Subtypes and phylogenetic analysis of blastocystis sp. Isolates from west ismailia, egypt
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT In Egypt, _Blastocystis_ sp. is not yet on the diagnostic list of parasitology reports, and information about its subtypes (STs) is scarce. This study investigated its prevalence
and its STs/alleles, performed phylogenetic analysis, and considered the distribution of risk factors associated with _Blastocystis_ sp. infections in West Ismailia, Ismailia governorate.
Sociodemographic data, exposure factors, and previous parasitic infection status were recorded for symptomatic and asymptomatic individuals. Microscopy, polymerase chain reaction,
sequencing, and phylogenetic analysis for _Blastocystis_ sp. isolated from fecal samples were performed. Eighty _Blastocystis_ sp.-infected individuals (15.3%) were examined. The age of the
individuals ranged between 0.60 and 85.0 (mean 17.10 ± 15.70), the male/female ratio was 33/47, and the asymptomatic/symptomatic ratio was 55/25. The findings demonstrate clear evidence of
direct contact with animals, poor water quality, and previous parasitic infections. Eleven samples yielded three _Blastocystis_ STs (ST1: allele 4, ST2: alleles 9 and 12, and ST3: allele
34), with ST3 (45.5%) representing the most common subtype. Phylogenetic analysis with a robust bootstrap revealed three distinct clades for isolates of each subtype. This study updates the
epidemiological knowledge of the distribution of _Blastocystis_ sp. STs in Egypt and expands the current understanding of the prevalence, risk factor frequencies, and genetic diversity of
this protist in the studied area. SIMILAR CONTENT BEING VIEWED BY OTHERS DETECTION AND MOLECULAR CHARACTERIZATION OF _BLASTOCYSTIS_ SP., _ENTEROCYTOZOON BIENEUSI_ AND _GIARDIA DUODENALIS_ IN
ASYMPTOMATIC ANIMALS IN SOUTHEASTERN IRAN Article Open access 20 February 2025 PREVALENCE AND SUBTYPING OF _BLASTOCYSTIS_ SP. IN RUMINANTS IN SOUTHWESTERN, IRAN Article Open access 31
August 2024 PREVALENCE AND GENETIC CHARACTERISTICS OF _BLASTOCYSTIS HOMINIS_ AND _CYSTOISOSPORA BELLI_ IN HIV/AIDS PATIENTS IN GUANGXI ZHUANG AUTONOMOUS REGION, CHINA Article Open access 05
August 2021 INTRODUCTION _Blastocystis_ sp. is a unicellular eukaryotic protist in the stramenopile family that lives in the guts of humans and animals. _Blastocystis_ sp. has become a much
more common issue for public health than previously thought because it is widely distributed with a high incidence and a high degree of genetic diversity1,2,3. There are about 28 recognized
_Blastocystis_ sp. lineages. These subtypes (STs) are defined based on the genetic diversity of their small-subunit ribosomal RNA (SSU) genes. By 2013, 17 different STs (ST1 to ST17) had
been identified. Although 11 further STs (ST18 to ST28) have been proposed, the validity of four of these (ST18, ST19, ST20, and ST22) remains contested3,4. The first nine subtypes and ST12
were isolated from the gastrointestinal tract of humans, with single instances of ST10, ST13, ST14, and ST16 also having been observed3,5,6,7,8,9,10. ST3, ST1, ST2, and ST4 are the most
prevalent subtypes in humans. Rodents, birds, pigs, and other primates have all been colonized by different _Blastocystis_ sp. subtypes3,5,6,7,8. _Blastocystis_ sp. is considered a parasite,
and scientific consensus classifies it as a commensal and potentially even beneficial resident of the gut11,12. The hypothesis that certain strains within subtypes are pathogenic is under
investigation10,13. Previous studies have speculated that _Blastocystis_ sp. interacts with the host’s gut microbiota14,15. However, detailed insights remain lacking. Although _Blastocystis_
sp. infections are generally asymptomatic in humans, common symptoms include nausea, anorexia, stomach discomfort, flatulence, and acute or chronic diarrhea. Such clinical manifestations
have been suggested to result from the proteases and gut-microbiome dysbiosis caused by _Blastocystis_ sp. colonization16. _Blastocystis_ sp. transmission is not clearly defined, but a human
transmission cycle has been proposed17,18,19. Nonetheless, studies of family units in developed and developing countries have indicated that this pathway remains to be conclusively
demonstrated20,21. Additional sources of infections appear to be contaminated water22,23,24,25, close contact with animals1, and contaminated soil20. A lack of sanitation and clean water
means that most Egyptian governorates are probably at high risk of _Blastocystis_ infections, with several studies of the country linking _Blastocystis_ sp. to urticaria, irritable bowel
syndrome, asthma, and iron deficiency anemia, with infections diagnosed in both healthy (asymptomatic) and symptomatic individuals26,27,28. Still, _Blastocystis_ sp. is not listed as a
pathogen in Egypt’s parasitological reports. According to a study on the diagnosis of gastrointestinal parasites by primary health care technicians in El-Kassassin, West Ismailia,
_Blastocystis_ sp. and _Giardia duodenalis_ are completely missing from parasitological diagnosis results29. Studies are lacking in Egypt concerning the epidemiology, molecular genetic
diversity, and prevalence of _Blastocystis_ sp. in carriers and non-carriers. Given the risk factors in Egypt and the current discussion of _Blastocystis_ pathogenicity, this parasite should
be included in the Egyptian “medical diagnostic radar.” Egypt has 27 governorates, one of which is Ismailia. The West Ismailia governorate has municipal divisions with rural areas, where
domestic farm animals and birds share homes with residents. Inadequate hygiene is predicted, given the low socioeconomic status of the area. However, few molecular studies have been
conducted to ascertain the incidence and subtype distribution of _Blastocystis_ sp. in the governorate. Therefore, the present study aims to focus more closely on the prevalence, risk factor
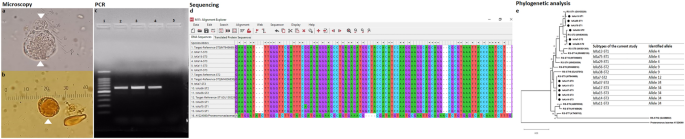
distribution, subtypes, and phylogenetics of _Blastocystis_ sp. in a rural Egyptian community. RESULTS Microscopy, polymerase chain reaction (PCR), sequencing, and phylogenetic analysis
were performed for _Blastocystis_ sp. from fecal samples (Fig. 1). Descriptive data concerning patient sociodemographics and the distribution of risk factors is illustrated for the 80
_Blastocystis-_positive samples (Tables 1 and 2). Microscopic and molecular examinations of _Blastocystis-_positive samples are shown in detail (Table 3, Fig. 2, and Table 4). Of the 15
samples sent for sequencing, 11 were successfully sequenced with high-quality yield (see "Methods" section for details). The genetic diversity and genotype distribution of
_Blastocystis_ sp. isolates are detailed in Table 5. Information regarding _Blastocystis_ sp. STs in the current investigation, compared against studies of different Egyptian governorates,
appears in Table 6. The current study’s sequence isolates were submitted to phylogenetic analysis (Fig. 3). _Blastocystis_ sp. was identified in a total of 80/520 (15.3%) fecal samples,
according to direct light microscopy of smears (saline and iodine examination). The 80 _Blastocystis_-positive subjects included those with no available data (NAD = 47) and those with
complete historical data available (SAD = 33). For NAD samples, the only available data were age, sex, and symptomatology status, with no information on exposure factors or previous
parasitic infections (PPIs). Both SAD and NAD subjects were classified as “symptomatic” (gastrointestinal symptoms) or “asymptomatic” (no gastrointestinal symptoms), based on responses to a
standardized questionnaire (Table 1). The 80 _Blastocystis_-positive individuals ranged in age from 0.6 to 85 years. Most patients (55/80) had no gastrointestinal symptoms; those who did
mostly complained of abdominal pain and diarrhea. Other non-specific symptoms such as vomiting, dehydration, and fever were also described (Table 1). Those aged between 5 and 18 (52/80) were
most affected by _Blastocystis_ sp. infection (Table 1). Approximately 60% (31) of this group were asymptomatic; the remainder were symptomatic. Females in this group represented 55.8%
(29), and males represented 44.2% (23). Most of the SAD subjects reported owning domestic animals (poultry, livestock, and pets). About 76.6% reported direct contact with their animals and
that the floors of their animal farms were covered with sand. The water supplies at the studied subjects’ residences appeared mostly clear. However, some participants mentioned that the
water sometimes became yellow and turbid with a smell (Table 1). Almost all SAD individuals––31/33 (93.9%)––had recorded previous parasitic infections (PPI), including _Entamoeba_ sp.,
_Schistosoma_ sp., and _Hymenolepis nana_, in the previous year. Only 14 patients had received the anti-parasitic treatment they needed, with schistosomiasis patients, in particular, mostly
not taking their medications or following their prescribed treatment regimens (Table 2). According to microscopic wet-mount examinations of their fecal samples, 41.3% of participants
recorded mixed parasitic infections (pathogenic and non-pathogenic). Polyparasitism included infections that were doubled, tripled, or even quadrupled. Other protozoan and helminthic
parasites were found in _Blastocystis_-infected subjects, including _Entamoeba coli_, _Entamoeba histolytica_/_dispar_/_moshkovskii_, _Chilomastix mesnili_, _Giardia duodenalis_, and
_Hymenolepis nana_ (Table 3, Fig. 2). Only 11 samples were successfully amplified, producing high-quality sequences that identified three STs (ST1, ST2, and ST3). _Blastocystis_ ST3 was the
most common ST in the West Ismailia population (45.5%), followed by ST2 and ST1 (27.3% each) (Table 4). Tests performed using BLAST software revealed that each of the SSU rRNA gene sequences
obtained in this study demonstrated a high degree of homology with previously reported sequences from other _Blastocystis_ sp. isolates. Although ST1 and ST2 sequences showed some
single-nucleotide polymorphism mutations against the reference strains, ST3 demonstrated 100% homology with the reference strain. Furthermore, 10/11 sequenced isolates belonged to
asymptomatic subjects, with only one isolate (ST1) belonging to a symptomatic subject (Table 5). The alignment of _Blastocystis_ sequences showed three to five nucleotide differences. Three
samples––IsKa 7 (ST2), IsKa 38 (ST2), and IsKa 75 (ST1)––demonstrated three nucleotide differences. Despite these differences, the BLAST search revealed that the first two of these isolates
belong to the same _Blastocystis_ ST. One sample, IsKa 29 (ST1), showed four nucleotide differences, while two samples, IsKa 13 (ST1) and IsKa 56 (ST2), showed five nucleotide differences.
All of the sequence data for _Blastocystis_ nucleotides obtained from the current study were deposited in GenBank with accession numbers OL845600–OL845610 as follows: ST3 represented by five
samples (OL845600–OL845604), ST2 represented by three samples (OL845605–OL845607), and ST1 represented by three samples (OL845608–OL845610) (Table 5). In terms of _Blastocystis_ alleles,
ST3 and ST1 produced completely homogeneous isolates with alleles 34 and allele 4, respectively. Meanwhile, ST2 exhibited low genetic variation in one isolate (IsKa 7), which corresponded to
allele 12, with its remaining two isolates corresponding to allele 9. A phylogenetic tree was constructed using eleven nucleotide sequences representative of the current study and GenBank
database reference sequences, with _Proteromonas lacertae_ (U37108) used as the outgroup. Three subtypes were distinguishable: ST1, ST2, and ST3 (Fig. 1). DISCUSSION This study’s findings
revealed _Blastocystis_ sp. to be prevalent in 15.3% of the West Ismailia population, which is consistent with the observations of El-Badry et al. (2018). Other studies have revealed
variations in _Blastocystis_ prevalence27,30,31. PCR in Cairo revealed a prevalence of 35.5% for _Blastocystis_ in both patient and control groups30. Patients with irritable bowel syndrome
in Beni-Suef were found to have a prevalence of _Blastocystis_ of 16.5% by microscopy and 19.1% by culture27_._ In the same governorate, 53.6% of patients with acute diarrhoea had
_Blastocystis_31. Such disparities can be attributed to various epidemiological parameters, including the target population, detection method, and the presence or absence of symptoms. In
this study, _Blastocystis_ colonization was more common among children aged 6 to 18 than among children under five years old and adults, which aligns with two previous Egyptian studies
observing schoolchildren to be the most affected32,33. Infection with _Blastocystis_ sp. has also been demonstrated by global surveys to be prevalent among schoolchildren18,34,35,36, likely
owing to this age group’s lax hygiene standards. _Blastocystis_ infection age disparities may be influenced by associated exposure risk factors, children’s immunity, and environmental
variables34,35,36_._ This study classifies _Blastocystis_ infections as silent, with most infected subjects not exhibiting symptoms. Three factors may influence the asymptomatic status of
_Blastocystis_ infections among Egyptians: (a) awareness, which is limited in the rural Egyptian population, of the need to seek medical attention for diarrhea; (b) the diagnostic ability of
Egyptian laboratories to identify _Blastocystis_ sp. in primary care settings (i.e., primary health care units), leaving _Blastocystis_ outside their diagnostic scope29; and (c) in the case
of _Blastocystis_ infection, the concept that it is a commensal protist, which encourages Egyptian physicians to disregard treating patients despite the presence of distressing symptoms29.
These factors may enable _Blastocystis_ sp. to colonize the host for an extended period without causing disease. Most Egyptians, and particularly those living in rural areas, keep domestic
animals and birds in and around their homes. This is especially evident in West Ismailia, where domestic animals, especially poultry, coexist with humans in traditional Egyptian residences
without separate yards, exposing owners to high concentrations of _Blastocystis_ infective stages for extended periods. This has led to the prediction that the risk of _Blastocystis_
infection is tenfold greater in rural areas than in urban areas among the Egyptian population with irritable bowel syndrome27. Elsewhere, strong molecular evidence has confirmed zoonotic
transmission between animals and their human caregivers37,38. Notably, humans in direct contact with animals were found to have the same _Blastocystis_ STs in two studies conducted in
Northern and Eastern Egypt (ST1, ST2, ST3, and ST4)37,39. Additionally, poultry can harbor the human-transmissible _Blastocystis_ ST6 and ST740, although this has only been reported in a
recent Egyptian study of colorectal cancer patients41. The Sweet Water Canal serves as the primary water source for municipal divisions in West Ismailia and is used directly for animal
bathing, dishwashing, and laundry. Consequently, water used for residential or recreational purposes becomes contaminated, creating a risk of transmission of gastrointestinal diseases to
humans and animals alike. Rural communities in West Ismailia have been particularly hard-hit, with some settlements in remote locations lacking access to safe drinking water, which increases
the possibility of contamination during transportation and processing. Numerous protozoan contaminants, including _Blastocystis_ sp., have been detected in Egypt’s Dakahlia, Ismailia, and
El-Minia governorates in potable water, water tanks, pumps, waterworks, and surface water (i.e., River Nile, ponds, and canals)24,42,43. Most SAD residents were observed to utilize tap water
without a filter, with the water supplied appearing clear. However, because it is only used to remove rust, insects, and dust, the presence or absence of a filter would not substantially
impact the purification of water from protozoa stages. Thus, even if the water appears clear, it may contain _Blastocystis_ sp. Furthermore, in recent years, soil pollution has been
identified as a source of _Blastocystis_ sp. infection20; as a major farming region, soil is extremely likely to be a source of infection for the West Ismailia population. PPI are prevalent
in rural Ismailia (author’s observation, unpublished data); when questioned, this study’s participants were fully aware of this. Over half of participants failed to take their parasitic
infection medications as prescribed and did not complete the entire course of treatment (dose and duration). Consequently, infected individuals have acted as carriers facilitating
anthroponotic protist transmission, while also experiencing infection maintenance, chronicity, and consequences. This study’s investigations also revealed polyparasitism in the West Ismailia
population. Although most samples represented single _Blastocystosis_ infections, the presence of pathogenic (_G. duodenalis_, _E. histolytica_, _H. nana_) and non-pathogenic (_E. coli_,
_C. mesnilli_, non-pathogenic species of _E. histolytica_) parasites mixed with _Blastocystis_ sp. infection suggests multiple sources of infection. Mixed parasitic infections are highly
predicted in rural areas due to the presence of multiple risk factors, as has been previously documented44,45,46,47. Consumption of contaminated water and unwashed vegetables, lack of
fingernail trimming and hand washing, children playing in the dirt, barefoot walking, low socioeconomic status, lack of sanitation, and large numbers of family members sharing a single room
have all been observed among the residents of West Ismailia27,31,48,49. Furthermore, rats, cockroaches, fleas, ants, and flies were observed to spread in numerous locations, especially
during hot weather, alongside sewage rash. Such behavioral, social, and sanitary factors are almost certainly implicated in developing mixed parasitic infections and perpetuating the life
cycle of those infections. Thus, nearly all the transmission routes required for _Blastocystis_ sp. appear open. Three subtypes of _Blastocystis_ sp. were characterized in the current
investigation via the molecular analysis of isolates, namely, ST3, ST2, and ST1, with the latter two recording equal distribution. This study’s findings corroborate those of Souppart et al.
(2010), who discovered that ST3 had the highest prevalence (61.9%) and that ST1 and ST2 had equal prevalence (19.05%). Several Egyptian studies have subtyped and sequenced _Blastocystis_
sp.39,41,50, with five STs (ST1, ST2, ST3, ST4 and ST7) identified at varying frequencies in distinct Egyptian groups using PCR sequenced-tagged sites, PCR restriction fragment length
polymorphisms, and PCR sequencing (barcoding) (Table 6). The high prevalence of _Blastocystis_ sp. ST1–ST4 in the Egyptian community suggests that most infections are transmitted from person
to person. The current investigation has revealed that ST3 is responsible for the vast majority of _Blastocystis_ infections in West Ismailia, aligning with observations for other Egyptian
governorates across 12 other studies (Table 6), which have revealed ST3 to be the most prevalent _Blastocystis_ subtype in six distinct Egyptian locations; furthermore, it is the ST most
closely related to various gastrointestinal symptoms (Table 6). Other subtypes (ST1, ST2, ST4 and ST7) have been detected in the Egyptian community, with varying frequencies depending on the
sample size and testing technique used. ST1 and ST2 have been identified as relevant STs in a smaller number of Egyptian studies28,39. Notably, STs and their relative frequencies appear to
vary significantly within a single country (Table 6). Almost all the isolates sequenced in this investigation were asymptomatic, except for one patient who suffered diarrhea and abdominal
pain and was subtyped as ST1, an observation consistent with the findings of other studies10,55,56,57. _Blastocystis_ is more frequent in healthy individuals, with its existence also linked
to altered composition and increased richness of the bacterial gut microbiota14,15. It is unclear whether _Blastocystis_ directly promotes a healthy gut and microbiome or whether it prefers
to colonize and persist in a healthy gut environment. A study of _Blastocystis_ sp. ST3 indicated that _Blastocystis_ sp. may modify the gut ecosystem in a protective manner and facilitate
faster recovery from disturbances12. The presence of _Blastocystis_ among healthy individuals has also been linked to reduced levels of fecal calprotectin, a sign of intestinal inflammation
according to a comparative investigation conducted in Mexico56. In contrast, some researchers have suggested that particular _Blastocystis_ sp. isolates may produce an imbalance of the gut
microbiota58,59,60,61,62,63. The context of the environment and hosts must be considered when discussing whether _Blastocystis_ is a pathogen or a mutualist. The present study’s genetic
analysis reveals that all 11 isolates detected in _Blastocystis_ subtypes cross-corresponded to previously observed alleles. Intriguingly, ST3 isolates produced the highest frequency of
isolates matching allele 34, the most common variant found in humans worldwide, with ST2 isolates exhibiting low levels of genetic diversity and multiple nucleotide substitutions
corresponding to two different alleles (9 and 12). Although ST1 isolates demonstrated limited genetic diversity, all isolates corresponded to allele 4. In Egyptian isolates from Cairo,
genetic diversity was detected in three subtypes in the same pattern, with ST1 and ST2 exhibiting nucleotide differences ranging from 1 to 11 and ST3 exhibiting reduced genetic variability
of up to four nucleotide differences50. However, no further allelic analysis was conducted. On the contrary, there was no evidence of genetic diversity in the _Blastocystis_ subtypes (ST3
and ST1) isolated from individuals with irritable bowel syndrome in the Beni-Suef governorate27. A phylogenetic tree demonstrated that the 11 nucleotide sequences in this study clustered
into the same subtype cluster, with high bootstrap support, and could be classified into three subtypes: ST1, ST2, and ST3. Each _Blastocystis_ sp. ST formed a distinct clade, implying that
the West Ismailian _Blastocystis_ population can be divided into three subgroups. Another Egyptian study found the same phylogenetic distribution pattern for ST1 and ST3 subtype clusters in
patients with irritable bowel syndrome27. Among the drawbacks of the present investigation is the infeasibility of sequencing every isolate. Because samples were collected via a large-scale
survey, there were no epidemiological data for some participants, which hindered presenting comprehensive information. _Blastocystis_ sp. infections are significantly under- and
mis-diagnosed in Egypt, particularly in rural and remote areas such as West Ismailia. Additional research can illuminate the epidemiological situation of _Blastocystis_ sp. in Egypt,
enabling more effective control efforts against _Blastocystis_ sp. infections and other parasitic disorders. CONCLUSIONS The current study updates the epidemiological situation and
distribution of _Blastocystis_ STs in Egypt. Phylogenetic analysis has revealed three distinct clades for isolates pertaining to each subtype, adding to our current understanding of
_Blastocystis_’s prevalence and genetic diversity. The widespread presence of ST3 in the West Ismailia population and throughout Egypt necessitates subtyping analysis, which has become
indispensable for elucidating the relationship between _Blastocystis_ subtypes and pathogenicity in the Egyptian population. We highlight the need to invest in parasite education programs,
specific to _Blastocystis_ sp., that involve the general public along with doctors and laboratory technicians. Moreover, further studies are needed in the underrepresented areas of Egypt to
verify the distribution of _Blastocystis_ sp. throughout the country. METHODS STUDY AREA AND SAMPLE COLLECTION The Ministry of Health and Population in West Ismailia’s municipal divisions
(villages around the Sweet Water Canal’s geographical line) conducted a screening survey of 598 fecal samples for schistosomiasis eradication campaigns. Samples were collected randomly,
without regard for age or gender, from the nearest sampling area. Thus, this descriptive study used data from screens for gastrointestinal parasites of individuals in the municipal divisions
of West Ismailia (El-Kassassin, El-Mahsama, El-Talelkbeer, and Abu-Suwayr). A total of 80 fecal samples positive for _Blastocystis_ sp. were analyzed. The flowchart in Fig. 4 describes the
current study’s process. Five hundred and twenty samples were selected after considering their suitability based on inclusion and exclusion criteria. The 520 fecal samples and their
questionnaire forms were sent to Suez Canal University’s Parasitology Laboratory for ovum and parasite examination, using wet mount and iodine microscopy to screen for gastrointestinal
parasites. _Blastocystis_-positive samples were separated and selected for the current study analysis. The amount of the received fecal samples (a full tablespoon, i.e., 15–20 gr) was used
as a guideline for rolling in or rolling out the received fecal samples. To collect and transfer the fecal samples, participants were given a clean, labeled plastic container with an
applicator stick. Patients were given verbal explanations in Arabic regarding the collection and transfer processes and the amount of stool sample required. Stool samples were excluded if
contaminated with urine and water or if the amount was too small (less than a full tablespoon, i.e., 15–20 gr). Subsequently, the parasitology laboratory at El-Mahsama Family Practice Center
divided the stool samples into two parts: one part for the eradication program campaign (primary health care unit at the parasitology laboratory) and one part for processing at the Suez
Canal University’s Medical Department’s parasitology laboratory in Ismailia. MICROSCOPY OF FECAL SAMPLES A teaspoon-sized fecal sample was combined with 50 mL saline. In a 15 mL conical
plastic tube, the mixture was strained using gauze. One mL of the filtered fecal mixture was pipetted into Eppendorf tubes and frozen. For _Blastocystis_ form diagnosis, microscopic
examination was conducted using a direct smear. To view other parasitic stages under the microscope, the formalin-ethyl acetate sedimentation procedure was employed to concentrate the
strained mixture64. Wet-mount analysis was performed and Lugol’s iodine added to the slides for microscopic examination. EXTRACTION OF GENOMIC DNA Before extraction, the preserved 1 mL fecal
samples were washed and centrifuged several times in phosphate-buffered saline until the supernatant became clear. Then, the supernatant was discarded, and 200 µL of the 1 mL sediment was
exposed to the InhibitEX lysis buffer from the Qiagen DNA Stool Mini Kit (Qiagen, Germany, GmbH) according to the manufacturer’s protocol. Using 100 µL of elution buffer, the protocol was
slightly modified, and the extracted DNA was stored at -20 °C for further molecular investigation. PCR AMPLIFICATION AND SEQUENCING Financial restrictions meant that only 15 samples were
sent to the Istituto Superiore di Sanità in Rome, Italy, for molecular characterization. For amplification of the SSU rRNA region in _Blastocystis_ isolates, Blasto F
(5′-TCTGGTTGATCCTGCCAGT-3′) and Blasto R (5′-AGCTTTTTAACTGCAACAACG-3′) primers were used according to the protocol described by Meloni et al.65. A proofreading enzyme (GoTaq Promega) was
used to amplify the PCR. The Promega Wizard SV Gel and PCR Clean-Up System kit was used to purify the obtained 600 bp amplicon, and purified products were sent for MGW Sanger sequencing. The
sequences obtained were compared with other _Blastocystis_ SSU rRNA gene sequences available in the National Center for Biotechnology Information database using the BLAST tool
(http://www.ncbi.nlm.nih.gov). Multiple nucleotide sequence alignments were performed using the MUSCLE algorithm
(https://www.megasoftware.net/web_help_10/Part_II_Assembling_Data_For_Analysis/Building_Sequence_Alignments/MUSCLE/About_Muscle.htm) of the MEGA software (https://www.megasoftware.net/)3,66.
Additionally, _Blastocystis_ alleles were identified by determining their closest similarity to known _Blastocystis_ sequences using the _Blastocystis_ PubMLST database
(https://pubmlst.org/organisms/blastocystis-spp). PHYLOGENETIC ANALYSIS Molecular and Evolution Genetic Analysis software (https://www.megasoftware.net/) was used to produce a phylogenetic
tree for nucleotide sequences using the maximum likelihood method. One thousand bootstrap replicates were used to test the phylogenetic tree’s reliability and the statistical support for the
topology. Evolutionary distances were calculated using the Tamura-3 parameter model. STATISTICAL ANALYSIS A descriptive analysis was used to report sociodemographic characteristics and the
frequency and distribution of _Blastocystis_ subtypes among the affected subjects. After uploading all questionnaire data, the IBM SPSS software package version 26.0 was used to analyze the
data (IBM Corporation, Armonk, NY). Numbers and percentages were used to represent categorical data; mean and standard deviation were used to represent numerical data. The chi-squared test
was used to examine the relationship between a PPI and its treatment as two qualitative variables. The _p_-value was statistically significant at 0.05. ETHICAL CONSIDERATIONS Fecal sample
collection and medical history questionnaires were reviewed and approved by the Scientific Research Committee and Bioethics Board of Suez Canal University, Faculty of Medicine, Egypt
(approval no. 5089). All methods were performed in accordance with the relevant guidelines. Participants in the study were asked to sign a written informed consent form that clearly detailed
in Arabic the study’s objectives, sociodemographic questionnaire, symptomatology, exposure factors, and procedures. The collected data were kept private during and after the collection and
analysis. The study’s participants were informed that they could withdraw at any time. Patients whose fecal specimens were positive for gastrointestinal parasites were referred to physicians
for further treatment. DATA AVAILABILITY All data generated or analyzed during this study are included in this published article. REFERENCES * Ahmed, S. A. & Karanis, P. _Blastocystis_
spp., ubiquitous parasite of human, animals and environment. in _Reference Module in Earth Systems and Environmental Sciences_ 1–6 (Elsevier, 2019).
https://doi.org/10.1016/B978-0-12-409548-9.10947-9. * Scanlan, P. D. _et al._ The microbial eukaryote _Blastocystis_ is a prevalent and diverse member of the healthy human gut microbiota.
_FEMS Microbiol. Ecol._ 90, 326–330 (2014). Article CAS Google Scholar * Stensvold, C. & Clark, C. G. Pre-empting pandora’s box: _Blastocystis_ subtypes revisited. _Trends Parasitol._
36, 229–232 (2020). Article Google Scholar * Hublin, J. S. Y., Maloney, J. G. & Santin, M. _Blastocystis_ in domesticated and wild mammals and birds. _Res. Vet. Sci._ 135, 260–282
(2021). Article CAS Google Scholar * Maloney, J. G., da Cunha, M. J. R., Molokin, A., Cury, M. C. & Santin, M. Next-generation sequencing reveals wide genetic diversity of
_Blastocystis_ subtypes in chickens including potentially zoonotic subtypes. _Parasitol. Res._ 120, 2219–2231 (2021). Article Google Scholar * Maloney, J. G., Jang, Y., Molokin, A.,
George, N. S. & Santin, M. wide genetic diversity of _Blastocystis_ in white-tailed deer (Odocoileus virginianus) from Maryland, USA. _Microorganisms_ 9, (2021). * Maloney, J. G.,
Molokin, A., da Cunha, M. J. R., Cury, M. C. & Santin, M. _Blastocystis_ subtype distribution in domestic and captive wild bird species from Brazil using next generation amplicon
sequencing. _Parasite Epidemiol. Control_ 9, (2020). * Higuera, A. _et al._ Identification of multiple _Blastocystis_ subtypes in domestic animals from Colombia using amplicon-based next
generation sequencing. _Front. Vet. Sci._ 8, 932 (2021). Article Google Scholar * Ramírez, J. D. _et al._ Geographic distribution of human _Blastocystis_ subtypes in South America.
_Infect. Genet. Evol._ 41, 32–35 (2016). Article Google Scholar * Tito, R. Y. _et al._ Population-level analysis of _Blastocystis_ subtype prevalence and variation in the human gut
microbiota. _Gut_ 68, 1180–1189 (2019). Article CAS Google Scholar * Mülayim, S. _et al._ investigation of isolated _Blastocystis_ subtypes from cancer patients in Turkey. _Acta
Parasitol._ 66, 584–592 (2021). Article Google Scholar * Billy, V. _et al. Blastocystis_ colonization alters the gut microbiome and, in some cases, promotes faster recovery from induced
colitis. _Front. Microbiol._ 12, (2021). * Betts, E. L. _et al._ Metabolic fluctuations in the human stool obtained from _Blastocystis_ carriers and non-carriers. _Metabolites_ 11, 883
(2021). Article CAS Google Scholar * Andersen, L. O. brie. & Stensvold, C. R. _Blastocystis_ in health and disease: Are we moving from a clinical to a public health perspective? _J.
Clin. Microbiol._ 54, 528 (2016). * Stensvold, C. R. _et al._ Differentiation of _Blastocystis_ and parasitic archamoebids encountered in untreated wastewater samples by amplicon-based
next-generation sequencing. _Parasite Epidemiol. Control_ 9, e00131 (2020). Article Google Scholar * Mirjalali, H. _et al._ Distribution and phylogenetic analysis of _Blastocystis_ sp.
subtypes isolated from IBD patients and healthy individuals in Iran. _Eur. J. Clin. Microbiol. Infect. Dis._ 36, 2335–2342 (2017). * Paulos, S. _et al._ Occurrence and subtype distribution
of _Blastocystis_ sp. in humans, dogs and cats sharing household in northern Spain and assessment of zoonotic transmission risk. _Zoonoses Public Health_ 65, 993–1002 (2018). Article CAS
Google Scholar * Muadica, A. S. _et al._ Molecular diversity of _Giardia duodenalis_, _Cryptosporidium_ spp. and _Blastocystis _sp in asymptomatic school children in Leganés Madrid Spain.
_Microorganisms_ 8, 466 (2020). Article CAS Google Scholar * Khaled, S. _et al._ _Blastocystis_ sp. prevalence and subtypes distribution amongst Syrian refugee communities living in North
Lebanon. _Microorganisms_ 9, 1–13 (2021). Article Google Scholar * Jinatham, V., Maxamhud, S., Popluechai, S., Tsaousis, A. D. & Gentekaki, E. _Blastocystis_ one health approach in a
rural community of Northern Thailand: Prevalence, subtypes and novel transmission routes. _Front. Microbiol._ 12, 746340 (2021). Article Google Scholar * Lhotská, Z. _et al._ A study on
the prevalence and subtype diversity of the intestinal protist _Blastocystis_ sp in a gut-healthy human population in the Czech Republic. _Front. Cell. Infect. Microbiol._ 10, 544335 (2020).
Article Google Scholar * Taamasri, P. _et al._ Transmission of intestinal blastocystosis related to the quality of drinking water - PubMed. _Southeast Asian J Trop Med Public Heal._ 31,
112–117 (2000). CAS Google Scholar * Leelayoova, S. _et al._ Evidence of waterborne transmission of _Blastocystis hominis_–PubMed. _Am. J. Trop. Med. Hyg._ 70, 658–662 (2004). Article
Google Scholar * Elshazly, A., Elsheikha, H., Soltan, D., Mohammad, K. & Morsy, T. Protozoal pollution of surface water sources in Dakahlia Governorate, Egypt–PubMed. _J. Egypt. Soc.
Parasitol._ 37, 51–74 (2007). Google Scholar * Angelici, M. C., Nardis, C., Scarpelli, R. & Ade, P. _Blastocystis hominis_ transmission by non-potable water: A case report in
Italy–PubMed. _New Microbiol._ 41, 173–177 (2018). Google Scholar * Abdel-Hameed, D. M. A., Hassanin, O. M. & Zuel-Fakkar, N. M. Association of _Blastocystis_ _hominis_ genetic subtypes
with urticaria. _Parasitol. Res._ 108, 553–560 (2011). Article Google Scholar * El-Badry, A. A., Abd El Wahab, W. M., Hamdy, D. A. & Aboud, A. _Blastocystis_ subtypes isolated from
irritable bowel syndrome patients and co-infection with _Helicobacter pylori_. _Parasitol. Res._ 117, 127–137 (2018). Article Google Scholar * Deeb El, H. K. & Khodeer, S.
_Blastocystis_ spp: Frequency and subtype distribution in iron deficiency anemic versus non-anemic subjects from Egypt. _J. Parasitol._ 99, 599–602 (2013). Article Google Scholar * Ahmed,
S. A., Mohamed, S. F., Fouad, A. M. & Karanis, P. Gastrointestinal parasites diagnoses at the primary health care units: A comparative analysis of diagnostic abilities of parasitology
staff technicians versus medical parasitologists in Ismailia, Egypt. _R. Soc. Trop. Med. Hyg._ trac072 (2022). * Zuel-Fakkar, N. M., Abdel Hameed, D. M. & Hassanin, O. M. Study of
_Blastocystis_ _hominis_ isolates in urticaria: A case-control study. _Clin. Exp. Dermatol._ 36, 908–910 (2011). Article CAS Google Scholar * Hamdy, D. A., Abd El Wahab, W. M., Senosy, S.
A. & Mabrouk, A. G. _Blastocystis_ spp. and _Giardia intestinalis_ co-infection profile in children suffering from acute diarrhea. _J. Parasit. Dis._ 44, 88–98 (2020). Article Google
Scholar * Abu El-Fetouh, N., Abdelmegeed, E., Attia, R., El-Dosoky, I. & Azab, M. Genotyping of _Blastocystis hominis_ symptomatic isolates and kinetics of associated local CD3 and CD20
cell infiltrate. _Parasitol. United J._ 8, 122 (2015). Google Scholar * El Saftawy, E. A., Amin, N. M., Hamed, D. H., Elkazazz, A. & Adel, S. The hidden impact of different
_Blastocystis_ genotypes on C-3 and IgE serum levels: A matter of debate in asthmatic Egyptian children. _J. Parasit. Dis._ 43, 443–451 (2019). Article Google Scholar * Bertozzo, T. V.,
David, É. B., Oliveira-Arbex, A. P., Victória, C. & Guimarães, S. Frequency, spatial distribution, and genetic diversity of _Blastocystis_ among referred individuals to a clinical
laboratory: First report of subtype 9 in Brazil. _Acta. Trop._ 234, (2022). * Khaled, S. _et al._ Prevalence and subtype distribution of _Blastocystis_ sp. in Senegalese school children.
_Microorganisms_ 8, 1408 (2020). Article Google Scholar * Cinek, O. _et al. Blastocystis_ in the faeces of children from six distant countries: Prevalence, quantity, subtypes and the
relation to the gut bacteriome. _Parasit. Vectors_ 14, (2021). * Mokhtar, A. & Youssef, A. Subtype analysis of _Blastocystis_ spp isolated from domestic mammals and poultry and its
relation to transmission to their incontact humans in Ismailia governorate, Egypt. _Parasitol. United J._ 11, 1687–7942 (2018). Article Google Scholar * Udonsom, R. _et al._ _Blastocystis_
infection and subtype distribution in humans, cattle, goats, and pigs in central and western Thailand. _Infect. Genet. Evol._ 65, 107–111 (2018). Article Google Scholar * Abdo, S. M. _et
al._ Detection and molecular identification of _Blastocystis_ isolates from humans and cattle in northern Egypt. _J. Parasit. Dis._ 45, 738–745 (2021). Article Google Scholar *
Rauff-Adedotun, A. A., Mohd Zain, S. N. & Farah Haziqah, M. T. Current status of _Blastocystis_ sp. in animals from Southeast Asia: A review. _Parasitol. Res._ 119, 3559–3570 (2020).
Article Google Scholar * Ali, S. H. _et al._ an association between _Blastocystis_ subtypes and colorectal cancer patients: A significant different profile from non-cancer individuals.
_Acta Parasitol._ 67, 752–763 (2022). Article Google Scholar * Rayan, H. Z., Eida, O. M., El-hamshary, E. M. & Ahmed, S. A. Detection of human _Cryptosporidium_ species in surface
water sources in Ismailia using polymerase chain reaction. _Parasitol. United J._ 2, 119–126 (2009). Google Scholar * Khalifa, R. M. A., Ahmad, A. K., Abdel-Hafeez, E. H. & Mosllem, F.
A. Present status of protozoan pathogens causing water-borne disease in Northern part of El-Minia governorate Egypt. _J. Egypt. Soc. Parasitol._ 44, 559–566 (2014). Google Scholar *
Fernández-Niño, J. A. _et al._ Profiles of intestinal polyparasitism in a community of the Colombian Amazon region. _Biomedica_ 37, 368–377 (2017). Article Google Scholar * Pagheh, A. S.
_et al._ A cross-sectional analysis of intestinal parasitic infections among the general population in north of Iran. _J. Infect. Dev. Ctries._ 12, 120–126 (2018). Article Google Scholar *
Dawaki, S., Al-Mekhlafi, H. M. & Ithoi, I. The burden and epidemiology of polyparasitism among rural communities in Kano State Nigeria. _Trans. R. Soc. Trop. Med. Hyg._ 113, 169–182
(2019). Article Google Scholar * Weerakoon, K. G. _et al._ Co-parasitism of intestinal protozoa and _Schistosoma japonicum_ in a rural community in the Philippines. _Infect. Dis. Poverty_
7, (2018). * Elmonir, W. _et al._ Prevalence of intestinal parasitic infections and their associated risk factors among preschool and school children in Egypt. _PLoS ONE_ 16, e0258037
(2021). Article CAS Google Scholar * Yones, D., Zaghlol, K., Abdallah, A. & Galal, L. Effect of enteric parasitic infection on serum trace elements and nutritional status in upper
Egyptian children. _Trop. Parasitol._ 5, 35 (2015). Article Google Scholar * Souppart, L. _et al._ Subtype analysis of _Blastocystis_ isolates from symptomatic patients in Egypt.
_Parasitol. Res._ 106, 505–511 (2010). Article Google Scholar * Abaza, S., Rayan, H., Soliman, R., Nemr, N. & Mokhtar, A. Subtype analysis of _Blastocystis_ spp. isolates from
symptomatic and asymptomatic patients in Suez Canal University Hospitals, Ismailia. _Egypt. Parasitol. United J._ 7, 56–67 (2014). Article Google Scholar * El-Taweel, H. _et al._
Restriction fragment length polymorphism RFLP analysis of _Blastocystis_ spp. in symptomatic and asymptomatic individuals from Alexandria. _Egypt. Parasitol. United J._ 13, 164–171 (2020).
Article Google Scholar * Fouad, S. A., Basyoni, M. M. A., Fahmy, R. A. & Kobaisi, M. H. The pathogenic role of different _Blastocystis hominis_ genotypes isolated from patients with
irritable bowel syndrome. _Arab J. Gastroentrol._ 12, 194–200 (2011). Article Google Scholar * Hussein, E. M., Hussein, A. M., Eida, M. M. & Atwa, M. M. Pathophysiological variability
of different genotypes of human _Blastocystis hominis_ Egyptian isolates in experimentally infected rats. _Parasitol. Res._ 102, 853–860 (2008). Article Google Scholar * Mardani Kataki,
M., Tavalla, M. & Beiromvand, M. Higher prevalence of _Blastocystis hominis_ in healthy individuals than patients with gastrointestinal symptoms from Ahvaz, southwestern Iran. _Comp.
Immunol. Microbiol. Infect. Dis._ 65, 160–164 (2019). Article Google Scholar * Nieves-Ramírez, M. E. _et al._ Asymptomatic intestinal colonization with protist _Blastocystis_ is strongly
associated with distinct microbiome ecological patterns. _mSystems_ 3, e00007-18 (2018). * Kesuma, Y., Firmansyah, A., Bardosono, S., Sari, I. P. & Kurniawan, A. _Blastocystis_ ST-1 is
associated with irritable bowel syndrome-diarrhoea (IBS-D) in Indonesian adolescences. _Parasite Epidemiol. Control_ 6, (2019). * Yason, J. A., Liang, Y. R., Png, C. W., Zhang, Y. & Tan,
K. S. W. Interactions between a pathogenic _Blastocystis_ subtype and gut microbiota: In vitro and in vivo studies. _Microbiome_ 7, 30 (2019). Article Google Scholar * Stensvold, C. R.,
Arendrup, M. C., Nielsen, H. V., Bada, A. & Thorsen, S. Symptomatic infection with _Blastocystis_ sp. subtype 8 successfully treated with trimethoprim-sulfamethoxazole. _Ann. Trop. Med.
Parasitol._ 102, 271–274 (2008). Article CAS Google Scholar * Stensvold, C. R., Lebbad, M. & Verweij, J. J. The impact of genetic diversity in protozoa on molecular diagnostics.
_Trends Parasitol._ 27, 53–58 (2011). Article CAS Google Scholar * Mattiucci, S., Crisafi, B., Gabrielli, S., Paoletti, M. & Cancrini, G. Molecular epidemiology and genetic diversity
of _Blastocystis_ infection in humans in Italy. _Epidemiol. Infect._ 144, 635–646 (2016). Article CAS Google Scholar * Gabrielli, S. _et al._ Molecular Subtyping of _Blastocystis_ sp.
isolated from farmed animals in Southern Italy. _Microorganisms_ 9, (2021). * Stensvold, C. R., Alfellani, M. & Clark, C. G. Levels of genetic diversity vary dramatically between
_Blastocystis_ subtypes. _Infect. Genet. Evol._ 12, 263–273 (2012). Article Google Scholar * Cociancic, P., Rinaldi, L., Zonta, M. L. & Navone, G. T. Formalin-ethyl acetate
concentration, FLOTAC pellet and anal swab techniques for the diagnosis of intestinal parasites. _Parasitol. Res._ 117, 3567–3573 (2018). Article Google Scholar * Meloni, D. _et al._
Molecular subtyping of _Blastocystis_ sp. isolates from symptomatic patients in Italy. _Parasitol. Res._ 109, 613–619 (2011). Article ADS Google Scholar * Stensvold, C. R. _et al._
Terminology for _Blastocystis_ subtypes–a consensus. _Trends Parasitol._ 23, 93–96 (2007). Article Google Scholar Download references ACKNOWLEDGEMENTS The study was supported by Taif
University Researchers Supporting Project (TURSP-2020/152), Taif University, Taif, Saudi Arabia. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Parasitology, Faculty of
Medicine, Suez Canal University, Ismailia, 41522, Egypt Shahira A. Ahmed * Department of Animal Hygiene, Zoonoses and Animal Behaviour and Management, Faculty of Veterinary Medicine, Suez
Canal University, Ismailia, 41522, Egypt Heba S. El-Mahallawy * Department of Family Medicine, Faculty of Medicine, Suez Canal University, Ismailia, 41522, Egypt Samar Farag Mohamed *
Department of Environment and Health, Istituto Superiore Di Sanità, Rome, Italy Maria Cristina Angelici * Department of Basic and Clinical Sciences, University of Nicosia Medical School,
24005, Nicosia, Cyprus Kyriacos Hasapis * Department of Clinical Laboratory Sciences, College of Applied Medical Sciences, Taif University, P.O. Box 11099, Taif, 21944, Saudi Arabia Taisir
Saber * Medical Faculty and University Hospital, University of Cologne, Cologne, Germany Panagiotis Karanis * Department of Basic and Clinical Sciences, University of Nicosia Medical School,
24005, CY-1700, Nicosia, Cyprus Panagiotis Karanis Authors * Shahira A. Ahmed View author publications You can also search for this author inPubMed Google Scholar * Heba S. El-Mahallawy
View author publications You can also search for this author inPubMed Google Scholar * Samar Farag Mohamed View author publications You can also search for this author inPubMed Google
Scholar * Maria Cristina Angelici View author publications You can also search for this author inPubMed Google Scholar * Kyriacos Hasapis View author publications You can also search for
this author inPubMed Google Scholar * Taisir Saber View author publications You can also search for this author inPubMed Google Scholar * Panagiotis Karanis View author publications You can
also search for this author inPubMed Google Scholar CONTRIBUTIONS S.A. conception of the study, study design, methodology, formal analysis and investigation, interpretation of the results,
writing original draft of manuscript; H.E., S.M., M.A., K.H., P.K. methodology, formal analysis and investigation, interpretation of the results, editing manuscript; S.A., H.E., S.M., M.A
and T.S. resources of the experiment. P.K. and T.S language profiling. P.K. mentoring, manuscript editing-revision, supervision. All authors have read and agreed to the published version of
the manuscript. CORRESPONDING AUTHOR Correspondence to Shahira A. Ahmed. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION
PUBLISHER'S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. RIGHTS AND PERMISSIONS OPEN ACCESS This article
is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give
appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in
this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's
Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Ahmed, S.A., El-Mahallawy, H.S., Mohamed,
S.F. _et al._ Subtypes and phylogenetic analysis of _Blastocystis_ sp. isolates from West Ismailia, Egypt. _Sci Rep_ 12, 19084 (2022). https://doi.org/10.1038/s41598-022-23360-0 Download
citation * Received: 23 May 2022 * Accepted: 31 October 2022 * Published: 09 November 2022 * DOI: https://doi.org/10.1038/s41598-022-23360-0 SHARE THIS ARTICLE Anyone you share the following
link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature
SharedIt content-sharing initiative