Preliminary results of the cross-sectional associations of sedentary behavior and physical activity with serum brain-derived neurotrophic factor in adults with coronary heart disease
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT This is the first study to analyze the association of accelerometer-measured patterns of habitual physical activity (PA) and sedentary behavior (SB) with serum BDNF in individuals
with coronary heart disease. A total of 30 individuals (M = 69.5 years; 80% men) participated in this pre-post study that aimed to test a multi-behavioral intervention. All participants
underwent standardized measurement of anthropometric variables, blood collection, self-administered survey, and accelerometer-based measurement of PA and SB over seven days. Serum BDNF
concentrations were measured using enzyme-linked immunosorbent assay kit. We applied separate multiple linear regression analysis to estimate the associations of baseline SB pattern
measures, light and moderate-to-vigorous PA with serum BDNF (n = 29). Participants spent 508.7 ± 76.5 min/d in SB, 258.5 ± 71.2 min/d in light PA, and 21.2 ± 15.2 min/d in
moderate-to-vigorous PA. Per day, individuals had 15.5 ± 3.2 numbers of 10-to-30 min bouts of SB (average length: 22.2 ± 2.1 min) and 3.4 ± 1.2 numbers of > 30 min bouts of SB (average
length: 43.8 ± 2.4 min). Regression analysis revealed no significant associations between any of the accelerometer-based measures and serum BDNF. The findings of this study did not reveal an
association of accelerometer-measured PA and SB pattern variables with serum BDNF in individuals with coronary heart disease. In addition, our data revealed a considerable variation of PA
and SB which should be considered in future studies. SIMILAR CONTENT BEING VIEWED BY OTHERS THE ASSOCIATION BETWEEN OBJECTIVELY-MEASURED SEDENTARY BEHAVIOR PATTERNS AND PREDICTED 10-YEAR
ASCVD RISK Article Open access 30 July 2024 LONGITUDINAL ASSOCIATION BETWEEN PHYSICAL ACTIVITY AND THE RISK OF INCIDENT METABOLIC SYNDROME IN MIDDLE-AGED ADULTS IN GERMANY Article Open
access 12 November 2022 BOTH SEDENTARY TIME AND PHYSICAL ACTIVITY ARE ASSOCIATED WITH CARDIOMETABOLIC HEALTH IN OVERWEIGHT ADULTS IN A 1 MONTH ACCELEROMETER MEASUREMENT Article Open access
25 November 2020 INTRODUCTION Being physically inactive, characterized as a combination of low levels of moderate-to-vigorous physical activity (MVPA) and high amounts of sedentary behavior
(SB), has been linked to detrimental effects on physical1,2 and brain health3,4,5. Regular physical activity (PA) has been shown to increase brain functions such as cognition, memory, and
attention3,4. Evidence suggests that brain-derived neurotrophic factor (BDNF) may play a fundamental role in mediating this association.6,7 Aside from its important role in the development,
maintenance, and plasticity of the neurons and the regulation of energy homeostasis, BDNF is also produced peripherally (i.e., skeletal muscle, adipose tissue)8. There is increasing evidence
that PA stimulates the synthesis of BDNF due to its function as a contraction-inducible protein in the skeletal muscle7. Given the amount of studies that examined the effects of PA on BDNF,
a distinction between different paradigms is necessary7,9. While studies have consistently emphasized that acute PA increases concentrations of BDNF in healthy adults7,9,10, exercise
training seems to have varying effects on resting BDNF concentrations7,10. In healthy adults, the brain seems to contribute to increases in BDNF during exercise, but the source of
circulating BDNF remains controversial8,11. From a public health perspective, it is important to consider that individuals spend the majority of the day in SB and comparatively little time
in MVPA. The lack of PA characterized by a sedentary lifestyle increases with age12 and accelerates the development of chronic diseases such as cardiovascular disease (CVD)13. Moreover,
individuals with CVD typically report low levels of MVPA and high levels of SB compared to healthy controls14,15,16, which may enhance the risk for CVD progression and mortality17,18. In the
past years, research focus has shifted from total time spent sitting to the importance of considering an accumulation pattern of SB19,20, but there is insufficient information about
distinct patterns of PA and SB, particularly in individuals with CVD. In a community-dwelling sample of 5638 American women from the Objective Physical Activity and Cardiovascular Health
(OPACH) study, results showed that women who accumulated sedentary time in longer, uninterrupted sedentary bouts seem to have a higher risk for CVD compared to those who had shorter
sedentary bout durations16. Further, in a sample of 248 Dutch adults, 131 individuals with CVD showed significantly more prolonged uninterrupted sedentary bouts compared to 117 healthy
controls14. In particular, prolonged periods of sitting have been found to have adverse effects on vascular function and blood pressure21. Recent evidence has identified BDNF as a protective
factor in the pathogenesis of CVD due to its involvement in cardiac processes such as angiogenesis, vascular growth, and survival of cardiomyocytes22,23,24. In line with these findings,
high levels of BDNF were found to be associated with reduced risk of CVD and mortality25. Furthermore, low BDNF levels seem to be associated with increased risk of future adverse
cardiovascular events26 and mortality in individuals with angina pectoris27 and heart failure28. Therefore, it is important to examine how habitual PA and SB are associated with resting BDNF
levels. Studies investigating the effect of different types of accelerometer-measured habitual PA and SB (e.g., total time spent sedentary, or different lengths of sedentary bouts) with
resting BDNF are rare and the findings are inconsistent. The controversial results of current research might be due to differences regarding sample characteristics, medical conditions, and
age group29,30,31,32,33,34. In an adult population, one study did not find significant associations between habitual PA (light physical activity [LPA], MVPA) and resting BDNF33, whereas
another study showed a positive association of MVPA with resting BDNF and an inverse association of total time spent sitting with resting BDNF31. To our knowledge, only Júdice and colleagues
examined the relationship between patterns of SB and resting BDNF in individuals with type 2 diabetes and found that prolonged uninterrupted periods in sedentary time (bouts > 15 min),
but not total time spent sitting, was negatively associated with resting BDNF33. However, evidence on associations of combined accelerometer-measured patterns of habitual PA and SB with BDNF
in adults with CVD is lacking. Therefore, the aim of this study was to analyze the association between accelerometer-measured habitual PA (LPA, MVPA) and SB (total sitting time and
different lengths of sedentary bouts) with resting levels of serum BDNF in individuals aged ≥ 60 years and a history of coronary heart disease (CHD). METHODS STUDY DESIGN AND PARTICIPANTS
For this non-controlled pre-post study, individuals who have had an inpatient treatment within the last 24 months prior to the start of the study (November 2017) at the Department of
Internal Medicine B at the University Medicine Greifswald, were recruited. Eligibility criteria for the study participation are shown in Table 1. A total of 164 individuals with CHD were
contacted by mail and invited to participate in the study that aimed to test the feasibility of a multi-behavioral lifestyle intervention. Among those, 127 individuals declined to
participate due to several reasons such as (i) a lack of interest (n = 87) or (ii) time and physical demands of intervention or data assessment (n = 24 too ill, n = 8 barriers to reach the
training- and cardiovascular examination center, n = 6 heavy work schedules, n = 2 other barriers). One individual agreed to participate, but did not complete enrolment because of severe
illness. Thus, the final sample comprised 36 individuals. The focus of the lifestyle intervention was PA and nutrition. Views on ageing as a psychological component formed the framework of
the intervention35. The program of the intervention consisted of group and individual sessions and took part at the cardiovascular training and examination center of the University Medicine
Greifswald for a duration of 12 weeks. Participants exercised twice a week (from week 1 to 6) or once a week (from week 6 to 12) in a group setting, respectively (overall: 18 sessions).
Views on ageing were discussed with the participants in three group sessions and one individual session. Furthermore, participants received four education group sessions focusing on healthy
eating from week 6 to 11. At week 6 and 12, an individual session was carried out in order to receive process information on participants’ motivation, study quality, and potential for
optimization of the study. From week 12 to 24, participants had the opportunity to exercise once a week in a group at the cardiovascular examination center on a voluntary basis. Each session
lasted 60 to 90 min. This study was conducted in accordance with the Declaration of Helsinki and current guidelines of good clinical practice. The ethics committee of the University
Medicine Greifswald approved the study protocol (number BB138/17). Written informed consent was obtained before inclusion of study participants. PROCEDURES This study is a secondary analysis
of baseline data of an intervention study (“ReStart 60 + ”) with a duration of 12 months. All participants were invited to the cardiovascular training- and examination center of the
University Medicine Greifswald and underwent the following procedure: (i) standardized measurement of anthropometrical data, (ii) blood collection, (iii) resting electrocardiogram recording,
and (iv) self-administered assessments. Detailed information on current medical status and medications was collected. Body weight and height as well as waist and hip circumference were
assessed. Waist circumference was measured midway between the lowest rib and the iliac crest using an inelastic tape. Hip circumference was measured about two inches below the iliac crest.
Blood samples were drawn in the supine position from participants who gave additional informed consent. The sampling was carried out between 7:30am and 1:00 pm. Study participants provided
fasting (> 8 h) or non-fasting blood samples. Immediately after sampling, the serum tubes were cooled down to 4 °C. An hourly transport to the central laboratory (Institute for Clinical
Chemistry and Laboratory Medicine, University Medicine Greifswald) was organized. Upon arrival at the laboratory, the samples were immediately stored at −80 °C in the Integrated Research
Biobank (LiCONiC, Lichtenstein). Trained and certified study staff performed all measurements and blood sampling based on established standard operating procedures. The day following the
cardiovascular examination program, the accelerometer had to be worn for ten consecutive days. Study participants were instructed to wear the accelerometer on their hip with an elastic band
after getting dressed in the morning and to take the device off for night’s sleep and water-based activities. The study was conducted between November 2017 and September 2019. MEASURES SERUM
BRAIN-DERIVED NEUROTROPHIC FACTOR CONCENTRATION To determine the resting serum levels of BDNF, human serum samples were diluted 1:20 with the appropriate buffer. Human serum BDNF
concentrations were measured in duplicates using enzyme-linked immunosorbent assay (ELISA) kit according to the instructions of the manufacturer (Quantikine Human Free BDNF ELISA kit,
R&D systems, Minneapolis, MN). Using this assay the minimum detectable dose of human free BDNF is less than 20 pg/ml. The intra-assay coefficient of variability lies between 3.8% and
6.2% while the inter-assay precision is between 7.6 and 11.3%. BDNF levels were calculated using a standard curve supplied by the ELISA kit. ACCELEROMETER-MEASURED PHYSICAL ACTIVITY AND
SEDENTARY BEHAVIOR The accelerometers were initialized at a sampling rate of 30 Hertz and raw data were integrated into 60-s epochs36. Data from the vertical axis were used. For statistical
analysis, data from the accelerometers were downloaded and processed using ActiLife software (Version 6.13.3; ActiGraph). Time spent in SB, LPA, MVPA, and accelerometer wear time was
determined by minutes per day (min/d). Non‐wear time was defined as at least 60 consecutive minutes of zero activity counts, with allowance for ≤ 2 counts per minute (cpm) between 0 and 100.
We used cut points according to different intensity threshold criteria. Values < 100 cpm were defined as SB, values between 100 and 1951 cpm as light PA, and values ≥ 1952 cpm as MVPA37.
In order to minimize measurement bias of reactivity38 and due to missing values, we decided not to include data of the first, second, and last accelerometer wearing day. Therefore, seven
consecutive days of accelerometer wearing were included for data analyses. Further, we analyzed data only among those who wore the accelerometer on ≥ 4 days per week for ≥ 10 h per day (n =
30)36. COVARIATES Additional variables including sex and age (years) were assessed as covariates by self-administered questionnaire. There is evidence that BDNF levels are associated with
body weight39. Thus, statistical models were adjusted for body mass index (BMI) which was calculated as body weight divided by height squared (kg/m2) and used as a continuous variable.
Accelerometer-based patterns of SB and PA have shown to be associated with accelerometer wear time40 and therefore, wear time was included as a covariate. STATISTICAL ANALYSIS The model
assumptions for normality and homoscedasticity of residuals were tested using Shapiro–Wilk tests, White tests and graphic methods (histograms, kernel density plots, Q-Q plots,
residual-versus-fitted plots). The distributions of SB, LPA, MVPA, and accelerometer wear time approximated normality. Thus, untransformed values were used for analyses and participant
characteristics were described in means (M) with standard deviations (SD). Estimated daily averages of time spent in MVPA were calculated as total minutes of MVPA (min/d). Estimated daily
averages of time spent in LPA were calculated as total minutes of LPA (min/d). Estimated daily averages of time spent in SB were calculated as (i) total minutes of SB (min/d); (ii) number of
10-to-30 min bouts and of > 30 min bouts of SB per day, and (iii) average length of 10-to-30 min bouts and > 30 min bouts of SB. Separate multiple linear regression models were
applied for each accelerometer-based measure (time in SB, LPA, MVPA, SB bouts: numbers and lengths) with resting levels of serum BDNF as outcome variable. In a first step, all analyses were
adjusted for potential confounders (model 1: sex, age, BMI, and accelerometer wear time). In a second step, we additionally adjusted for SB in all regression models that included PA
variables (e.g., MVPA). However, due to multicollinearity, we did not adjust these models for other PA variables (e.g., LPA). Likewise, all regression models including SB were additionally
adjusted for MVPA (model 2). We used robust standard errors estimations to account for heteroscedasticity. _P_-values < 0.05 were considered statistically significant. All statistical
analyses were conducted using STATA version 15.1 (Stata-Corp, 2015). ETHICS APPROVAL AND CONSENT TO PARTICIPATE All procedures performed in studies involving human participants were in
accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Further, this study was conducted in accordance with the current guidelines of good clinical practice. The ethics committee of the University Medicine Greifswald approved the study protocol
(number BB 138/17). Written informed consent was obtained before inclusion of study participants. CONSENT FOR PUBLICATION Participants cannot be individually identified from data published
in this manuscript. Participants were made aware of the intent to publish this data when providing informed consent. RESULTS Table 2 provides descriptive characteristics of the study sample
(n = 30). The mean age was 69.5 years and 80% were males. The majority of the participants attended school for more than 10 years (58.6%), lived in a partnership (86.7%), and perceived their
health as very good or good (53.8%). In total, 18 individuals wore the accelerometer for seven valid days (60.0%), _n_ = 8 for six valid days (26.7%), _n_ = 3 for five valid days (10.0%),
and _n_ = 1 for four valid days (3.3%) with an average accelerometer wear time of over 13 h per day. Participants spent 508.7 min/d ± 76.5 min/d in SB, which corresponds to 64.5% of
accelerometer wear time, 258.5 min/d ± 71.2 min/d in LPA (32.8%), and 21.2 min/d ± 15.3 min/d in MVPA (2.7%). Accelerometer-based measures showed a large inter-individual variability across
the 7-day-assessment (Appendix, Fig. S1). Individuals had a daily number of 15.5 ± 3.2 10-to-30-min bouts of SB with an average length of a single bout of 22.2 ± 2.1 min. Further, they had a
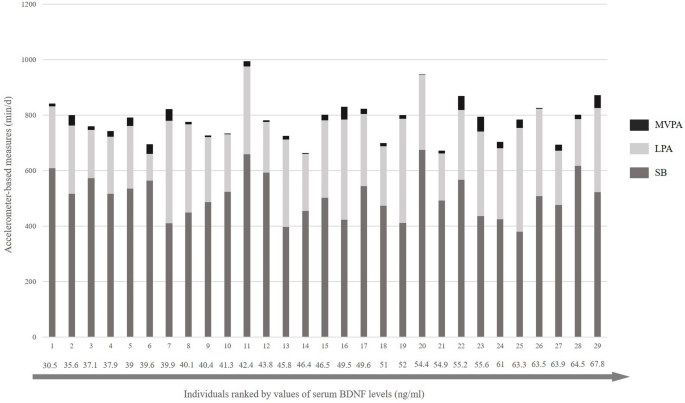
number of 3.4 ± 1.2 of > 30 min bouts of SB with an average length of 43.8 ± 2.4 min per single bout. The mean of the resting levels of serum BDNF was 48.7 ± 10.2 ng/ml. Figure 1
displays the total time spent in SB, LPA, and MVPA in individuals with CHD ranked by serum BDNF values. As shown in Table 3, regression analyses in 29 study participants revealed that there
were no statistically significant associations between any of the accelerometer-based measures with serum levels of BDNF either in model 1 or model 2 (all _P_-values > 0.05). DISCUSSION
In this study, we examined the association of accelerometer-measured habitual PA (LPA, MVPA) and SB (total sitting time as well as numbers and different length of sedentary bouts) with
resting levels of serum BDNF among individuals with CHD. Taken together, our results revealed two main findings: First, individuals with CHD spent most of their day in SB and little time in
MVPA. In addition, the distribution of time spent in different accelerometer-based measures showed a large inter-individual variability. Second, we could not reveal a statistically
significant associations of accelerometer-based measures with resting levels of serum BDNF. In line with previous research, we found a pattern characterized by low levels of MVPA and high
levels of SB among individuals with CHD14,16, despite the fact that sample characteristics of these studies such as age (mean age: 63 years;14 mean age: 79 years16) and proportion of sex
(e.g., only women16) differed notably from our study sample. Furthermore, our results revealed a large inter-individual variability in daily accelerometer-based measures. It could be
speculated that this is one of the reasons why we did not detect an association between accelerometer-based measures and resting levels of serum BDNF. Further research is needed to clarify
the effects of different intensities of habitual PA and SB accumulation pattern measures on levels of serum BDNF in individuals with CHD. Assuming that lifestyle behaviors such as habitual
PA and SB can influence cardio-protective factors such as BDNF in individuals with CHD, it might be considered as a strategy to maintain or increase physical and brain health status. As
pointed out in a systematic review9 and a meta-analysis7, studies regarding accelerometer-measured habitual PA and SB and resting BDNF in non-healthy adults (e.g., obesity, CVD, diabetes)
are limited and pattern variables of PA and SB (e.g., sedentary bouts) were rarely reported in these studies. Only the study by Júdice and colleagues examined accelerometer-measured habitual
PA and patterns of SB and resting BDNF in adults with type 2 diabetes mellitus (mean age: 58.3 years)33 which represents also a vulnerable group because these individuals are less active
and have lower levels of BDNF41. In line with their results, we could not find an association between habitual MVPA and resting BDNF. It should be noted that there is no scientific consensus
on how habitual PA or SB and resting levels of serum BDNF might be related and what the biological mechanism might be. Some have argued that resting levels of serum BDNF only increase
temporarily after acute bouts of PA, in an intensity- and duration-dependent manner10, and that BDNF levels return to pre-PA concentrations after 15 up to 60 min following PA cessation42,43.
Therefore, it seems to be important to distinguish evidence that reported effects of an acute bout of PA on the one hand and the effects of habitual PA on resting levels of BDNF on the
other hand. In contrast to our results, Júdice and colleagues found that the number of sedentary breaks were positively associated with plasma BDNF concentration, whereas number of longer
sedentary bouts per day (> 15 min) were negatively associated with plasma BDNF concentration33. Although sedentary breaks and sedentary bouts describe two different pattern parameters and
are therefore not interchangeable, both define the accumulation pattern of sitting time and are interdependent19. If individuals interrupt their total sitting time often (i.e., having many
breaks), the number of short sedentary bouts would increase and the number of longer sedentary bouts would decrease. Our findings may be different from those reported by Júdice and
colleagues due to methodological aspects such as sample characteristics (e.g., age, medical condition), accelerometer data processing criteria (e.g., 15-s epochs versus 60-s epochs),
operationalization of SB pattern measures (e.g., prolonged sedentary bouts > 15 min versus > 30 min), or BDNF measurement methods (e.g., plasma versus serum BDNF; different ELISA
kits). Given that also measurement errors in biological assays may occur due to serum collection or different methodological approaches44, the results of different studies on serum and
plasma BDNF levels might not be comparable45. Various strengths and limitations should be noted regarding the study design, study sample characteristics, and methodological issues. To our
knowledge, the present study is the first one that examined associations of accelerometer-measured habitual PA and SB with levels of serum BDNF in individuals with CHD. Further, it promotes
the understanding of patterns of activity, inactivity, and SB accumulation in individuals with CHD by combining information on duration of different PA intensities and SB measures (i.e.,
sedentary bouts). However, several limitations have to be discussed. First, individuals were assessed within a pre-post study aiming to test the implementation of a multi-modal intervention
program in individuals with CHD. The sample size was small. This might have limited the possibility to detect associations between accelerometer-based measures and serum BDNF (i.e.,
decreased statistical power). Further, the proportion of individuals who declined to participate (78%) was high, which may have led to a sample selection bias. Therefore, generalizability of
our results may be compromised. Secondly, there are methodological limitations regarding the measurement of BDNF44,45 and of accelerometer-based PA and SB46. We used hip-worn accelerometers
that may not accurately measure all types of PA (e.g., bicycling) or miss activities (e.g., swimming). Moreover, accelerometers cannot differentiate well enough between sitting and standing
because movement is determined by acceleration rather than body posture.47 Given that there are no validation or calibration studies in individuals with CVD48, we used the recommendations
for data collection and analysis that were developed for general populations36. Thirdly, there may be confounding variables that influence the association of habitual PA and SB and BDNF,
such as medications (e.g., antidepressants) or other lifestyle behaviors (e.g., smoking, diet, alcohol) that were not considered in the current analysis. Fourthly, the proportion of male
participants was high (80%). Although study participants with CHD in PA intervention studies appear to be more frequently male (69%)49, the low number of females did not allow to stratify
our results by sex. Recent evidence demonstrated that BDNF is a sexually dimorphic neurotrophin50 and Schmalhofer and colleagues have shown that there were significant sex-specific
associations between serum BDNF and cardiorespiratory fitness in women but not in men51. Therefore, the findings of our study should be interpreted cautiously and verified in a larger sample
of individuals with CHD using stratification by sex. Finally, due to the cross-sectional character of the study results, it is not allowed to drawn inferences about any possible causal
associations between the predictor and outcome variables. CONCLUSION To conclude, the cross-sectional findings of our study suggest that pattern variables of accelerometer-measured PA and SB
seems not to be related with serum BDNF in individuals with CHD. In addition, our data show a substantial inter-individual variability of habitual PA and SB. Given that this study is one of
the first to examine the association between different habitual accelerometer-based measures and serum BDNF, further research is needed to confirm our results. DATA AVAILABILITY The
datasets generated and/or analyzed during the current study are not publicly available due to restrictions associated with anonymity of participants but are available from the corresponding
author on reasonable request. The data is shared with researchers who submit a methodologically sound proposal to achieve the aims of the approved proposal., Requests in this regard should
be directed to the corresponding author to gain access. Requestors must sign a data access agreement ensuring data usage in compliance with the statement given in the informed consent
procedure and with the German data protection law, that the data will not be transferred to others, and that the data will be deleted after the intended analysis has been completed.
REFERENCES * Ekelund, U. _et al._ Dose-response associations between accelerometry measured physical activity and sedentary time and all cause mortality: Systematic review and harmonised
meta-analysis. _BMJ_ 366, l4570. https://doi.org/10.1136/bmj.l4570 (2019). Article PubMed PubMed Central Google Scholar * Young, D. R. _et al._ Sedentary behavior and cardiovascular
morbidity and mortality: A science advisory from the american heart association. _Circulation_ 134, e262–e279. https://doi.org/10.1161/cir.0000000000000440 (2016). Article PubMed Google
Scholar * Di Liegro, C. M., Schiera, G., Proia, P. & Di Liegro, I. Physical activity and brain health. _Genes_ 10, 720. https://doi.org/10.3390/genes10090720 (2019). Article CAS
PubMed Central Google Scholar * Umegaki, H., Sakurai, T. & Arai, H. Active life for brain health: A narrative review of the mechanism underlying the protective effects of physical
activity on the brain. _Front. Aging Neurosci._ 13, 761674. https://doi.org/10.3389/fnagi.2021.761674 (2021). Article CAS PubMed PubMed Central Google Scholar * Wheeler, M. J. _et al._
Sedentary behavior as a risk factor for cognitive decline? A focus on the influence of glycemic control in brain health. _Alzheimers Dement_ 3, 291–300.
https://doi.org/10.1016/j.trci.2017.04.001 (2017). Article Google Scholar * Swardfager, W. _et al._ Brain derived neurotrophic factor, cardiopulmonary fitness and cognition in patients
with coronary artery disease. _Brain Behav. Immun._ 25, 1264–1271. https://doi.org/10.1016/j.bbi.2011.04.017 (2011). Article CAS PubMed PubMed Central Google Scholar * Szuhany, K. L.,
Bugatti, M. & Otto, M. W. A meta-analytic review of the effects of exercise on brain-derived neurotrophic factor. _J. Psychiatr. Res._ 60, 56–64.
https://doi.org/10.1016/j.jpsychires.2014.10.003 (2015). Article PubMed Google Scholar * Walsh, E. I., Smith, L., Northey, J., Rattray, B. & Cherbuin, N. Towards an understanding of
the physical activity-BDNF-cognition triumvirate: A review of associations and dosage. _Ageing Res. Rev._ 60, 101044. https://doi.org/10.1016/j.arr.2020.101044 (2020). Article PubMed
Google Scholar * Huang, T., Larsen, K. T., Ried-Larsen, M., Møller, N. C. & Andersen, L. B. The effects of physical activity and exercise on brain-derived neurotrophic factor in healthy
humans: A review. _Scand J. Med. Sci. Sports_ 24, 1–10. https://doi.org/10.1111/sms.12069 (2014). Article PubMed Google Scholar * Dinoff, A. _et al._ The effect of exercise training on
resting concentrations of peripheral brain-derived neurotrophic factor (BDNF): A meta-analysis. _PLoS ONE_ 11, e0163037–e0163037. https://doi.org/10.1371/journal.pone.0163037 (2016). Article
CAS PubMed PubMed Central Google Scholar * Rasmussen, P. _et al._ Evidence for a release of brain-derived neurotrophic factor from the brain during exercise. _Exp. Physiol._ 94,
1062–1069. https://doi.org/10.1113/expphysiol.2009.048512 (2009). Article CAS PubMed Google Scholar * Harvey, J. A., Chastin, S. F. & Skelton, D. A. Prevalence of sedentary behavior
in older adults: A systematic review. _Int. J. Environ. Res. Public Health_ 10, 6645–6661. https://doi.org/10.3390/ijerph10126645 (2013). Article PubMed PubMed Central Google Scholar *
Hajduk, A. M. & Chaudhry, S. I. Sedentary behavior and cardiovascular risk in older adults: A scoping review. _Curr. Cardiovasc. Risk. Rep._ https://doi.org/10.1007/s12170-016-0485-6
(2016). Article PubMed PubMed Central Google Scholar * Bakker, E. A. _et al._ Sedentary behaviour in cardiovascular disease patients: Risk group identification and the impact of cardiac
rehabilitation. _Int. J. Cardiol._ 326, 194–201. https://doi.org/10.1016/j.ijcard.2020.11.014 (2021). Article PubMed Google Scholar * Barker, J. _et al._ Physical activity of UK adults
with chronic disease: cross-sectional analysis of accelerometer-measured physical activity in 96 706 UK Biobank participants. _Int. J. Epidemiol._ 48, 1167–1174.
https://doi.org/10.1093/ije/dyy294 (2019). Article PubMed PubMed Central Google Scholar * Bellettiere, J. _et al._ Sedentary behavior and cardiovascular disease in older women: The
objective physical activity and cardiovascular health (OPACH) Study. _Circulation_ 139, 1036–1046. https://doi.org/10.1161/circulationaha.118.035312 (2019). Article CAS PubMed PubMed
Central Google Scholar * Jeong, S. W. _et al._ Mortality reduction with physical activity in patients with and without cardiovascular disease. _Eur. Heart J._ 40, 3547–3555.
https://doi.org/10.1093/eurheartj/ehz564 (2019). Article CAS PubMed PubMed Central Google Scholar * Moholdt, T., Wisløff, U., Nilsen, T. I. & Slørdahl, S. A. Physical activity and
mortality in men and women with coronary heart disease: A prospective population-based cohort study in Norway (the HUNT study). _Eur. J. Cardiovasc. Prev. Rehabil._ 15, 639–645.
https://doi.org/10.1097/HJR.0b013e3283101671 (2008). Article PubMed Google Scholar * Boerema, S. T., van Velsen, L., Vollenbroek, M. M. & Hermens, H. J. Pattern measures of sedentary
behaviour in adults: A literature review. _Digit. Health_ 6, 2055207620905418. https://doi.org/10.1177/2055207620905418 (2020). Article PubMed PubMed Central Google Scholar * Kim, Y.,
Welk, G. J., Braun, S. I. & Kang, M. Extracting objective estimates of sedentary behavior from accelerometer data: Measurement considerations for surveillance and research applications.
_PLoS ONE_ 10, e0118078. https://doi.org/10.1371/journal.pone.0118078 (2015). Article CAS PubMed PubMed Central Google Scholar * Carter, S., Hartman, Y., Holder, S., Thijssen, D. H.
& Hopkins, N. D. Sedentary behavior and cardiovascular disease risk: Mediating mechanisms. _Exerc. Sport Sci. Rev._ 45, 80–86. https://doi.org/10.1249/jes.0000000000000106 (2017).
Article PubMed Google Scholar * Amadio, P. _et al._ Patho- physiological role of BDNF in fibrin clotting. _Sci. Rep._ 9, 389. https://doi.org/10.1038/s41598-018-37117-1 (2019). Article
ADS CAS PubMed PubMed Central Google Scholar * Jin, H. _et al._ Association between brain-derived neurotrophic factor and von Willebrand factor levels in patients with stable coronary
artery disease. _BMC Cardiovasc. Disord._ 18, 23. https://doi.org/10.1186/s12872-018-0762-z (2018). Article CAS PubMed PubMed Central Google Scholar * Pius-Sadowska, E. &
Machaliński, B. BDNF—A key player in cardiovascular system. _J. Mol. Cell Cardiol._ 110, 54–60. https://doi.org/10.1016/j.yjmcc.2017.07.007 (2017). Article CAS PubMed Google Scholar *
Kaess, B. M. _et al._ Circulating brain-derived neurotrophic factor concentrations and the risk of cardiovascular disease in the community. _J. Am. Heart Assoc._ 4, e001544.
https://doi.org/10.1161/jaha.114.001544 (2015). Article PubMed PubMed Central Google Scholar * Rahman, F. _et al._ Serum brain-derived neurotrophic factor and risk of atrial
fibrillation. _Am. Heart J._ 183, 69–73. https://doi.org/10.1016/j.ahj.2016.07.027 (2017). Article CAS PubMed Google Scholar * Jiang, H., Liu, Y., Zhang, Y. & Chen, Z. Y. Association
of plasma brain-derived neurotrophic factor and cardiovascular risk factors and prognosis in angina pectoris. _Biochem. Biophys. Res. Commun._ 415, 99–103.
https://doi.org/10.1016/j.bbrc.2011.10.020 (2011). Article CAS PubMed Google Scholar * Takashio, S. _et al._ Significance of low plasma levels of brain-derived neurotrophic factor in
patients with heart failure. _Am. J. Cardiol._ 116, 243–249. https://doi.org/10.1016/j.amjcard.2015.04.018 (2015). Article CAS PubMed Google Scholar * Arvidsson, D. _et al._ A
longitudinal analysis of the relationships of physical activity and body fat with nerve growth factor and brain-derived neural factor in children. _J. Phys. Act. Health_ 15, 620–625.
https://doi.org/10.1123/jpah.2017-0483 (2018). Article PubMed Google Scholar * Beltran-Valls, M. R., Adelantado-Renau, M. & Moliner-Urdiales, D. Association between objectively
measured physical activity and plasma BDNF in adolescents: DADOS study. _J. Mol. Neurosci._ 65, 467–471. https://doi.org/10.1007/s12031-018-1122-2 (2018). Article CAS PubMed Google
Scholar * Engeroff, T. _et al._ Is objectively assessed sedentary behavior, physical activity and cardiorespiratory fitness linked to brain plasticity outcomes in old age?. _Neuroscience_
388, 384–392. https://doi.org/10.1016/j.neuroscience.2018.07.050 (2018). Article CAS PubMed Google Scholar * Huang, T. _et al._ Cross-sectional associations of objectively measured
physical activity with brain-derived neurotrophic factor in adolescents. _Physiol. Behav._ 171, 87–91. https://doi.org/10.1016/j.physbeh.2016.12.026 (2017). Article CAS PubMed Google
Scholar * Júdice, P. B., Magalhães, J. P., Hetherington-Rauth, M., Correia, I. R. & Sardinha, L. B. Sedentary patterns are associated with BDNF in patients with type 2 diabetes
mellitus. _Eur. J. Appl. Physiol._ 121, 871–879. https://doi.org/10.1007/s00421-020-04568-2 (2021). Article CAS PubMed Google Scholar * Mora-Gonzalez, J. _et al._ Sedentarism, physical
activity, steps, and neurotrophic factors in obese children. _Med. Sci. Sports Exerc._ 51, 2325–2333. https://doi.org/10.1249/mss.0000000000002064 (2019). Article CAS PubMed Google
Scholar * Wurm, S., Diehl, M., Kornadt, A. E., Westerhof, G. J. & Wahl, H. W. How do views on aging affect health outcomes in adulthood and late life? Explanations for an established
connection. _Dev. Rev._ 46, 27–43. https://doi.org/10.1016/j.dr.2017.08.002 (2017). Article PubMed PubMed Central Google Scholar * Migueles, J. H. _et al._ Accelerometer data collection
and processing criteria to assess physical activity and other outcomes: A systematic review and practical considerations. _Sports Med._ 47, 1821–1845.
https://doi.org/10.1007/s40279-017-0716-0 (2017). Article PubMed PubMed Central Google Scholar * Freedson, P. S., Melanson, E. & Sirard, J. Calibration of the computer science and
applications Inc. accelerometer. _Med. Sci. Sports Exerc._ 30, 777–781. https://doi.org/10.1097/00005768-199805000-00021 (1998). Article CAS PubMed Google Scholar * Baumann, S. _et al._
Pitfalls in accelerometer-based measurement of physical activity: The presence of reactivity in an adult population. _Scand J. Med. Sci. Sports_ 28, 1056–1063.
https://doi.org/10.1111/sms.12977 (2018). Article CAS PubMed Google Scholar * Lommatzsch, M. _et al._ The impact of age, weight and gender on BDNF levels in human platelets and plasma.
_Neurobiol. Aging_ 26, 115–123. https://doi.org/10.1016/j.neurobiolaging.2004.03.002 (2005). Article CAS PubMed Google Scholar * Herrmann, S. D., Barreira, T. V., Kang, M. &
Ainsworth, B. E. Impact of accelerometer wear time on physical activity data: A NHANES semisimulation data approach. _Br. J. Sports Med._ 48, 278–282.
https://doi.org/10.1136/bjsports-2012-091410 (2014). Article PubMed Google Scholar * Bellettiere, J. _et al._ Sedentary behavior and prevalent diabetes in 6166 older women: The objective
physical activity and cardiovascular health study. _J. Gerontol. A Biol. Sci. Med. Sci._ 74, 387–395. https://doi.org/10.1093/gerona/gly101 (2019). Article PubMed Google Scholar * Walsh,
J. J. _et al._ Neurotrophic growth factor responses to lower body resistance training in older adults. _Appl. Physiol. Nutr. Metab._ 41, 315–323. https://doi.org/10.1139/apnm-2015-0410
(2016). Article CAS PubMed Google Scholar * Walsh, J. J. & Tschakovsky, M. E. Exercise and circulating BDNF: Mechanisms of release and implications for the design of exercise
interventions. _Appl. Physiol. Nutr. Metab._ 43, 1095–1104. https://doi.org/10.1139/apnm-2018-0192 (2018). Article PubMed Google Scholar * Naegelin, Y. _et al._ Measuring and validating
the levels of brain-derived neurotrophic factor in human serum. _eNeuro_ https://doi.org/10.1523/eneuro.0419-17.2018 (2018). Article PubMed PubMed Central Google Scholar * Serra-Millàs,
M. Are the changes in the peripheral brain-derived neurotrophic factor levels due to platelet activation?. _World J. Psychiatry_ 6, 84–101. https://doi.org/10.5498/wjp.v6.i1.84 (2016).
Article PubMed PubMed Central Google Scholar * Migueles, J. H. _et al._ GRANADA consensus on analytical approaches to assess associations with accelerometer-determined physical
behaviours (physical activity, sedentary behaviour and sleep) in epidemiological studies. _Br. J. Sports Med._ https://doi.org/10.1136/bjsports-2020-103604 (2021). Article PubMed Google
Scholar * Heesch, K. C., Hill, R. L., Aguilar-Farias, N., van Uffelen, J. G. Z. & Pavey, T. Validity of objective methods for measuring sedentary behaviour in older adults: A systematic
review. _Int. J. Behav. Nutr. Phys. Act._ 15, 119. https://doi.org/10.1186/s12966-018-0749-2 (2018). Article PubMed PubMed Central Google Scholar * Vetrovsky, T. _et al._ Advances in
accelerometry for cardiovascular patients: A systematic review with practical recommendations. _ESC Heart Fail_ 7, 2021–2031. https://doi.org/10.1002/ehf2.12781 (2020). Article PubMed
PubMed Central Google Scholar * Marzolini, S., Oh, P. I. & Brooks, D. Effect of combined aerobic and resistance training versus aerobic training alone in individuals with coronary
artery disease: A meta-analysis. _Eur. J. Prev. Cardiol._ 19, 81–94. https://doi.org/10.1177/1741826710393197 (2012). Article PubMed Google Scholar * Yang, X. _et al._ Muscle-generated
BDNF is a sexually dimorphic myokine that controls metabolic flexibility. _Sci. Signal_ https://doi.org/10.1126/scisignal.aau1468 (2019). Article PubMed PubMed Central Google Scholar *
Schmalhofer, M.-L. _et al._ Sex-specific associations of brain-derived neurotrophic factor and cardiorespiratory fitness in the general population. _Biomolecules_ 9, 630.
https://doi.org/10.3390/biom9100630 (2019). Article CAS PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS We thank Elke Bandelin for her support in recruitment and data
collection. Further, we thank Sophia Lein and Silke Liermann for the organization and implementation of the comprehensive intervention program. We thank the nurses at the cardiovascular
examination center of the DZHK partner site Greifswald for collecting the socio-demographic and anthropometric data. Finally, we acknowledge the support of all study participants. FUNDING
Open Access funding enabled and organized by Projekt DEAL. This study was supported and funded by grants from the Federal Ministry of Education and Research as part of the German Centre of
Cardiovascular Research (DZHK; Grand No. D347000002). The DZHK had no direct role in the development of methodology, the acquisition, analysis, and interpretation of data or in writing the
manuscript. In addition, the study was supported and funded by grants from the Research Network Community Medicine of the University Medicine Greifswald. AUTHOR INFORMATION AUTHORS AND
AFFILIATIONS * Department of Prevention Research and Social Medicine, Institute for Community Medicine, University Medicine Greifswald, Walther-Rathenau-Str. 48, 17475, Greifswald, Germany
Antje Ullrich, Lisa Voigt, Susanne Wurm & Sabina Ulbricht * German Centre for Cardiovascular Research (DZHK), Partner Site Greifswald, Greifswald, Germany Antje Ullrich, Kristin Wenzel,
Martin Bahls, Lisa Voigt, Stephanie Könemann, Marcus Dörr & Sabina Ulbricht * Department of Internal Medicine B, University Medicine Greifswald, Greifswald, Germany Kristin Wenzel,
Martin Bahls, Stephanie Könemann & Marcus Dörr Authors * Antje Ullrich View author publications You can also search for this author inPubMed Google Scholar * Kristin Wenzel View author
publications You can also search for this author inPubMed Google Scholar * Martin Bahls View author publications You can also search for this author inPubMed Google Scholar * Lisa Voigt View
author publications You can also search for this author inPubMed Google Scholar * Stephanie Könemann View author publications You can also search for this author inPubMed Google Scholar *
Marcus Dörr View author publications You can also search for this author inPubMed Google Scholar * Susanne Wurm View author publications You can also search for this author inPubMed Google
Scholar * Sabina Ulbricht View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS M.D., S.W. and S.U. planned und designed the study. M.D. and S.U.
also managed participants’ recruitment and data collection. A.U., K.W., M.B., and S.U. analyzed and interpreted the data. A.U. drafted the manuscript. S.U. supervised the writing and M.B.,
K.W., S.K., L.V., M.D., and S.W. provided additional input. All authors read, critically revised, and approved the final version of the manuscript. CORRESPONDING AUTHOR Correspondence to
Antje Ullrich. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE Springer Nature remains neutral with regard to
jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed
under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate
credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article
are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons
licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of
this licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Ullrich, A., Wenzel, K., Bahls, M. _et al._ Preliminary results
of the cross-sectional associations of sedentary behavior and physical activity with serum brain-derived neurotrophic factor in adults with coronary heart disease. _Sci Rep_ 12, 19685
(2022). https://doi.org/10.1038/s41598-022-23706-8 Download citation * Received: 16 August 2022 * Accepted: 03 November 2022 * Published: 16 November 2022 * DOI:
https://doi.org/10.1038/s41598-022-23706-8 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative