Diabetes mellitus degenerates cisplatin-induced nephrotoxicity in short hydration method: a propensity score-matching analysis
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Cisplatin (CDDP)-induced nephrotoxicity (CIN) is dose-limiting. We revealed that co-administration of non-steroid anti-inflammatory drugs and baseline comorbidity of diabetes
mellitus (DM) are associated with CIN development in the short hydration method; however, the results were accessorily obtained without appropriate power calculation. This study aimed to
demonstrate the influence of DM complications on CIN incidence in a real-world setting. Lung cancer patients receiving CDDP (≥ 75 mg/m2)-containing regimens with a short hydration method (n
= 227) were retrospectively evaluated. The patients were divided into control and baseline DM complication groups. The primary endpoint was the evaluation of CIN incidence between the
groups. Propensity score-matching was performed to confirm the robustness of the primary analysis results. CIN occurred in 6.8% of control and 27.0% of DM patients, respectively, with a
significant difference in all-patient populations (_P_ = 0.001). In addition, variation of serum creatinine and creatinine clearance significantly worsened in DM patients. Similar results
were obtained in a propensity-matched population. Multivariate logistic regression analysis found that DM complication is a singular risk factor for CIN development (adjusted odds ratio;
4.31, 95% confidence interval; 1.62–11.50, _P_ = 0.003). In conclusion, our study revealed that baseline DM complications significantly worsen CIN. SIMILAR CONTENT BEING VIEWED BY OTHERS
RISK FACTOR ANALYSIS FOR CISPLATIN-INDUCED NEPHROTOXICITY WITH THE SHORT HYDRATION METHOD IN DIABETIC PATIENTS Article Open access 10 October 2023 COMPARISON OF PREVENTIVE EFFECTS OF
COMBINED FUROSEMIDE AND MANNITOL VERSUS SINGLE DIURETICS, FUROSEMIDE OR MANNITOL, ON CISPLATIN-INDUCED NEPHROTOXICITY Article Open access 07 May 2024 USE OF HIGH-DOSE MESNA AND
HYPERHYDRATION LEADS TO LOWER INCIDENCE OF HEMORRHAGIC CYSTITIS AFTER POSTTRANSPLANT CYCLOPHOSPHAMIDE-BASED ALLOGENEIC TRANSPLANTATION Article 09 June 2021 INTRODUCTION Chemotherapy is the
main treatment strategy for advanced lung cancer1,2, and managing chemotherapy-induced adverse effects is necessary for safe, effective treatment and patient quality of life. Cisplatin
(CDDP) is a key chemotherapeutic agent in treating lung cancer in combination with radiotherapy and surgery1,2. In contrast, it induces nausea, vomiting, hearing impairment, neuropathy, and
nephrotoxicity3. CDDP-induced nephrotoxicity (CIN) is dose-relatedly reversible and is known to be its dose-limiting toxicity3. CIN used to occur in 30–40% of the patients3. However, the
progress of CIN management, including magnesium supplementation, quality antiemetic therapy, and appropriate diuretics administration, significantly reduced its occurrence to
0–10%3,4,5,6,7,8,9,10,11. Many reports evaluate CIN risk factors, although most were conducted using old CDDP administration methods without sufficient CIN prophylaxis described above,
suggesting some do not reflect real-world settings12,13,14,15,16,17,18,19,20,21,22. We have revealed that co-administration of non-steroid anti-inflammatory drugs (NSAIDs) and baseline
comorbidity of diabetes mellitus (DM) are significantly associated with CIN incidence in the short hydration method, the most advanced CDDP administration method4. However, the results were
obtained without sufficient power calculation. The prevalence rate of DM is increasing worldwide, and people with prediabetes are also growing23. Therefore, the CDDP administration to DM
patients is increasing. It is important to manage CIN in higher-risk patients although there is a lack of evidence in the population. This study aimed to determine the real-world impact of
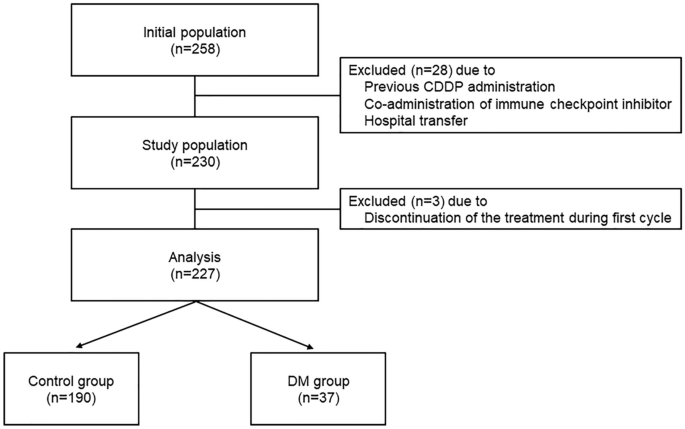
DM complications on CIN incidence. RESULTS PATIENT CHARACTERISTICS In total, 227 patients were enrolled in this study based on its eligibility criteria (Fig. 1). The baseline patient
characteristics (all-patient population and propensity score-matched population) are shown in Table 1. Patients in the DM group were significantly older and staged earlier than controlled
patients. In addition, patients with cardiovascular diseases (controlled hypertension and ischemic heart disease, according to a previous report19) were more included in the DM group than
control patients. In contrast, no background differences were confirmed between the groups in the propensity score-matched population. Patients with co-administered NSAIDs, an independent
CIN risk factor, accounted for 13.7% of the control group and 13.5% of the DM group in all populations, and 6.1% and 12.1% in propensity-matched populations, respectively, without
significant difference. Total administration cycles were not different between the groups (median cycles 4 [interquartile range 2–4 cycles] for both groups in the all-patient population, _P_
= 0.54; 4 cycles [2–4 cycles] for propensity-matched population, _P_ = 0.58). All DM patients were type 2 with well-controlled HbA1c (median 6.6%, range 5.4–9.3%). During the evaluation
periods, none of the patients had type 1 DM. Detailed DM medication is shown in Supplemental Table 1. COMPARISON OF CIN INCIDENCE AND VARIATION OF SERUM CREATININE LEVELS AND CREATININE
CLEARANCE CIN occurred in 6.8% of control and 27.0% of DM patients, respectively, with a significant difference in the all-patient population, meeting the study's primary endpoint (Fig.
2A). In addition, variation of serum creatinine (SCr) levels and creatinine clearance (CCr) significantly worsened in DM patients (Fig. 2A,B). Similar results were also obtained in
propensity-matched populations, with significant differences (Fig. 2C,D). INCIDENCE AND SEVERITY OF GASTROINTESTINAL SYMPTOMS AND HEARING IMPAIRMENT Management of chemotherapy-induced nausea
and vomiting (CINV) according to current guidelines24 is important for sufficient oral hydration, which is an important hydration method. Therefore, in addition to hearing impairment, we
also evaluated nausea, vomiting, and anorexia (Table 2). The incidence and severity of gastrointestinal adverse effects and hearing impairment were similar in both populations. RISK FACTOR
ANALYSIS FOR CIN DEVELOPMENT Multivariate logistic regression analysis in the all-patient population revealed that DM complication is a singular risk factor associated with CIN development
(adjusted odds ratio; 4.31, 95% confidence interval; 1.62–11.50, _P_ = 0.003, Table 3). Patients with NSAIDs co-administration had a higher risk of CIN, but this was not statistically
significant. DISCUSSION DM is associated with many complications, including renal toxicity, which affects pharmacokinetics and chemotherapy toxicities25, and we sometimes administer CDDP to
DM patients. Mathe et al. also reported that patients with cardiovascular diseases are at a higher risk for CIN development, and complication of DM and cardiovascular diseases further
aggravates CIN19. However, they did not evaluate the aggravating action of DM alone, and an advanced CIN management strategy using magnesium supplementation was not conducted in the study.
Our previous study suggested baseline DM complication as an independent risk factor for CIN development under CDDP short hydration although there was less power to evaluate the association4.
This study aimed to evaluate whether CIN more frequently occurs in DM patients. CIN incidence was significantly higher in DM complicated patients than in non-DM patients. In addition, SCr
and CCr aggravation varied significantly in DM patients than in the control patients. These results were confirmed in all and propensity score-matched patient populations. Multivariate
logistic regression analysis also suggested that DM complication is a singular risk factor for CIN, although cardiovascular diseases were not associated. Oral hydration substitutes for
intravenous hydration in the short hydration method4. Therefore, CINV control is important for sufficient oral hydration. However, the incidence and severity of CINV between the groups
suggest that oral hydration may have been similar. Consequently, it was suggested that DM directly worsened CIN. This is the first report suggesting that DM complication significantly
degenerates CIN in advanced management. We should frequently monitor renal function in DM patients and administer additional hydration for early countermeasures in case of CIN incidence. DM
has been reported to accelerate kidney aging26,27, and long-term DM degenerates intrarenal arterial sclerosis28. Furthermore, CIN worsens in autophagy-deficient mice compared to controls29.
We showed that celecoxib, a cyclooxygenase-2 selective NSAIDs, exhibits a nephroprotective effect against CIN by activating autophagy and suppressing oxidative stress30. These results
suggest that autophagy works nephroprotective against CIN. In addition, Sakai et al. have reported that autophagic activity differs in type 1 and 2 diabetic nephropathies, and its induction
is significantly suppressed in type 2 DM31. Most DM patients are diagnosed with type 223, and all patients in the present study were type 2. Consequently, we speculate that CIN is additively
or synergistically worsened by DM-induced renal impairment or autophagy suppression. In contrast, aging was not associated with CIN development in this study. In general, aging reduces
renal function19,26,27. However, it has been suggested that the organic cation transporter 2 (OCT2), which transports CDDP to the proximal tubule, has a critical role in CDDP
accumulation32,33, and its expression level reportedly decreases with aging34. These suggest that there is compensation between renal function decline and CDDP accumulation reduction due to
aging. Further studies evaluating these mechanisms are necessary for better CIN management in DM patients. This study has some limitations. First, this study was retrospectively conducted
with a relatively small population from a single institution. Second, as all DM patients in this study were type 2, and renal pathology differs between type 1 and 2 DM31, the results may
vary for type 1 DM. Third, in all-patient population analysis, patients in the DM group were significantly older than the control patients. Aging is associated with glomerulosclerosis and
arteriosclerosis of intrarenal vessels, causing nephron losss19. However, age was not a risk factor, and CIN was more common in DM patients, even in propensity-matched groups in this study.
Evaluation of a balanced population in all-patient population analysis was desirable. Finally, it has been suggested that OCT2 has single-nucleotide polymorphisms (SNPs)35 and that the 808G
> T SNP in OCT2 ameliorates CIN without alteration of disposition36. We did not assess patients' genetic backgrounds in this study so they might have affected the results. In
conclusion, our study revealed that DM complications significantly worsen CIN. Therefore, we should monitor DM patients cautiously and evaluate its mechanisms and countermeasures for
appropriate CIN management. METHODS PATIENTS This retrospective observational study enrolled lung cancer patients who received CDDP (≥ 75 mg/m2) from May 2014 to December 2021. We evaluated
CDDP-including regimens such as CDDP (75 mg/m2, day 1) + pemetrexed (PEM, 500 mg/m2, day 1) ± bevacizumab (BV, 15 mg/kg, day 1), CDDP (80 mg/m2, day 1) + etoposide (ETP, 100 mg/m2, days 1–3)
± radiation, and CDDP (80 mg/m2, day 1) + vinorelbine (VNR, 20–25 mg/m2, days 1, 8) ± radiation. All patients met the following baseline criteria: (1) age ≥ 20 years; (2) detailed patient
information available from medical records; (3) 0 to 2 Eastern Cooperative Oncology Group performance status (ECOG-PS); (4) sufficient renal or liver function for CDDP-containing treatment
induction. Patients who were previously administered CDDP, transferred hospital during the chemotherapy, could not complete the first cycle, and those who received dose reduction from
treatment initiation were excluded. Patients administered immune checkpoint inhibitors were also excluded as we evaluated direct DM influence on CIN. The patients were divided into two
groups: the DM group, which includes patients who required pharmacotherapy for DM treatment at baseline between May 2014 and December 2021, and the controls, who did not exhibit DM during
the treatment between May 2014 and March 2021. If the patient had uncontrolled DM, they were treated by diabetologists before treatment induction. We hypothesized that the CIN incidence
would be 10% in the control group and 30% in the DM group, with a patient ratio of 5:1, according to our previous research4. To achieve 80% power with an alpha error of 5%, the required
sample size was 190 subjects in the control group and 38 subjects in the DM group. Finally, 190 patients in the control group and 37 in the DM group were analyzed for eligibility. The
present study was approved by the Ethical Review Board for Life Science and Medical Research of Hokkaido University Hospital (Approval Number: 022-0107) and was carried out following the
Declaration of Helsinki and the STROBE statement. However, given the retrospective nature of the study, informed consent from the subjects was waived by the Ethical Review Board for Life
Science and Medical Research of Hokkaido University Hospital. TREATMENT METHODS OF PROPHYLACTIC SUPPORTIVE CARE All patients received the CDDP short hydration method, including 8 mEq of
magnesium sulfate administration as described previously4. In addition, all regimens included the same antiemetic therapy according to the current guidelines24; intravenous palonosetron 0.75
mg on day 1, oral aprepitant 125 mg on day 1 and 80 mg on days 2, 3, and dexamethasone 9.9 mg infusion on day 1 and 8 mg orally on days 2–4. EVALUATION OF CIN AND OTHER ADVERSE EFFECTS
Toxicities in all subsequent treatment cycles were evaluated under the Common Terminology Criteria for Adverse Events, version 5.0, by physicians and pharmacists. Pharmacists confirmed
appropriate CDDP administration, including oral hydration in each treatment cycle. Renal function was assessed based on the SCr variation measured by an enzymatic method. CCr was calculated
using the Cockcroft-Gault formula. CIN was defined as grade 2 or higher SCr elevation in this study as well as our previous reports3,4. The primary endpoint of this study was the evaluation
of CIN incidence between the groups. Secondary endpoints were assessment of SCr and CCr variation and CDDP-related adverse effects. Furthermore, propensity score-matching was performed to
adjust the baseline factors between the two groups, and matched data were additionally analyzed to confirm the robustness of the primary analysis results. STATISTICAL ANALYSIS The
differences in baseline clinical characteristics between control and DM groups were assessed using Fisher’s exact probability test for categorical outcome variables and the Mann–Whitney _U_
test for continuous parameters. Incidence of CIN was compared using Fisher's exact probability test, and differences in variation of SCr and CCr between the two groups were assessed
using the Mann–Whitney _U_ test. Assessment of adverse effects other than CIN was conducted using Fisher's exact probability test for the incidence and the Mann–Whitney _U_ test for
severity. The univariate and multivariate logistic regression analyses were used to identify the independent risk factor(s) for CIN incidence. We included the following baseline covariates:
sex, age, ECOG PS, staging, adjuvant chemotherapy setting, radiation combination setting, prior chemotherapy existence, body surface area (BSA), hemoglobin, serum albumin levels,
co-administration of proton pump inhibitors (PPI) and NSAIDs, complication of DM and cardiovascular diseases according to previous reports4,12,13,14,15,16,17,18,19,20,21,22. Variables that
demonstrated potential associations with CIN incidence in univariate logistic regression analysis (_P_ < 0.10) were considered when building the multivariable model. Propensity
score-matching was performed using the following baseline variables: sex, age, ECOG PS, staging, adjuvant chemotherapy setting, prior treatment existence, BSA, baseline hemoglobin, albumin,
SCr levels, NSAIDs and PPI co-administration, and cardiovascular disease complication. To reduce bias with these potential confounding factors, 1:1 matching (without replacement) in the two
groups was achieved using the nearest neighbor method with a 0.20- width caliper of the standard deviation of the logit of propensity scores. All analyses were performed using JMP version
16.2 statistical software (SAS Institute Japan, Tokyo, Japan). Differences were considered statistically significant when _P_-values were less than 0.05. ETHICS APPROVAL AND CONSENT TO
PARTICIPATE All procedures performed in this study were carried out in accordance with the ethical standards of the institutional and/or national research committee and the 1964 Helsinki
declaration and its later amendments or comparable ethical standards. The study was approved by the Ethical Review Board for Life Science and Medical Research of Hokkaido University Hospital
(Approved Number: 022-0107). Given the retrospective nature of the study, informed consent from the subjects was waived by the Ethical Review Board for Life Science and Medical Research of
Hokkaido University Hospital. DATA AVAILABILITY The datasets used and/or analyzed in the current study are available from the corresponding author on reasonable request. REFERENCES * Hanna,
N. H. _et al._ Therapy for stage IV non-small-cell lung cancer without driver alterations: ASCO and OH (CCO) joint guideline update. _J. Clin. Oncol._ 38(14), 1608–1632.
https://doi.org/10.1200/JCO.19.03022,Pubmed:31990617 (2020). Article ADS Google Scholar * Dingemans, A. C. _et al._ Small-cell lung cancer: ESMO clinical practice guidelines for
diagnosis, treatment and follow-up☆. _Ann. Oncol._ 32(7), 839–853. https://doi.org/10.1016/j.annonc.2021.03.207,Pubmed:33864941 (2021). Article Google Scholar * Saito, Y. _et al._
Premedication with intravenous magnesium has a protective effect against cisplatin-induced nephrotoxicity. _Support. Care Cancer_ 25(2), 481–487.
https://doi.org/10.1007/s00520-016-3426-5,Pubmed:27699503 (2017). Article Google Scholar * Saito, Y. _et al._ Suitability of oral rehydration solution (ORS) for use in the cisplatin short
hydration method. _Anticancer Res._ 42(6), 3185–3193. https://doi.org/10.21873/anticanres.15808 (2022). Article CAS Google Scholar * Tiseo, M. _et al._ Short hydration regimen and
nephrotoxicity of intermediate to high-dose cisplatin-based chemotherapy for outpatient treatment in lung cancer and mesothelioma. _Tumori_ 93(2), 138–144 (2007). Article CAS Google
Scholar * Horinouchi, H. _et al._ Short hydration in chemotherapy containing cisplatin (≥75 mg/m2) for patients with lung cancer: A prospective study. _Jpn. J. Clin. Oncol._ 43(11),
1105–1109. https://doi.org/10.1093/jjco/hyt122,Pubmed:24006505 (2013). Article Google Scholar * Yamaguchi, T. _et al._ Short hydration regimen with magnesium supplementation prevents
cisplatin-induced nephrotoxicity in lung cancer: a retrospective analysis. _Support. Care Cancer_ 25(4), 1215–1220. https://doi.org/10.1007/s00520-016-3512-8,Pubmed:27966021 (2017). Article
Google Scholar * Sakaida, E. _et al._ Safety of a short hydration method for cisplatin administration in comparison with a conventional method-a retrospective study. _Jpn. J. Clin.
Oncol._ 46(4), 370–377. https://doi.org/10.1093/jjco/hyv203,Pubmed:26755829 (2016). Article Google Scholar * Ninomiya, K. _et al._ Short-term low-volume hydration in cisplatin-based
chemotherapy for patients with lung cancer: The second prospective feasibility study in the Okayama Lung Cancer Study Group Trial 1201. _Int. J. Clin. Oncol._ 21(1), 81–87.
https://doi.org/10.1007/s10147-015-0860-1,Pubmed:26093520 (2016). Article CAS Google Scholar * Hotta, K. _et al._ Reappraisal of short-term low-volume hydration in cisplatin-based
chemotherapy: Results of a prospective feasibility study in advanced lung cancer in the Okayama Lung Cancer Study Group Trial 1002. _Jpn. J. Clin. Oncol._ 43(11), 1115–1123.
https://doi.org/10.1093/jjco/hyt128 (2013). Article Google Scholar * Horinouchi, H. _et al._ Oral rehydration solution (OS-1) as a substitute of intravenous hydration after cisplatin
administration in patients with lung cancer: A prospective multicenter trial. _ESMO Open_ 3(1), e000288. https://doi.org/10.1136/esmoopen-2017-000288 (2018). Article Google Scholar *
Okamoto, K. _et al._ Non-steroidal anti-inflammatory drugs are a risk factor for cisplatin-induced nephrotoxicity: A meta-analysis of retrospective studies. _Anticancer Res._ 40(3),
1747–1751. https://doi.org/10.21873/anticanres.14128 (2020). Article CAS Google Scholar * Yoshida, T. _et al._ Protective effect of magnesium preloading on cisplatin-induced
nephrotoxicity: A retrospective study. _Jpn. J. Clin. Oncol._ 44(4), 346–354. https://doi.org/10.1093/jjco/hyu004 (2014). Article Google Scholar * de Jongh, F. E. _et al._ Weekly high-dose
cisplatin is a feasible treatment option: Analysis on prognostic factors for toxicity in 400 patients. _Br. J. Cancer_ 88(8), 1199–1206. https://doi.org/10.1038/sj.bjc.6600884 (2003).
Article CAS Google Scholar * Anand, A. J. & Bashey, B. Newer insights into cisplatin nephrotoxicity. _Ann. Pharmacother._ 27(12), 1519–1525. https://doi.org/10.1177/106002809302701219
(1993). Article CAS Google Scholar * Madias, N. E. & Harrington, J. T. Platinum nephrotoxicity. _Am. J. Med._ 65(2), 307–314. https://doi.org/10.1016/0002-9343(78)90825-2 (1978).
Article CAS Google Scholar * Haas, A., Anderson, L. & Lad, T. The influence of aminoglycosides on the nephrotoxicity of cis-diamminedichloroplatinum in cancer patients. _J. Infect.
Dis._ 147(2), 363. https://doi.org/10.1093/infdis/147.2.363 (1983). Article CAS Google Scholar * Launay-Vacher, V. _et al._ Prevention of cisplatin nephrotoxicity: state of the art and
recommendations from the European Society of Clinical Pharmacy Special Interest Group on Cancer Care. _Cancer Chemother. Pharmacol._ 61(6), 903–909. https://doi.org/10.1007/s00280-008-0711-0
(2008). Article CAS Google Scholar * Máthé, C. _et al._ Cisplatin nephrotoxicity aggravated by cardiovascular disease and diabetes in lung cancer patients. _Eur. Respir. J._ 37(4),
888–894. https://doi.org/10.1183/09031936.00055110 (2011). Article CAS Google Scholar * Galfetti, E., Cerutti, A., Ghielmini, M., Zucca, E. & Wannesson, L. Risk factors for renal
toxicity after inpatient cisplatin administration. _BMC Pharmacol. Toxicol._ 21(1), 19. https://doi.org/10.1186/s40360-020-0398-3 (2020). Article CAS Google Scholar * Stewart, D. J. _et
al._ Association of cisplatin nephrotoxicity with patient characteristics and cisplatin administration methods. _Cancer Chemother. Pharmacol._ 40(4), 293–308.
https://doi.org/10.1007/s002800050661 (1997). Article CAS Google Scholar * Sato, K. _et al._ Nephrotoxicity of cisplatin combination chemotherapy in thoracic malignancy patients with CKD
risk factors. _BMC Cancer_ 16, 222. https://doi.org/10.1186/s12885-016-2271-8 (2016). Article CAS Google Scholar * Saeedi, P. _et al._ Global and regional diabetes prevalence estimates
for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. _Diabetes Res. Clin. Pract._ 157, 107843.
https://doi.org/10.1016/j.diabres.2019.107843 (2019). Article Google Scholar * Aogi, K. _et al._ Optimizing antiemetic treatment for chemotherapy-induced nausea and vomiting in Japan:
Update summary of the 2015 Japan Society of Clinical Oncology Clinical Practice Guidelines for Antiemesis. _Int. J. Clin. Oncol._ 26(1), 1–17. https://doi.org/10.1007/s10147-020-01818-3
(2021). Article Google Scholar * Deshpande, A. D., Harris-Hayes, M. & Schootman, M. Epidemiology of diabetes and diabetes-related complications. _Phys. Ther._ 88(11), 1254–1264.
https://doi.org/10.2522/ptj.20080020 (2008). Article Google Scholar * Perkins, B. A. & Krolewski, A. S. Early nephropathy in type 1 diabetes: The importance of early renal function
decline. _Curr. Opin. Nephrol. Hypertens._ 18(3), 233–240. https://doi.org/10.1097/MNH.0b013e3283293db1 (2009). Article CAS Google Scholar * Hernández-Marco, R. _et al._
Oxidant/antioxidant status and hyperfiltration in young patients with type 1 diabetes mellitus. _Pediatr. Nephrol._ 24(1), 121–127. https://doi.org/10.1007/s00467-008-0961-4 (2009). Article
Google Scholar * Takazakura, E. _et al._ Intrarenal vascular changes with age and disease. _Kidney Int._ 2(4), 224–230. https://doi.org/10.1038/ki.1972.98,Pubmed:4657923 (1972). Article
CAS Google Scholar * Kaushal, G. P. & Shah, S. V. Autophagy in acute kidney injury. _Kidney Int._ 89(4), 779–791. https://doi.org/10.1016/j.kint.2015.11.021,Pubmed:26924060 (2016).
Article CAS Google Scholar * Okamoto, K. _et al._ Comparison of the nephroprotective effects of non-steroidal anti-inflammatory drugs on cisplatin-induced nephrotoxicity in vitro and in
vivo. _Eur. J. Pharmacol._ 884, 173339. https://doi.org/10.1016/j.ejphar.2020.173339,Pubmed:32726655 (2020). Article CAS Google Scholar * Sakai, S. _et al._ Proximal tubule autophagy
differs in type 1 and 2 diabetes. _J. Am. Soc. Nephrol._ 30(6), 929–945. https://doi.org/10.1681/ASN.2018100983 (2019). Article CAS Google Scholar * Saito, Y. _et al._ Magnesium
attenuates cisplatin-induced nephrotoxicity by regulating the expression of renal transporters. _Eur. J. Pharmacol._ 811, 191–198. https://doi.org/10.1016/j.ejphar.2017.05.034 (2017).
Article CAS Google Scholar * Saito, Y. _et al._ Magnesium co-administration decreases cisplatin-induced nephrotoxicity in the multiple cisplatin administration. _Life Sci._ 189, 18–22.
https://doi.org/10.1016/j.lfs.2017.08.028 (2017). Article CAS Google Scholar * Xu, Y. J. _et al._ Age-associated differences in transporter gene expression in kidneys of male rats. _Mol.
Med. Rep._ 1, 474–482. https://doi.org/10.3892/mmr.2016.5970 (2017). Article CAS Google Scholar * Fukushima-Uesaka, H. _et al._ Fourteen novel single nucleotide polymorphisms in the
SLC22A2 gene encoding human organic cation transporter (OCT2). _Drug Metab. Pharmacokinet._ 19(3), 239–244. https://doi.org/10.2133/dmpk.19.239 (2004). Article CAS Google Scholar * Iwata,
K. _et al._ Effects of genetic variants in SLC22A2 organic cation transporter 2 and SLC47A1 multidrug and toxin extrusion 1 transporter on cisplatin-induced adverse events. _Clin. Exp.
Nephrol._ 16(6), 843–851. https://doi.org/10.1007/s10157-012-0638-y (2012). Article CAS Google Scholar Download references FUNDING This work was funded by JSPS KAKENHI Grant Number
22K15310. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Pharmacy, Hokkaido University Hospital, Kita 14-Jo, Nishi 5-Chome, Kita-Ku, Sapporo, 060-8648, Japan Yoshitaka Saito,
Tatsuhiko Sakamoto, Yoh Takekuma, Keisuke Okamoto & Mitsuru Sugawara * Laboratory of Clinical Pharmaceutics & Therapeutics, Faculty of Pharmaceutical Sciences, Hokkaido University,
Kita 12-Jo, Nishi 6-Chome, Kita-Ku, Sapporo, 060-0812, Japan Masaki Kobayashi * Department of Respiratory Medicine, Faculty of Medicine, Hokkaido University, Kita 15-Jo, Nishi 7-Chome,
Kita-Ku, Sapporo, 060-8638, Japan Naofumi Shinagawa * Department of Medical Oncology, Faculty of Medicine and Graduate School of Medicine, Hokkaido University, Kita 15-Jo, Nishi 7-Chome,
Kita-Ku, Sapporo, 060-8638, Japan Yasushi Shimizu & Ichiro Kinoshita * Laboratory of Pharmacokinetics, Faculty of Pharmaceutical Sciences, Hokkaido University, Kita 12-Jo, Nishi 6-Chome,
Kita-Ku, Sapporo, 060-0812, Japan Mitsuru Sugawara Authors * Yoshitaka Saito View author publications You can also search for this author inPubMed Google Scholar * Tatsuhiko Sakamoto View
author publications You can also search for this author inPubMed Google Scholar * Yoh Takekuma View author publications You can also search for this author inPubMed Google Scholar * Masaki
Kobayashi View author publications You can also search for this author inPubMed Google Scholar * Keisuke Okamoto View author publications You can also search for this author inPubMed Google
Scholar * Naofumi Shinagawa View author publications You can also search for this author inPubMed Google Scholar * Yasushi Shimizu View author publications You can also search for this
author inPubMed Google Scholar * Ichiro Kinoshita View author publications You can also search for this author inPubMed Google Scholar * Mitsuru Sugawara View author publications You can
also search for this author inPubMed Google Scholar CONTRIBUTIONS Designed the study: Y.S. and M.K. Performed the research: Y.S. and T.S. Analyzed the data: Y.S. Contributed new methods or
models: Y.S. and M.K. Wrote the paper: Y.S. All authors have read and approved the manuscript. CORRESPONDING AUTHOR Correspondence to Mitsuru Sugawara. ETHICS DECLARATIONS COMPETING
INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and
institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0
International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the
source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's
Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not
permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Saito, Y., Sakamoto, T., Takekuma, Y. _et al._ Diabetes mellitus degenerates
cisplatin-induced nephrotoxicity in short hydration method: a propensity score-matching analysis. _Sci Rep_ 12, 21819 (2022). https://doi.org/10.1038/s41598-022-26454-x Download citation *
Received: 11 September 2022 * Accepted: 14 December 2022 * Published: 17 December 2022 * DOI: https://doi.org/10.1038/s41598-022-26454-x SHARE THIS ARTICLE Anyone you share the following
link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature
SharedIt content-sharing initiative