. The plugs are relatively dense at the top (opening) of the dentin tubules,
but are relative loose inside the tubules. These plugs should be able to block fluid from flowing into the dentin tubule and treating dentin hypersensitivity. The occlusion depth of the
dentin disk, which is the length of the plug inside the dentin tubule, was quantitatively measured and plotted as shown in Fig. 5. Nine valid data points were obtained from the 3-D volume,
indicating the minimum depth of the occlusion is 13.5 µm and the maximum depth of the occlusion is 16.5 µm. The average occlusion depth of 15.3 µm can be calculated with a standard deviation
of 1.3 µm. This measured depth of the plugs can be used to evaluate the efficacy of different toothpastes for anti-hypersensitivity by dentin occlusion. DISCUSSION The occlusion of dentinal
tubules has been widely used to treat dentin hypersensitivity by directly interrupting the fluid movement within the dentinal tubules, according to the “hydrodynamic theory” that
demonstrates the pain-producing mechanism from the exterior stimulus26. In the in vitro study, a versatile method to evaluate the material occluding the tubules is of great importance.
Conventional X-ray imaging techniques, such as Computed tomography (CT), 3-D X-ray microcopy, and confocal microscopy, have been used widely to evaluate dentin occlusion in this in vitro
study. However, the resolution of these techniques is not high enough to reveal the detailed micro-/nanostructures of most of the material occluding the dentin tubule, as the particle sizes
of these material (e.g., silica) are usually in a few tens of nanometers. Although imaging based on synchrotron technology has been claimed with a resolution of 30 nm, the instrument is not
readily available. Also, to achieve such a high resolution, the sample thickness needs to be trimmed below 100 µm, which requires FIB milling and extensive preparatory work. With the
advances made in FIB-SEM instrumentation, a very high resolution can be achieved from SEM and a milling thickness of 3 nm can be obtained using FIB. Combining the high-resolution FIB milling
and SEM imaging can reveal more significant details between the major tubules of dentin, such as interconnected branching11 that includes major branches (0.5–1.0 μm), fine branches (300–700
nm), and microbranches (25–200 nm). These branches can potentially present large amounts of canalicular and anastomosing network. Therefore, study of the intricate intratubular dentin
structure is imperative. Serial slices (images) can be automatically obtained and reconstructed by the instrument. By a further imaging process, the structures of interest can be segmented
out and displayed individually. In this work, 860 SEM images were obtained with a pixel size of 10 nm × 10 nm and a slice thickness of 20 nm. The images were reconstructed into a 3-D volume,
and the material occluded into the dentin tubules were segmented out and demonstrated. The 3-D FIB-SEM tomography can reveal the dentin structure and occluded material with nanoscale
resolution, as shown in Fig. 3. The occluded material shows a relatively dense plugging on the top of the dentin tubules. In deeper regions of the tubules, the plugs are not continuous.
These detailed structures are directly related the efficacy of the applied toothpaste for treating dentin hypersensitivity by interfering the fluid motion in the dentin tubules. Compared
with the 2-D SEM images, 3-D visualization of dentin occlusion can provide quantitative information of the material occluded in the tubules. As shown Fig. 5, the lengths of the occluding
plugs can be quantitatively analyzed from the captured 3-D image. To show the occlusion efficacy of an oral care product or compare the occlusion efficacy of different oral care products, a
quantitative analysis is always desired. However, there are not many methods available. This is largely due to the fact that the quantitative analysis requires a high-resolution technique to
reveal the nanoparticles in the occluding material. Although X-ray technologies, or the synchrotron technology have been claimed to have the resolution of sub-microns or a few tens of
nanometers16,17, they still cannot match the sub-nanometer resolution offered by SEM. In this work, we show the process for obtaining SEM images with a pixel size of 10 nm × 10 nm and a
slice thickness of 20 nm. Visualization of the particles occluding the dentin tubules was achieved, and the obtained images showed that the occluding material is not continuous inside the
tubules. These details, which may not be obtained by other technique, provide the direct reference information for developing toothpastes to treat dentin hypersensitivity. Most of the
advanced SEM/FIB systems can achieve a 5 nm × 5 nm pixel size and 5 nm slice thickness for analyzing dentine structure27. However, we noticed some challenges when acquiring serial SEM images
from dentin structures using the best resolutions. First, when a small pixel size and slice thickness are used, the image acquisition time is significantly increased, taking one or two days
to obtain 2000 images. Because the dentin structure is an organic–inorganic composite, the long imaging time will cause severe change and accumulated heat28. As a result, the significant
drift will occur, and the automatic tracking of the sample drift may be failed, causing the interruption of the image acquisition process. Thus, when the targeted size (volume) of interest
is determined, the pixel size and slice thickness should be reasonably increased to minimize the sample drift. On top of the sample drift during image acquisition, the organic–inorganic
composite nature of dentin may also cause it to receive beam damage from the high energy ions of FIB. The high energy beam can burn out the organics, such as collagen fibers from the dentin
structure, and leave micro-pores as shown in Fig. 2b-ii. Thus, when interpreting the cross-sectional SEM images of dentin structure obtained by FIB, the micro-pores generated by ion beam
damage may need to be considered as artifacts. To reduce the drift and beam damage, a cryogenic stage or a special sample process can be used if a large volume of interest is desired29. The
3-D reconstruction of SEM images has been improved due to the improvement of commercial software packages. However, there are still challenges for automatically segmenting the region of
interest (ROI) from the occluded dentin. One such challenge is that, referring to Fig. 4b, there is not significant contrast difference between the occluding material and dentin. Thus, the
segmentation has to be manually achieved from each slice. The deep learning process30,31 potentially enables to automatically segment the ROI. However, whether the deep learning process may
obtain the desired segmentation results is highly dependent on the ROI. For example, the occluding plugs inside the tubules are most likely to be porous. The deep learning process is often
unable to differentiate between the part of the pore that lies in the background of the sliced cross-section and the part of the pore wall that is in that sliced cross-section. This results
in artifacts that requires extensive corrections. In summary, a reliable and desirable 3-D characterization method for dentinal occlusion has been reported here to study the penetration and
distribution of applied toothpaste into dentin tubules. The 3-D image can provide detailed information about the dentin occlusion. Moreover, this method could be applied for the quantitative
measurements of volumetric occlusions within the tubules, or other microstructural parameters such as porosity, diameter of tubules, and the ratio of peritubular dentin. Dentin specimens
analyzed by this 3-D structural visualization approach may help elucidate the changes in dentinal blockage of specimens via testing various occlusion-based treatments in advanced dental
research. This method will facilitate understanding the fundamental information of the occlusion efficacy of toothpaste, provide guidance for product development, and better communicate with
consumers and professionals. MATERIALS AND METHODS All methods were carried out in accordance with relevant guidelines and regulations. All experimental protocols were reviewed and approved
by the Comitato Etico Romano Institutional Review Board. The samples were from male and female adults from the ages of 18 through 70 years (inclusive) and all participants signed an
informed consent form. DENTINAL SPECIMEN PREPARATION The human teeth were cut to several cross-sectional slices (thickness: 600 µm) by using a dicing saw (Buehler IsoMet High Speed Pro,
Buehler). Then, these disc-shaped dentin specimens were sanded and polished on a polishing grinder (EcoMet III, Buehler) to create the smooth surface. The polished specimens were etched in
1% citric acid solution for a period of 5 min at room temperature under sonication, followed by cleaning them with the deionized (DI) water for 1 min. Toothpaste slurry was then brushed onto
the polished surface of the specimen by a small brush and the specimen was washed in DI water. This process was repeated five times to obtain the final specimen. TREATMENT PROCEDURE At
least 3 tested specimens were brushed with the toothpaste slurries for 30 s, by using a microbrush. The tested toothpastes were created by mixing phosphate-buffered saline (PBS) and original
toothpaste in a 1:3 ratio. After the occlusion procedure, the tested specimens were placed in 30 mL PBS solution for 15 min at room temperature, followed by stirring at 130 rpm for 15 min.
Finally, these treated specimens were rinsed and dried for surface analysis and 3-D FIB-SEM tomography. FIB-SEM TOMOGRAPHY The Zeiss@ Crossbeam 540 workstation was used to characterize the
surface of specimens before and after the test toothpaste treatment without metal coating. The accelerating voltage and beam current were optimized and set to 0.5 kV and 100 pA,
respectively. To obtain the serial FIB/SEM images (or slice-and-view), the specimen was mounted on a SEM stub using silver paste and coated with a 200 nm thick platinum (Pt) film. The sample
was loaded into the chamber, and the stage was tilted at an angle of 54° to allow the ion beam to be perpendicular to the specimen surface. The accelerating voltage and current of the ion
beam were 30 kV and 700 pA, respectively. ZEISS Atlas 5 was used to set up the automatic acquisition of the SEM images of the slices (cross-sections) with a slice thickness of 20 nm, and the
pixel size was 10 nm × 10 nm. Dragonfly@ was further used to process and reconstruct the SEM images to the 3D volume. DATA AVAILABILITY The datasets generated during and/or analyzed during
the current study are available from the corresponding author on reasonable request. REFERENCES * FavaroZeola, L., Soares, P. V. & Cunha-Cruz, J. Prevalence of dentin hypersensitivity:
Systematic review and meta-analysis. _J. Dent._ 81, 1–6 (2019). Article Google Scholar * Rees, J. S. The prevalence of dentine hypersensitivity in general dental practice in the UK. _J.
Clin. Periodontol._ 27, 860–865 (2000). Article CAS Google Scholar * Rees, J. S. & Addy, M. A cross-sectional study of dentine hypersensitivity. _J. Clin. Periodontol._ 29, 997–1003
(2002). Article CAS Google Scholar * Addy, M. Dentine hypersensitivity: New perspectives on an old problem. _Int. Dent. J._ 52, 367–375 (2002). Article Google Scholar * Brännström, M.
Dentin sensitivity and aspiration of odontoblasts. _J. Am. Dent. Assoc._ 66, 366–370 (1963). Article Google Scholar * Holland, G. R., Narhi, M. N., Addy, M., Gangarosa, L. &
Orchardson, R. Guidelines for the design and conduct of clinical trials on dentine hypersensitivity. _J. Clin. Periodontol._ 24, 808–813 (1997). Article CAS Google Scholar * Gillam, D. G.
& Orchardson, R. Advances in the treatment of root dentine sensitivity: mechanisms and treatment principles. _Endod. Top._ 13, 13–33 (2006). Article Google Scholar * Davari, A.,
Ataei, E. & Assarzadeh, H. Dentin hypersensitivity: Etiology, diagnosis and treatment. A literature review. _J. Dent._ 14, 136–145 (2013). Google Scholar * Kara, C. & Orbak, R.
Comparative evaluation of Nd:YAG laser and fluoride varnish for the treatment of dentinal hypersensitivity. _J. Endodont._ 35, 971–974 (2009). Article Google Scholar * Stead, W. J.,
Orchardson, R. & Warren, P. B. A mathematical model of potassium ion diffusion in dentinal tubules. _Arch. Oral Biol._ 41, 679–687 (1996). Article CAS Google Scholar * Mjör, I. A.
& Nordahl, I. The density and branching of dentinal tubules in human teeth. _Arch. Oral Biol._ 41, 401–412 (1996). Article Google Scholar * Sauro, S., Watson, T. F. & Thompson, I.
Dentine desensitization induced by prophylactic and air-polishing procedures: An in vitro dentine permeability and confocal microscopy study. _J. Dent._ 38, 411–422 (2010). Article Google
Scholar * Hines, D. _et al._ Effect of a stannous fluoride toothpaste on dentinal hypersensitivity: In vitro and clinical evaluation. _J. Am. Dent. Assoc._ 150, S47–S59 (2019). Article
Google Scholar * Kim, S., Kim, E., Kim, D. & Lee, I. The evaluation of dentinal tubule occlusion by desensitizing agents: A real-time measurement of dentinal fluid flow rate and
scanning electron microscopy. _Oper. Dent._ 38, 419–428 (2013). Article CAS Google Scholar * Chen, C. L., Parolia, A., Pau, A. & Porto, I. C. M. Comparative evaluation of the
effectiveness of desensitizing agents in dentine tubule occlusion using scanning electron microscopy. _Austral. Dent. J._ 60, 65–72 (2015). Article CAS Google Scholar * Kawabata, M. _et
al._ Diffusive transport within dentinal tubules: An X-ray microtomographic study. _Arch. Oral Biol._ 53, 736–743 (2008). Article Google Scholar * Davis, G. & Mills, D. High-contrast
X-ray microtomography in dental research. _Dev. X-Ray Tomogr. XI_ 10391, 187–194 (2017). Google Scholar * Knott, G., Marchman, H., Wall, D. & Lich, B. Serial section scanning electron
microscopy of adult brain tissue using focused ion beam milling. _J. Neurosci._ 28, 2959–2964 (2008). Article CAS Google Scholar * Ohta, K. _et al._ Beam deceleration for block-face
scanning electron microscopy of embedded biological tissue. _Micron_ 43, 612–620 (2012). Article CAS Google Scholar * Besinis, A., van Noort, R. & Martin, N. Infiltration of
demineralized dentin with silica and hydroxyapatite nanoparticles. _Dent. Mater._ 28, 1012–1023 (2012). Article CAS Google Scholar * Toledano-Osorio, M. _et al._ Improved reactive
nanoparticles to treat dentin hypersensitivity. _Acta Biomater._ 72, 371–380 (2018). Article CAS Google Scholar * Narayan, K. & Subramaniam, S. Focused ion beams in biology. _Nat.
Methods_ 12, 1021–1031 (2015). Article CAS Google Scholar * Stachewicz, U., Szewczyk, P. K., Kruk, A., Barber, A. H. & Czyrska-Filemonowicz, A. Pore shape and size dependence on cell
growth into electrospun fiber scaffolds for tissue engineering: 2D and 3D analyses using SEM and FIB-SEM tomography. _Mater. Sci. Eng. C_ 95, 397–408 (2019). Article CAS Google Scholar *
Cantoni, M. & Holzer, L. Advances in 3D focused ion beam tomography. _MRS Bull._ 39, 354–360 (2014). Article CAS Google Scholar * van der Hoeven, J. E. S. _et al._ Bridging the gap:
3D real-space characterization of colloidal assemblies via FIB-SEM tomography. _Nanoscale_ 11, 5304–5316 (2019). Article Google Scholar * Cummins, D. Dentin hypersensitivity: From
diagnosis to a breakthrough therapy for everyday sensitivity relief. _J. Clin. Dent._ 20, 1–9 (2009). Google Scholar * De Portu, G., Chevalier, J., Douillard, T., Gremillard, L. &
Bonnefont, G. Can (Mg, Y)-PSZ: Spinel composites be a valuable option for dental application?. _Int. J. Appl. Ceram. Technol._ 15, 873–883 (2018). Article Google Scholar * Kubota, Y.,
Sohn, J. & Kawaguchi, Y. Large volume electron microscopy and neural microcircuit analysis. _Front. Neural Circuits_ 12, 98 (2018). Article CAS Google Scholar * Xu, S., Stranick, M.,
Hines, D., Du, K. & Pan, L. _Super High-Quality SEM/FIB Imaging of Dentine Structures Without Collagen Fiber Loss Through a Metal Staining Process_. (2021). * Fend, C., Moghiseh, A.,
Redenbach, C. & Schladitz, K. Reconstruction of highly porous structures from FIB-SEM using a deep neural network trained on synthetic images. _J. Microsc._ 281, 16–27 (2021). Article
CAS Google Scholar * Fend, C., Moghiseh, A., Redenbach, C. & Schladitz, K. Machine learning for reconstruction of highly porous structures from FIB-SEM nano-tomographic data. In
_Machine Learning for Cyber Physical Systems_ (eds Beyerer, J. _et al._) 123–130 (Springer, 2021). https://doi.org/10.1007/978-3-662-62746-4_13. Chapter Google Scholar Download references
ACKNOWLEDGEMENTS The authors thank Xian Boles at RIT for the schematic drawing. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Colgate Technology Center, Piscataway, NJ, 08854, USA Xinye
Chen, Kaleigh M. Ryan, Deon Hines, Long Pan & Shiyou Xu * Microsystems Engineering, Rochester Institute of Technology, Rochester, NY, 14623, USA Xinye Chen * Chemical and Environmental
Engineering, University of California Riverside, Riverside, CA, 92508, USA Ke Du * Department of Materials Science and Engineering, Rutgers University, Piscataway, NJ, 08854, USA Kaleigh M.
Ryan Authors * Xinye Chen View author publications You can also search for this author inPubMed Google Scholar * Kaleigh M. Ryan View author publications You can also search for this author
inPubMed Google Scholar * Deon Hines View author publications You can also search for this author inPubMed Google Scholar * Long Pan View author publications You can also search for this
author inPubMed Google Scholar * Ke Du View author publications You can also search for this author inPubMed Google Scholar * Shiyou Xu View author publications You can also search for this
author inPubMed Google Scholar CONTRIBUTIONS X.C. contributed to carrying on part of the experiment and drafting the manuscript. S.X. conceived the experiments. S.X. and D.H. contributed to
the experiments and FIB-SEM observations. X.C., K.R., L.P., K.D. and S.X. analyzed the results and revised the manuscript. All authors discussed the obtained results and reviewed the
manuscript. CORRESPONDING AUTHORS Correspondence to Ke Du or Shiyou Xu. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION
PUBLISHER'S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION Supplementary Video 1.
RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and
reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes
were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material.
If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to
obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS
ARTICLE Chen, X., Ryan, K.M., Hines, D. _et al._ Three-dimensional visualization of dentine occlusion based on FIB-SEM tomography. _Sci Rep_ 13, 2270 (2023).
https://doi.org/10.1038/s41598-023-29155-1 Download citation * Received: 19 September 2022 * Accepted: 31 January 2023 * Published: 08 February 2023 * DOI:
https://doi.org/10.1038/s41598-023-29155-1 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative