Antarctic aldehyde dehydrogenase from flavobacterium pl002 as a potent catalyst for acetaldehyde determination in wine
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Latest solutions in biotechnologies and biosensing targeted cold-active extremozymes. Analysis of acetaldehyde as a relevant quality indicator of wine is one example of application
that could benefit from using low-temperatures operating catalysts. In search of novel aldehyde dehydrogenases (ALDH) with high stability and activity at low temperatures, the recombinant
S2-ALDH from the Antarctic _Flavobacterium_ PL002 was obtained by cloning and expression in _Escherichia coli_ BL21(DE3). Structural and phylogenetic analyses revealed strong protein
similarities (95%) with psychrophilic homologs, conserved active residues and structural elements conferring enzyme flexibility. Arrhenius plot revealed a conformational shift at 30 °C,
favoring catalysis (low activation energy) at lower temperatures. In addition to a broad substrate specificity with preference for acetaldehyde (Km = 1.88 mM), this enzyme showed a high
tolerance for ethanol (15%) and several salts and chelators (an advantage for wine analysis), while being sensitive to mercury (I50 = 1.21 µM). The neutral optimal pH (7.5) and the stability
up to 40 °C and after lyophilization represent major assets for developing S2-ALDH-based sensors. An enzymatic electrochemical assay was developed for acetaldehyde detection in wines with
proven accuracy in comparison with the reference spectrophotometric method, thus evidencing the potential of S2-ALDH as effective biocatalyst for industry and biosensing. SIMILAR CONTENT
BEING VIEWED BY OTHERS SCREENING METHODS FOR ENZYME-MEDIATED ALCOHOL OXIDATION Article Open access 22 February 2022 ASSOCIATION BETWEEN ORGANIC NITROGEN SUBSTRATES AND THE OPTICAL PURITY OF
D-LACTIC ACID DURING THE FERMENTATION BY _SPOROLACTOBACILLUS TERRAE_ SBT-1 Article Open access 08 May 2024 BIOCATALYTIC CHARACTERIZATION OF AN ALCOHOL DEHYDROGENASE VARIANT DEDUCED FROM
_LACTOBACILLUS KEFIR_ IN ASYMMETRIC HYDROGEN TRANSFER Article Open access 12 October 2023 INTRODUCTION Aldehydes are volatile, reactive carbonyl compounds, widely used in the food and
cosmetics industries as important contributors to emerging olfactory technologies. This class of chemical compounds is relevant for assessing the human health status and used as indicators
of the quality of environmental air1, food2 and beverages3. Among these compounds, acetaldehyde is known to affect the color, stability and aroma of alcoholic beverages3. This aldehyde is
present in wine at concentrations up to 211 mg/L, while it could reach 63 mg/L and 1159 mg/L in beers and spirits, respectively4. In wine, acetaldehyde is formed during alcoholic
fermentation, and also produced in later stages by acetic bacteria. This compound binds to bisulfite used as stabilizer, thus preventing its antimicrobial and antioxidant actions5. Moreover,
acetaldehyde forms bridges between polyphenolic compounds such as flavanols and anthocyanins (e.g. catechin and malvidin-3-_O_-glucoside) with formation of oligomeric compounds responsible
for the change of color and aroma in wines6. Aldehydes bound in adducts with bisulfite can be liberated during the wine ageing when the sulfite gets oxidized, thus the dynamics of this class
of reactive carbonyl compounds critically influences the wine organoleptic characteristics7. In addition to acetaldehyde, other aldehydes present at µg/L concentrations contribute to wine
aroma, counting isovaleraldehyde, isobutyraldehyde, 2-methylbutanal, octanal, nonanal, decanal, phenylacetaldehyde and benzaldehyde7,8. In liquid phase, the aldehydes content could be
measured by a variety of methods counting chromatography with different detection modes (UV–VIS spectrometry, fluorescence, mass spectrometry), titration, and enzymatic assays3,9,10. In
these cases, specific detection requires separation and quantitation of individual aldehydes. In addition to the time consuming and complexity of many tests, they require expensive
laboratory equipment. Alternatively, the level of aldehydes in beverages, food products, and pharmaceutical ingredients could be measured using enzyme-based biosensors relying on various
enzyme such as aldehyde dehydrogenases (ALDH) from different sources, aldehyde oxidoreductase PaoABC from _E. coli_, or alcohol dehydrogenases (ADH) based on the reverse
reaction11,12,13,14,15,16,17,18. The main advantages of biosensors as compared to the classical analytical methods reside on their ability for real-time measurements, being compatible with
portable cost effective equipment for on-site measurements. Aldehyde dehydrogenase superfamily catalyzes the NAD(P)+-dependent oxydation of highly reactive aliphatic and aromatic aldehydes
to the corresponding carboxylic acid19,20. Monitoring the aldehydes concentration relies on the quantification of NADH+ formed during the enzymatic reaction, and is commonly carried out by
spectrometry and fluorimetry20. In comparison with these methods, electrochemical assays and biosensors utilization provided several advantages related to their reduced cost and portability.
While the field of biosensing advanced considerably towards miniaturized and user friendly transducer devices21, the search for new enzymes with improved characteristics including variable
substrate specificity and high catalytic efficiency for targeted substrates, lack of sensitivity to ions and compounds typically found in real samples, and high stability and enzymatic
activity in a wide range of temperatures is still ongoing22.For the last decades, microbial catalysts were extensively used for a variety of industrial and biosensing applications, most of
them originating from mesophilic strains23. More recently, extremophilic microorganisms thriving under extreme condition of temperature, salinity, pH, hydrostatic pressure, radiation,
etc.24,25,26,27 showed a rapid development in using their molecules adapted to cope with a wide range of biophysical parameters28,29. Among extremophilic microorganisms hosting these potent
enzymes (extremozymes), thermophilic and hyperthermophilic strains isolated from hot environments were among the main source of industrial catalysts due to their high stability and
distinctive substrate utilization profile30,31. Alternatively, cold-active enzymes were actively screened for a wide range of applications in32,33,34,35,36, being active at low temperatures
and presenting different substrate specificity as compared to enzymes from mesophilic and thermophilic counterparts37,38. Our group has previously reported the production and
characterization of a novel cold-active recombinant aldehyde dehydrogenase from the Antarctic _Flavobacterium_ PL002, highlighting its applicatve potential for the electrochemical detection
of benzaldehyde in pharmaceutical ingredients15,39,40. However, to date, limited data on cold-active oxidoreductases is available for developing catalysts for industrial applications36,41.
In this context, the current study reported the cloning and characterization of a new recombinant aldehyde dehydrogenase from the Antarctic _Flavobacterium_ PL002 strain (S2-ALDH) as a
valuable bacterial cold-active catalyst for measuring acetaldehyde in liquid phase, highlighting for the first time the enhanced characteristics of a cold-active-ALDH-based assay for
measurements in wine. Specifically suitable for this application, S2-ALDH enables measurements at wine cellar temperatures and is not sensitive to ethanol and phenolic compounds. The assay
uses electrochemical detection based on screen-printed carbon nanotubes electrodes as transducers. The accuracy of the new system was confirmed in comparison with a reference
spectrophotometric method. RESULTS AND DISCUSSION SEQUENCE ANALYSIS OF S2-ALDH BLAST screening of the _Flavobacterium_ PL002 genome sequence42 led to identification of a coding region
homologous to aldehyde dehydrogenase gene (1365 bp) coding for the hypothetical enzyme S2-ALDH of 454 amino acids, with calculated MW 49,062 (Supplementary Fig. 1S). Phylogenetic analysis of
the S2-ALDH amino acid sequence in relation with aldehyde dehydrogenases from genus _Flavobacterium_ showed three distinct clusters mainly composed of psychrophilic and psychrotolerant
species (Supplementary Fig. 2S). Among these, Clade I (bootstrap = 98) comprised one strain from Arctic soil (WP132111999) and an Antarctic sub-clade (bootstrap 94) composed of three
_Flavobacterium_ strains originating from sea ice (WP091086047, WP074724235, WP101136856), and from a meromictic lagoon (WP016989072). Clade II (bootstrap 100) also comprised Antarctic
strains isolated from sea ice (WP 007139642) and lake microbial mat (WP091433283), while Clade III (bootstrap 99) was composed of Antarctic marine strains. Within the latter one, S2-ALDH
showed high similarity (> 99%) with closely related sequences of an Antarctic unclassified _Flavobacterium_ strain (WP173857136) (100%) and _Flavobacterium_ 28A (WP173851899) (99%),
clustering with the well supported sub-clade (bootstrap 86) of the psychrophilic _F. faecale_ (WP108740064) isolated from Antarctic penguin feces and _F. frigidarium_ (WP026707351) retrieved
from Antarctic marine sediment, and with the psychrotolerant _F. kayseriense_ (WP187011286) originating from a farmed rainbow trout (Supplementary Fig. 2S). Pair alignment of the S2-ALDH
amino acid sequence with homologous enzymes from psychrophilic, mesophilic and (hyper)thermophilic species (Table 1) confirmed the strong resemblance with the psychrophilic enzyme, and
highlighted relatively low similarity and identity percentages with ALDHs from species thriving at higher temperatures. However, their sequence identity showed an overall descending trend
with the increase of the environmental growth temperature of the host, varying from 90.1% (psychrophiles) to 30.2–40.2% (mesophiles) and 28–33.3% (hyper/thermophiles) (Table 1), in support
of the presence of common structural features of cold-adapted enzymes43. The aminoacid composition of this Antarctic enzyme indicated a lower number of cysteine residues as compared to that
of mesophilic ALDHs, that could induce a higher protein flexibility at low temperatures by a reduced disulfide bond formation44, similar to homologous enzymes from the analyzed thermophilic
and hyperthermophilic species (Supplementary Table 1S). Meanwhile, the low content of prolines that could rigidify flexible regions45 as compared to ALDHs from both mesophilic46,47 and
(hyper)thermophilic48,49 species could also contribute to enhance enzyme flexibility at low temperature. The total number of positively (Arg + Lys) and negatively (Asp + Glu) charged
residues of S2-ALDH occupied an intermediary position between that of the _E. coli_ and _T. thermophilus_ ALDHs (Supplementary Table 1S), suggesting improved ion interactions relative to the
mesophilic enzyme, but reduced as complared with the thermophilic ones. Considering the ratio of Arg/(Arg + Lys) residue numbers, where a relatively low value suggests a low presence of ion
interactions50, the estimated salt bridges formed in S2-ALDH (0.2692) appeared to be reduced as compared to that of mesophilic (0.3396–0.5870) and (hyper)thermophilic (0.4355–0.6557)
homologous enzymes (Supplementary Table 1S), in support of a higher flexibility in the case of the cold-active extremozymes to cope with low temperature catalysis43. The multiple alignment
of these ALDHs (Supplementary Fig. 3S) revealed the presence of all catalytic residues universally conserved, Asn131, Glu228, Gly259 and Cys262 (numbers in S2-ALDH), in support of a
functional Antarctic enzyme. Moreover, the substrate binging site of S2-ALDH was highly conserved among the investigated homologous enzymes, comprising identical (Pro129, Lys154, Thr205,
Gly206, Leu229, Gly230, Glu359, Phe361), and partially conserved (Trp130, Leu204, Ala210, Met214, Phe425) residues. Among these, all the NAD+ cofactor binding sites Trp130, Lys154, Gly206,
Glu359 and Phe361 were fully conserved. Based on sequence homology with _S. aureus_ ALDH51, the oligomerizations domains (Phe101-Gly122) and (Asn447-Ser454) exhibited a partial conservation,
in particular between the enzymes originating from psychrophilic hosts (Supplementary Fig. 3S). The Phe101-Gly122 region showed a high content of Ser (13.6%) and Thr (17.4%) residues for
S2-ALDH and _F. frigidarium_ ALDH, respectively, while charged residues (13–13.6%) and hydrophobic residues Leu, Ile, Val (13.6%) were highly represented in the mesophilic and thermophilic
counterparts. The Asn447-Ser454 stretch was characterized by a high content of positively charged residues (37.5%) in comparison with mesophilic enzymes from _E. coli_ (20%) and _S. aureus_
(9.7%), and displayed close values of hydrophobic residue content (37.5%) with those of mesophilic enzymes (31.6%-40%), while significantly reduced relative to that of the hyperthermophilic
enzyme (60%) (Supplementary Fig. 3S). CLONING, EXPRESSION AND PURIFICATION OF THE COLD-ACTIVE ENZYME The S2-ALDH gene encoding the hypothetical S2-ALDH _Flavobacterium_ PL002 aldehyde
dehydrogenase was synthesized and inserted into the pHAT2 expression vector using NcoI/BamHI cloning sites (ATG: Biosynthetics GmbH, Merzhausen, Germany), in order to append a His-tag to the
amino end of the recombinant protein and facilitate purification. The heterologously expressed enzyme in _Escherichia coli_ BL21 (DE3) was purified from the soluble fractions in a single
step by Ni2+ affinity chromatography, with a yield of 1.02 ± 0.12 µg S2-ALDH /L culture (Supplementary Fig. 4S). The specific activity of the purified enzyme measured at 25 °C in the
presence of 1 mM acetaldehyde and 10 mM NAD+ cofactor was of 0.51 ± 0.15 U/mg. BIOCHEMICAL CHARACTERIZATION OF THE RECOMBINANT S2-ALDH _Substrate specificity._ The catalysis of different
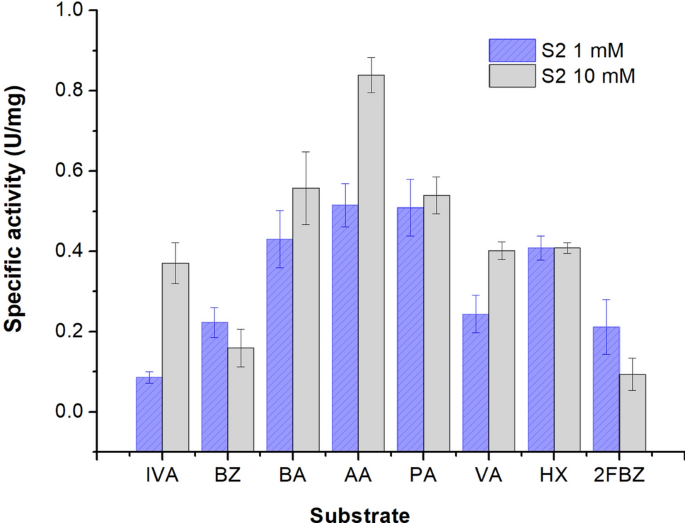
aliphatic and aromatic aldehydes by this cold-active dehydrogenase showed a variable substrate specificity in the presence of both high and low concentrations of aldehydes (Fig. 1). When
using 10 mM aldehydes, the enzyme showed a preference for saturated aliphatic aldehydes, with the highest activity measured for acetaldehyde (0.84 U/mg), while at lower substrate
concentration (1 mM), both acetaldehyde and propionaldehyde provided the highest S2-ALDH activity (0.51 U/mg), followed by hexanal (0.41 U/mg) and butiraldehyde (0.43 U/mg). In the presence
of 10 mM substrate, the aliphatic aldehydes isovaleraldehyde, valeraldehyde and hexanal showed comparable activities. Noteworthy, the unsaturated aliphatic aldehyde hexanal appeared to be a
potent substrate for S2-ALDH at both low and high concentrations. Meanwhile, isovaleraldehyde was one of the substrates leading to the lowest activity when tested at low concentration (1
mM). Also, the aromatic aldehydes benzaldehyde and 2-fluorobanzaldehyde exhibited low ALDH activity at both 1 mM and 10 mM concentrations (Fig. 1). Substrate specificity of S2-ALDH was
different from that of the aldehyde dehydrogenase from the same Antarctic microorganism which preferred isovaleraldehyde among the aliphatic aldehyde substrates39, and also different from
that of the commercially available mesophilic aldehyde dehydrogenase from yeast52 and human53. Thus, the preference for acetaldehyde and the different substrate specificity indicated S2-ALDH
as a promising component of bioelectronic e-tongues, i.e. arrays of enzyme biosensors, each modified with a different enzyme with slightly different and overlapping specificities. Such
arrays are used to analyze a mixture of enzymatic substrates, enabling a specific and quantitative response of all or some of the substrates via a chemometric method54. _Optimal pH_
established for the NAD+-dependent reaction of propionaldehyde in the presence of various buffers covering the pH 6.0–10.5 interval (see “Methods”) indicated the highest activity of S2-ALDH
when using 100 mM potassium phosphate pH 7.5 as buffer (not shown). This high activity at neutral pH is advantageous for biosensing applications that include electrochemical mediators, as
well as for coupled cascade enzymatic reactions with enzymes having similar optimum pH. _Effect of metal ions and additives_ on the NAD-dependent activity when using propionaldehyde as
substrate showed an overall stable response (Fig. 2). No inhibitory effect was observed in the cases of EDTA (2 mM), Triton (1%) and betamercaptoethanol (1–10 mM), the first two tested
compounds inducing even a slight activation of 121.68% and 146.80%, respectively (Fig. 2A). This cold-active ALDH showed no inhibition in the presence of ethanol at concentrations up to 5%,
and a residual activity of 73% and 50% in the presence of 10% and 15% EtOH, respectively (Fig. 2B). NaCl and KCl concentrations of 100 mM and 200 mM induced a moderate activity loss up to
26%, while no inhibition was observed by 1 mM of both these monovalent ions (Fig. 2A). Moreover, the cold-active S2-ALDH had a different response to various divalent ions, being inhibited by
41% and 77% in the presence of 10 mM and 100 mM MgCl2, respectively, while CaCl2 had no impact up to 10 mM. In addition, 1 mM NiCl2 inhibited the S2-ALDH enzyme by 23%, with a slight
increase (35%) at concentrations of 10 mM (Fig. 2A). Such high resilience to monovalent and divalent ions of this cold-active enzyme was also observed in the case of the hyperthermophilic
_S. tokadaii_ ALDHs48, except for Mg2+ that showed no affect. Interestingly, in the case of HgCl2, S2-ALDH exhibited a high sensitivity to this divalent ion, with 15% residual activity in
the presence of 10 nM HgCl2, and a calculated I50 value of 1.21 µM (Fig. 2C). The total inhibition of both ALDH from the thermophilic _Geobacillus thermodenitrificans_ NG80-255 and the
cold-active F-ALDH originating from the same bacterial host strain as S2-by 1 mM and 0.5 mM HgCl2, respectively, revealed the strong response to this heavy metal of this class of enzymes
independent of their origin. Moreover, the high sensitivity to mercury of the novel S2-ALDH could constitute a starting point for develping stable sensors for detecting traces of this heavy
metal in various environments. STORAGE, LYOPHILIZATION AND THERMAL STABILITY OF S2-ALDH The stability of the purified S2-ALDH when stored as liquid and lyophlized forms under various
conditions, and the thermal stability after exposure to higher temperatures that those of the natural habitat of _Flavobacterium_ PL00242 were determined (Fig. 3) in order to evaluate the
potential of this cold-active enzyme for practical applications in analytical, industrial and biotechnological processes. Storage of S2-ALDH at 4 °C for 2 days in the presence and absence of
different additives showed full preservation of the enzyme activity for 24 h, with a partial activity loss (13–35%) after 48 h without additives, and in the presence of 20% glycerol, 1 M
trehalose, 1 M sucrose or 0.5 M mannose, respectively (Fig. 3A). Moreover, the lyophilized enzyme treated with 0.1 M or 0.5 M trehalose was fully stable for 24 h at − 20 °C, but loosed
43–56% activity when stored at 4 °C (Fig. 3A). These data constitute a good starting point for longer stability studies. Investigation of the thermal stability of the Antarctic ALDH after
exposure to different temperatures revealed a fully preserved activity up to 40 °C, followed by an abrupt decrease at above 45 °C and complete inactivation at 50 °C (Fig. 3B). This high
stability of this cold-active enzyme to temperatures above 25 °C commonly used in industrial applications was also observed for other ALDHs originating from Antarctic habitats, such as the
recently described F-ALDH from _Flavobacterium_ PL00239 and the homologous enzyme from the psychrotrophic marine _F. frigidimaris_ (_Cytophaga sp_)41. The temperature effect on the S2-ALDH
activity was determined by conducting the assays at variable temperatures ranging between 4 and 40 °C, taking into account the thermal stability of the enzyme (Fig. 3C). As expected, the
activity increased rapidly with the temperature and started to decrease above 35 °C when the protein began to be affected by thermal denaturation. The Arrhenius representation (Fig. 3C,
inset) corresponded to a bi-phasic plot with a calculated activation energy shift from Ea10–30 °C = 64.77 J/K mol to Ea30–35 °C = 18.72 J/K mol, suggesting a conformational change that
occurred at 30 °C. This temperature effect on enzymes’ tertiary and quaternary structure entrained a wider energy shift and a lower activation energy at low temperature in the case of
S2-ALDH as compared to that of the F-ALDH from the same host (from Ea10–30 °C = 76 J/K mol to Ea30–35 °C = 19 J/K mol) that occurred at the same temperature threshold (30 °C)39. These data
suggested similar overall intramolecular interactions involved in catalysis for the two cold-active ALDHs and similar activation energy required at higher temperatures, while a favored
catalysis (lower Ea) at low temperatures in the case of S2-ALDH. Moreover, the homologous enzyme from the psychrophilic _F. frigidimaris_41 required a higher activation energy of 27 J/K mol
above 30 °C and comparable (57 J/K mol) at low temperatures, as a hint for particular thermal adaptation mechanisms of these psychrotolerant and psychrophilic species. KINETIC PARAMETERS OF
RECOMBINANT S2-ALDH In order to determine the apparent affinity for acetaldehyde and the catalytic efficiency of this recombinant cold-active enzyme, saturation curves for the NAD+-dependent
reaction of acetaldehyde were carried out at 25 °C for both the substrate and cofactor. In this case, the calculated steady state kinetic parameters of the S2-ALDH (Table 2) indicated a
kcat value of 0.432 ± 0.025/s and Km of 1.88 ± 0.70 mM for acetaldehyde, corresponding to a catalytic efficiency of 229.8 ± 85.2/M s. Meanwhile, the kcat for NAD+ was of 0.121 ± 0.026/s and
Km in the mM range (2.87 ± 0.48 mM), with calculated catalytic efficiency of 42.16 ± 7.05/M s (Table 2). In comparison with the homologous enzymes from _Saccharomyces cerevisiae var.
boulardii_, NCYC 3264, the catalytic efficiency of S2-ALDH for the utilization of acetaldehyde at 25 °C was two-fold (14.3/M s) and three-fold (7.38/M s) higher, respectively, than that of
the mitochondrial (ALD4) and cytosolic (ALD6) ALDHs from yeast measured at 30 °C56, corresponding to a more potent catalyst al low temperatures than these mesophilic enzymes. Moreover, the
ALDH from the hyperthermophilic _S. tokadaii_ strain 7 had a threefold higher Km (6.38 mM) when oxidizing this substrate at 80 °C, and a slightly lower catalytic efficiency (180/M s) than
that of the cold-active counterpart, with a severe activity reduction (by 90%) at temperatures < 30 °C relative to the optimal of 80 °C for this enzyme48. In this context, the high
stability of the investigated S2-ALDH within a temperature range suitable for industrial and biosensing applications, and stability after lyophilization, as well as the high activity and
catalytic efficiency at 25 °C constituted cumulative favorable conditions for developing applicative model systems using this Antarctic enzyme for acetaldehyde monitoring in liquid phase. In
particular, the preference for acetaldehyde as substrate, combined with the high ratio between the typical levels of acetaldehyde and other aldehydes in wine and the lack of sensitivity
towards ethanol, recommended the recombinant cold-active S2-ALDH as an advantageous catalyst for acetaldehyde determination in wine. APPLICATION OF S2-ALDH IN ELECTROCHEMICAL ASSAYS FOR
ACETALDEHYDE DETERMINATION IN WINE In order to test the applicative potential of S2-ALDH as biocatalyst, an electrochemical assay for the determination of acetaldehyde in wines was
developped and optimized. This application was based on measuring the concentration of NADH formed in the S2-ALDH enzymatic reaction which was oxidized on CNT electrodes, the intensity of
the generated anodic current being proportional to the concentration of acetaldehyde in the sample. Carbon nanotubes electrodes were selected taking advantage of their antifouling surface,
electrocatalytic characteristics for the detection of NADH, and high sensitivity when implemented as electrochemical transducers in biosensors57. The preliminary characterization of the CNT
electrodes by Raman, SEM and cyclic voltammetry (Supplementary Fig. 5S) confirmed the advantages of these electrodes for electrochemical detection, considering the high surface to volume
ratio (specific surface BET of 300 m2/g), favorable morphology of the CNT layer for sensitive electrochemical detection, and the electrocatalytic effect for NADH oxidation. When used in
chronoamperometry measurements at 0.5 V, these electrodes responded linearly to NADH concentrations in the range of 12.5–250 µM (R2 = 0.9992), with a sensitivity of 1.15 ± 0.16 μA L/mmol and
a detection limit of 10 µM (Supplementary Table 2S). These features were similar to those reported for other sensors, and appropriate for substrates detection of NAD+-dependent
dehydrogenases40,58. These pre-requisits for acetaldehyde concentration measurement using S2-ALDH were verified by a typical detection scheme40 which minimized the reagents amount used for
the assay (Fig. 4A). Based on the current intensity increase (ΔI) with the concentration of acetaldehyde (Fig. 4B), an apparent Kmapp of 1.10 ± 0.52 mM was calculated for S2-ALDH (Fig. 4C).
Five calibrations were performed in the range of 0.025 mM 0.75 mM acetaldehyde over a 5-week period, with on average of 24 tests in the same day, using one sensor per calibration. (The
repeatability, evaluated based on the average RSD (n = 3 replicates) of responses measured with the same electrode for various concentrations of acetaldehyde within the linear range was
11.3%, varying in the 1.2–35.1% interval. The average assay sensitivity was 327.1 ± 75.0 nA L/µmol, and the calculated detection limit was 17 µM acetaldehyde based on the threefold standard
deviation of the blank divided by the slope of the calibration curve. These characteristics indicated appropriate ruggedness of the assay and suitability for the measurement of acetaldehyde
in wine, taking into considertion the average acetaldehyde content in this matrix of up to 4.8 mM4. OPTIMIZATION OF WINE PRE-TREATMENT FOR THE ELECTROCHEMICAL ASSAYS BASED ON S2-ALDH
Analysis of acetaldehyde concentration in wines was carried out after optimizing the experimental conditions and wine pre-treatment, taking into consideration the pH of various wines ranging
in the 3.3–3.6 interval, the ethanol content of 11–13%, and the presence of easily oxidizable species including high amounts of phenolics. Consequently, all acetaldehyde solutions used for
calibration were prepared in model wine solution to avoid any bias on the activity measurements of S2-ALDH. As previously shown (Fig. 2B), this cold-active ALDH preserved full activity in
the presence of 5% ethanol. Nonetheless, the presence of phenolic compounds oxidized at the relatively high potential (0.5 V) was likely to induce electrochemical interferences. Therefore,
to eliminate interferences59, the wine was filtered through a cartridge filled with PVPP and activated charcoal using an adaptation of a literature procedure10. The sample treatment
efficiency was evaluated at 0.5 V using CNT-electrodes (Fig. 5). Among variable conditions tested during optimization (reported in Supplementary information), the smallest anodic current at
0.5 V, potentially interfering with the analysis of acetaldehyde, was obtained for the wine filtered through a cartridge filled with PVPP and activated charcoal, and analyzed on a CNT
electrode “blocked” with BSA (Fig. 5A). The current intensity due to the oxidation of phenolic compounds from a red wine decreased by ~ 98% from 5.69 to 0.12 µA after filtration through the
PVPP/activated charcoal cartridge (Fig. 5A). The remaining small anodic current was still significantly higher than that recorded for a model wine solution under the same conditions, and was
stable after 100 s, indicating the fast oxidation of the residual phenolics in wine. The difference between the current intensity at 400 s and 100 s (7.6 ± 0.07 nA) was similar for the
model wine solution and the filtered red wine (Fig. 5B). Consequently, the current intensity at 100 s was considered as baseline, and the variation between 100 and 400 s (mainly due to NADH
formed in the S2-ALDH-catalyzed reaction) corresponded to the analytical signal. For acetaldehyde measurements, the calibration was performed at 17 °C in order to mimic the typical
temperatures in cellars and avoid evaporation of acetaldehyde (Supplementary Fig. 6SA). In this case, the sensitivity of the test was of 508.4 µA L/mmol. The determined linear range of the
assay (R2 = 0.9927) was of 0.125–2.5 mM acetaldehyde in the electrochemical cell (Supplementary Fig. 6SB). Considering the dilution, this corresponds to 0.5–10 mM (4.4–440 mg/L) in wines.
These corroborated data demonstrated that the assay was appropriate for testing wines, considering their average content of 34–211 mg/L acetaldehyde4. The accuracy of the electrochemical
assay of acetaldehyde in wines using this method was demonstrated in comparison with a reference method that relies on a commercial kit using an aldehyde dehydrogenase from yeast10 and
spectrophotometric detection (Table 3). In the case of all eigtht analyzed wine samples (2 white, 1 rosé and 5 red wines), the acetaldehyde concentration measured by the two methods was in
good agreement (Table 3), with a correlation coefficient of R2 = 0.9747 and a slope of 1.0183 (Supplementary Fig. 6SC). As compared to the spectrophotometric test, the proposed
electrochemical assay is simpler, faster, uses cost-effective and portable equipment, and can be easily adapted for in situ measurements in wine cellars. These corroborated data confirmed
the utilization of the novel cold-active ALDH as an effective biocatalyst for monitoring acetaldehyde in wine, with extensive applicative potential in biosensing. To further explore this
potential, immobilization of S2-ALDH will be conducted based on crosslinking with glutaraldehyde, as demonstrated for the homologous enzyme originating from the same bacterial species39 and
Ni-histidine affinity for developing enzymatic biosensors for detection of aldehydes in alcoholic beverages and other relevant industrial applications. CONCLUSIONS A new cold-active
bacterial aldehyde dehydrogenase was obtained originating from the Antarctic marine strain _Flavobacterium_ PL002 by cloning and expression in _E. coli_. This recombinant S2-ALDH enzyme
exhibited a broad substrate specificity encompassing aliphatic and aromatic aldehydes and particular functional characteristics as compared with ALDHs from other sources. In addition to the
high sensitivity to mercury ions with I50 in the µM range suggesting that S2-ALDH was a good candidate for the detection of this heavy metal, the functional profile of the novel cold-active
ALDH revealed compelling advantages for practical applications in biotechnology and biosensing. These features corroborate a high stability to storage and lyophilization, neutral optimum pH,
high substrate specificity for acetaldehyde (a particular asset for wine analysis), high tolerance for a series of compounds including ethanol, and relatively high catalytic efficiency at
low temperatures. The neutral optimum pH is in particular advantageous for mediator-based electrochemical sensors and coupled chemical and enzymatic reactions (e.g. for cascade reactions in
biocatalysis). The different substrate specificity and catalytic efficiency of the NAD+-dependent acetaldehyde oxidation as compared to both the commercial enzyme from yeast and the
previously characterized cold-active F-ALDH open the way for using this cold-active ALDH as component of bioelectronic tongues for the specific detection of aldehydes. The applicative
potential of the newly characterized cold-active microbial enzyme S2-ALDH as potent biocatalyst for industrial processes and biosensing was evidenced by the enzymatic electrochemical
detection of acetaldehydes in wines. The assay presented here is simple to perform, and the costs were decreased by the use of low fouling carbon nanotube electrodes, that are used for
multiple tests. These corroborated data support the use of S2-ALDH as valuable catalyst in developing easy to use kits for acetaldehyde measurements in wine. Alternatively, biosensors with
the immobilized recombinant Antarctic enzyme could be envisaged for various applications targetting other relevant aldehydes. Experiments for obtaining stable enzymatic inks based on S2-ALDH
for developing printed, highly sensitive detection interfaces are under way in our laboratory, demonstrating the applicative potential of this Antarctic enzyme. METHODS REAGENTS AND
MATERIALS Polyvinylpolypyrollidone (PVPP) was from Supelco, Switzerland. Activated charcoal, tartaric acid, sodium phosphate, monobasic and dibasic were from Sigma Aldrich (Merck), Germany.
Nicotinamide adenin dinucleotide (NAD+) and isopropyl-β-d-thiogalactoside (IPTG) were from Carl Roth, Germany. NADH and the spectrophotometric kit for acetaldehyde were from Roche (Merck),
Germany. Screen printed electrodes modified with carbon nanotubes (DRP 110 CNT) and polyamide membranes covering the screen-printed 3-electrode system for the analysis of small volumes of
sample (7.5–15 μL) were from Metrohm Dropsens, Oviedo, Spain. Ethanol was from Chimreactiv S.R.L. (Bucharest, Romania). The enzymatic UV spectrometric kit for acetaldehyde was from
R-Biopharm, Darmstadt, Germany. The aldehydes were from Acros Organics, Geel, Belgium. A model wine solution (5 g/L tartaric acid, 13% ethanol, pH 3.660 was used for preparing the standard
acetaldehyde solutions and for diluting the samples for the wine analysis. All acetaldehyde solutions were kept at 4 °C before use to avoid evaporation. The wines tested included two house
wines without added preservatives (one rosé, one red) and six commercial wines, kindly provided by the Research and Development Institute for Vine and Wine, Valea Calugareasca, Romania.
CLONING AND HETEROLOGOUS EXPRESSION OF _FLAVOBACTERIUM_ PL002 _S2-ALDH_ GENE The _S2-ALDH_ (1365 bp) gene (Supplementary Fig. S1) identified in the _Flavobacterium_ PL002 genome sequence42
was synthesized (ATG Biosynthetics GmbH, Merzhausen, Germany) and cloned into the pHAT2 His-tag expression vector (EMBL, Heidelberg, Germany) using the _Nco_I/_Bam_HI restriction sites. Gene
expression of the resulting pS2-ALDH recombinant plasmid was performed in _Escherichia coli_ BL21(DE3) (Thermo Fisher Scientific, Massachusetts, USA) after induction for 6 h at 25 °C in the
presence of 1 mM IPTG. The induced cells were separated by centrifugation at 9000 × _g_ for 10 min (4 °C) and stored at − 80 °C. PURIFICATION OF THE RECOMBINANT S2-ALDH The recombinant
enzyme S2-ALDH appending an N-terminal His-tag polypeptide to the psychrophilic protein was purified by affinity chromatography using Ni–NTA agarose (Qiagen, Hilden, Germany)61. After
induction (200 mL culture), the cells were resuspended in 6 mL of buffer A (100 mM TrisHCl pH 8, 200 mM NaCl) and dissrupted by 5 min ultrasonication cycles alternating 5 s pulses and 60 s
pauses, using a Sonopuls ultrasonic homogenizer (Bandelin, Berlin, Germany). Following the centrifugation of the extract at 16.000 × _g_ for 30 min at 4 °C, the soluble fraction was passed
on a 1-mL Ni–NTA agarose (Qiagen, Hilden, Germany) column equilibrated with buffer A and washed with 10 mL buffer A and 3 mL buffer A containing 30 mM imidazole. The recombinant S2-ALDH was
further eluted in the presence of 50–100 mM imidazole. The protein fractions were examined by SDS-PAGE and desalted using 7 K MWCO Zeba Spin Desalting columns (ThermoFisher Scientific,
Massachusetts, USA). The resulted enzyme fractions were store at – 20 °C in 100 mM TrisHCl pH 8 buffer containing 20% glycerol. ALDEHYDE DEHYDROGENASE ASSAYS The activity of free S2-ALDH was
measured spectrophotometrically in a FLUOstarOmega microplate reader (BMG Labtech, Offenburg, Germany) by monitoring the rate of NADH formation at OD340 nm62. The reaction was measured at
25 °C using a FLUOstarOmega microplate reader (BMG Labtech, Offenburg, Germany), in the presence of 10 mM aldehyde and 10 mM NAD+, using 100 mM potassium phosphate buffer pH 7.5, and
initiated with 6 µg S2-ALDH. One unit of ALDH was defined as the amount of enzyme producing 1 μmol NADH per minute, using the molar absorption coefficient εNADH = 6.22 × 103 L/mol cm. The
optimal pH was determined in the presence of various buffers encompassing 100 mM potassium phosphate buffer (pH 6.0–7.5), Tris buffer (pH 7.5–9), and glycine-KOH buffer (pH 9.0–10.5).
Substrate specificity was determined for a series of aliphatic (acetaldehyde, propionaldehyde, valeraldehyde, isovaleraldehyde, butyraldehyde, and hexanal) and aromatic (benzaldehyde,
2-fluorobenzaldehyde) aldehydes at both 1 mM and 10 mM, in the presence of 10 mM NAD+. The effect of 2 mM EDTA, 1% Triton, 1–10 mM betamercaptoethanol, 1–15% ethanol and 1–200 mM metal ions
(Na+, K+, Ca2+, Mg2+, Ni2+, Hg2+ on the activity of S2-ALDH was measured at 25 °C in the presence of varrious concentrations of salts and compounds, using 10 mM propionaldehyde and 10 mM
NAD+. The thermal stability was determined by incubating the enzyme for 15 min at various temperatures in the 4–50 °C interval, and further measuring their residual activity at 25 °C. The
effect of temperature on the reaction rate was obtained by measuring the activity at different temperatures ranging from 4 to 40 °C, in the presence of 10 mM propionaldehyde and 10 mM NAD+.
Arrhenius plot was used for calculating the activation energy of the S2-ALDH catalyzed reaction (Atkins and De Paula, 2006). S2-ALDH stability was determined after incubation at 4 °C for 24
h and 48 h in the presence and absence of 20% glycerol, 1 M trehalose, 0.5 M mannose, or 1 M sucrose. Lyophilization of S2-ALDH samples (100 µL) using a Martin Christ Alpha 1–4 LO plus
Freeze Dryer Lyophilizer was performed in the presence and absence of after adding 0.1 M trehalose and 0.5 M trehalose by freeze-dry for 3 h after incubation of the freshy purified enzyme at
− 20 °C for 16 h. Stability of the lyophilized enzyme was monitored after storage at 4 °C and − 20 °C for 24 h, respectively, by measuring the ALDH activity prior and post treatment under
standard conditions. The residual activity expressed as percentage relative to the ALDH activity of the untreated enzyme was measured under standard conditions. The saturation curves were
determined at 25 °C by alternatively varying the substrate acetaldehyde and cofactor NAD+ concentrations, while keeping saturating (10 mM) the alternative substrate, and fit to the
Michaelis–Menten equation for calculating the KM and Vmax kinetic parameters. SEQUENCE ANALYSES Primary structure analysis of S2-ALDH providing the theoretical molecular weight (MW),
isoelectric point (pI), and aminoacid composition was performed using ExPASy ProtParam platform (https://web.expasy.org/cgi-bin/protparam/protparam)63. Sequence similarity and identity
percentages between the Antarctic enzyme and homologous ALDHs were calculated using Emboss Needle pair alignment tool (http://www.ebi.ac.uk/Tools/psa/emboss_needle/)64. Multiple sequence
alignment of ALDHs primary structures was carried out using the CLUSTAL OMEGA EMBL-EBI (1.2.4) software64. Alignment and phylogenetic analysis of ALDH sequences were performed using the
MAFFT online service version 765,66. Phylogeny was carried out using 15 amino acid sequences with a total of 454 positions after elimination of all positions containing gaps and missing
data. Initial trees for the heuristic search were obtained by applying Neighbor-Join algorithm to a matrix of pairwise distances using a JTT model. The tree topography was evaluated using
the bootstrap analysis of 1000 repetitions. The Itol online service (https://itol.embl.de/) was used to visualize and edit the phylogenetic tree67. ANALYSIS OF WINE SAMPLES BY THE
SPECTROPHOTOMETRIC ALDEHYDE DEHYDROGENASE TEST The wines and the standard acetaldehyde solutions were pretreated immediately before the analysis by filtration through a 1 mL cartridge
containing 0.15 g PVPP and 0.025 g activated charcoal (the optimization of wine pre-treatment is reported in Supplementary Fig. 6S). A commercial kit from Roche (Germany) was used for
evaluating the acetaldehyde quantity by spectrophotometry using the yeast ALDH. Spectrophotometric measurements at 340 nm were performed at room temperature with a UV–VIS Evolution 600
spectrophotometer (Thermo Scientific, Loughborough, United Kingdom) equipped with VISION PRO software. ELECTROCHEMICAL ASSAYS A VSP potentiostat (BioLogic, France) equipped with the EC Lab
software was used for most electrochemical tests. A PalmSens4 potentiostat (PalmSens, Netherlands) equipped with the PSTrace software was also used for the amperometric tests at 17 °C. CNT
electrodes (catalog number DRP 110CNT, Metrohm Dropsens, Spain) consist in 3 coplanar electrodes printed on a ceramic support, with a 4 mm diameter working electrode made of carbon modified
with multi-walled carbon nanotubes, a carbon counter electrode and a silver reference electrode. Carbon electrodes (DRP110, Metrohm Dropsens, Spain) with similar characteristics but with the
working electrode made of bare carbon were also used in some tests. All measurements were performed in triplicate. The temperature and humidity during the electrochemical assays of
acetaldehyde in wines were monitored by a sensor placed in the vicinity of the electrode. Amperometric assays were performed at + 0.5 V with the screen-printed Ag electrode used as
reference. The electrodes were fixed horizontally and the sample was added at 60 s after applying the potential. The analytical signal consisted in the difference between the current
intensity at two different times, t1 and t0, which were optimized depending on the test. For the _c_hronoamperometric measurements of NADH, 75 µL buffer 0.2 M phosphate buffer pH 7.5 were
mixed with 25 µL NADH aqueous solutions of concentrations 2.5–250 µM. The difference between the current intensity at 180 s and 60 s was correlated with the NADH concentration. For
calculating the Km for acetaldehyde, the final concentrations in the electrochemical cell were 7.5 mM NAD+, 0.2 U/mL S2-ALDH and 0.025–12.5 mM acetaldehyde. The difference in current
intensity between 300 and 60 s was correlated with the concentration of acetaldehyde in the sample. For the enzymatic assay of acetaldehyde in wines at 17 °C, 75 µL of S2-ALDH (0.26U/mL) and
NAD+ (10 mM) in 0.2 M phosphate buffer pH 7.5 were mixed with 25 µL solution of acetaldehyde of different concentrations in the range 0.1–10 mM prepared in model wine solution. The
difference in current intensity between 400 and 100 s was taken as the analytical signal. RAMAN ANALYSIS Raman measurements were carried out with SPELEC RAMAN (Metrohm DropSens, Spain), a
compact instrument with a laser source of 785 nm. This instrument was connected to a bifurcated reflection probe (DRP-RAMANPROBE, Metrohm DropSens) and a specific cell for screen-printed
electrodes SPEs (DRP-RAMANCELL, Metrohm DropSens) was used. The SPELEC RAMAN instrument was controlled by DropView SPELEC software. Integration time for raman spectra was 20 s. SCANNING
ELECTRON MICROSCOPY Images of the electrode surfaces were obtained with a JEOL JSM-6100 scanning electron microscope (20 kV, Japan), after the sputtering of 20 nm gold layers over the
samples (Gold sputter coater Balzers SCD004, Liechtenstein). DATA AVAILABILITY All data supporting the conclusions of this article are included in the manuscript. The annotated genome
sequence of _Flavobacterium_ PL002 was deposited in DDBJ/ENA/GenBank under the accession number MQTN00000000.1. The corresponding aminoacid sequence of S2-ALDH used in the current study has
the NCBI Reference Sequence number WP_173857136.1. REFERENCES * Jones, A. W. Measuring and reporting the concentration of acetaldehyde in human breath. _Alcohol Alcohol_ 30, 271–285 (1995).
CAS PubMed Google Scholar * Sinharoy, P., McAllister, S. L., Vasu, M. & Gross, E. R. Environmental aldehyde sources and the health implications of exposure. _Adv. Exp. Med. Biol._
1193, 35–52. https://doi.org/10.1007/978-981-13-6260-6_2 (2019). Article CAS PubMed PubMed Central Google Scholar * Shin, K. S. & Lee, J. H. Acetaldehyde contents and quality
characteristics of commercial alcoholic beverages. _Food Sci. Biotechnol._ 28, 1027–1036. https://doi.org/10.1007/s10068-019-00564-1 (2019). Article CAS PubMed PubMed Central Google
Scholar * Lachenmeier, D. W. & Sohnius, E.-M. The role of acetaldehyde outside ethanol metabolism in the carcinogenicity of alcoholic beverages: Evidence from a large chemical survey.
_Food Chem. Toxicol._ 46, 2903–2911. https://doi.org/10.1016/j.fct.2008.05.034 (2008). Article CAS PubMed Google Scholar * Liu, S.-Q. & Pilone, G. J. An overview of formation and
roles of acetaldehyde in winemaking with emphasis on microbiological implications. _Int. J. Food Sci. Technol._ 35, 49–61. https://doi.org/10.1046/j.1365-2621.2000.00341.x (2000). Article
CAS Google Scholar * Liu, Y., Zhang, X.-K., Shi, Y., Duan, C.-Q. & He, F. Reaction kinetics of the acetaldehyde-mediated condensation between (−)-epicatechin and anthocyanins and their
effects on the color in model wine solutions. _Food Chem._ 283, 315–323. https://doi.org/10.1016/j.foodchem.2018.12.135 (2019). Article CAS PubMed Google Scholar * Bueno, M., Zapata, J.
& Ferreira, V. Simultaneous determination of free and bonded forms of odor-active carbonyls in wine using a headspace solid phase microextraction strategy. _J. Chromatogr. A_ 1369,
33–42. https://doi.org/10.1016/j.chroma.2014.10.004 (2014). Article CAS PubMed Google Scholar * Culleré, L., Ferreira, V. & Cacho, J. Analysis, occurrence and potential sensory
significance of aliphatic aldehydes in white wines. _Food Chem._ 127, 1397–1403. https://doi.org/10.1016/j.foodchem.2011.01.133 (2011). Article CAS PubMed Google Scholar * Kishikawa, N.,
El-Maghrabey, M. H. & Kuroda, N. Chromatographic methods and sample pretreatment techniques for aldehydes determination in biological, food, and environmental samples. _J. Pharm.
Biomed. Anal._ 175, 112782. https://doi.org/10.1016/j.jpba.2019.112782 (2019). Article CAS PubMed Google Scholar * R-Biopharm, B. M. In Product brochure, available online at
0668613Food_a.fm (r-biopharm.com) Vol. product brochure, available online at 0668613Food_a.fm (r-biopharm.com) (2022). * Badalyan, A., Neumann-Schaal, M., Leimkühler, S. & Wollenberger,
U. A biosensor for aromatic aldehydes comprising the mediator dependent PaoABC-aldehyde oxidoreductase. _Electroanalysis_ 25, 101–108. https://doi.org/10.1002/elan.201200362 (2013). Article
CAS Google Scholar * Iitani, K. _et al._ Improved sensitivity of acetaldehyde biosensor by detecting ADH reverse reaction-mediated NADH fluoro-quenching for wine evaluation. _ACS
Sensors_ 2, 940–946. https://doi.org/10.1021/acssensors.7b00184 (2017). Article CAS PubMed Google Scholar * Achmann, S., Hämmerle, M. & Moos, R. Amperometric enzyme-based gas sensor
for formaldehyde: Impact of possible interferences. _Sens. (Basel)_ 8, 1351–1365. https://doi.org/10.3390/s8031351 (2008). Article ADS CAS Google Scholar * Sadeghi, S., Fooladi, E. &
Malekaneh, M. A new amperometric benzaldhyde biosensor based on aldehyde oxidase immobilized on Fe3O4-grapheneoxide/polyvinylpyrrolidone/polyaniline nanocomposite. _Electroanalysis_ 27,
242–252 (2015). Article CAS Google Scholar * Titoiu, A. M. _et al._ Flow injection enzymatic biosensor for aldehydes based on a Meldola Blue-Ni complex electrochemical mediator.
_Microchim. Acta_ 187, 550. https://doi.org/10.1007/s00604-020-04477-3 (2020). Article CAS Google Scholar * Avramescu, A., Noguer, T., Avramescu, M. & Marty, J.-L. Screen-printed
biosensors for the control of wine quality based on lactate and acetaldehyde determination. _Anal. Chim. Acta_ 458, 203–213. https://doi.org/10.1016/S0003-2670(01)01580-X (2002). Article
CAS Google Scholar * Iitani, K., Hayakawa, Y., Toma, K., Arakawa, T. & Mitsubayashi, K. Switchable sniff-cam (gas-imaging system) based on redox reactions of alcohol dehydrogenase for
ethanol and acetaldehyde in exhaled breath. _Talanta_ 197, 249–256. https://doi.org/10.1016/j.talanta.2018.12.070 (2019). Article CAS PubMed Google Scholar * Ghica, M. E., Pauliukaite,
R., Marchand, N., Devic, E. & Brett, C. M. An improved biosensor for acetaldehyde determination using a bienzymatic strategy at poly(neutral red) modified carbon film electrodes. _Anal.
Chim. Acta_ 591, 80–86. https://doi.org/10.1016/j.aca.2007.03.047 (2007). Article CAS PubMed Google Scholar * Marchitti, S. A., Brocker, C., Stagos, D. & Vasiliou, V. Non-P450
aldehyde oxidizing enzymes: The aldehyde dehydrogenase superfamily. _Expert Opin. Drug Metab. Toxicol._ 4, 697–720. https://doi.org/10.1517/17425255.4.6.697 (2008). Article CAS PubMed
PubMed Central Google Scholar * Shortall, K., Djeghader, A., Magner, E. & Soulimane, T. Insights into aldehyde dehydrogenase enzymes: A structural perspective. _Front. Mol. Biosci._ 8,
410 (2021). Article Google Scholar * Liu, D. _et al._ Trends in miniaturized biosensors for point-of-care testing. _TrAC Trends Anal. Chem._ 122, 115701.
https://doi.org/10.1016/j.trac.2019.115701 (2020). Article CAS Google Scholar * Ashrafi, A. M., Bytesnikova, Z., Barek, J., Richtera, L. & Adam, V. A critical comparison of natural
enzymes and nanozymes in biosensing and bioassays. _Biosens. Bioelectron._ 192, 113494. https://doi.org/10.1016/j.bios.2021.113494 (2021). Article CAS PubMed Google Scholar * Kumar, P.
& Sharma, S. Enzymes in green chemistry: The need for environment and sustainability. _Int. J. Appl. Res._ 2, 337–341 (2016). Google Scholar * Deming, J. W. Psychrophiles and polar
regions. _Curr. Opin. Microbiol._ 5, 301–309. https://doi.org/10.1016/s1369-5274(02)00329-6 (2002). Article CAS PubMed Google Scholar * Merino, N. _et al._ Living at the extremes:
Extremophiles and the limits of life in a planetary context. _Front. Microbiol._ 10, 780 (2019). Article Google Scholar * Muthusamy, C. & Sukumaran, R. Marine microbial enzymes.
_Biotechnology_ 2010, 9 (2010). Google Scholar * Seckbach, J.O.A.S.-L.H. _Polyextremophiles : Life Under Multiple Forms of Stress_ (Springer, 2013). Book Google Scholar * Singh, R.,
Kumar, M., Mittal, A. & Mehta, P. K. Microbial enzymes: Industrial progress in 21st century. _3 Biotech_ 6, 174. https://doi.org/10.1007/s13205-016-0485-8 (2016). Article PubMed PubMed
Central Google Scholar * Di Donato, P. _et al._ Exploring marine environments for the identification of extremophiles and their enzymes for sustainable and green bioprocesses.
_Sustainability_ 11, 149 (2019). Article Google Scholar * Turner, P., Mamo, G. & Karlsson, E. N. Potential and utilization of thermophiles and thermostable enzymes in biorefining.
_Microb. Cell Fact._ 6, 9. https://doi.org/10.1186/1475-2859-6-9 (2007). Article CAS PubMed PubMed Central Google Scholar * Gomes, E. _et al._ In _Gene Expression Systems in Fungi:
Advancements and Applications_ (eds Monika Schmoll & Christoph Dattenböck) 459–492 (Springer International Publishing, 2016). * Santiago, M., Ramírez-Sarmiento, C. A., Zamora, R. A.
& Parra, L. P. Discovery, molecular mechanisms, and industrial applications of cold-active enzymes. _Front. Microbiol._ 7, 1408. https://doi.org/10.3389/fmicb.2016.01408 (2016). Article
PubMed PubMed Central Google Scholar * Tropeano, M. _et al._ Culturable heterotrophic bacteria from Potter Cove, Antarctica, and their hydrolytic enzymes production. _Polar Res._ 2012,
31. https://doi.org/10.3402/polar.v31i0.18507 (2012). Article Google Scholar * Danilovich, M., Sánchez, L., Acosta, F. & Delgado, O. Antarctic bioprospecting: In pursuit of
microorganisms producing new antimicrobials and enzymes. _Polar Biol._ 2018, 41. https://doi.org/10.1007/s00300-018-2295-4 (2018). Article Google Scholar * Georlette, D. _et al._ Some like
it cold: Biocatalysis at low temperatures. _FEMS Microbiol. Rev._ 28, 25–42. https://doi.org/10.1016/j.femsre.2003.07.003 (2004). Article CAS PubMed Google Scholar * Mangiagalli, M.,
Brocca, S., Orlando, M. & Lotti, M. The, “cold revolution”. Present and future applications of cold-active enzymes and ice-binding proteins. _New Biotechnol._ 55, 5–11.
https://doi.org/10.1016/j.nbt.2019.09.003 (2020). Article CAS Google Scholar * Siddiqui, K. S. & Cavicchioli, R. Cold-adapted enzymes. _Annu. Rev. Biochem._ 75, 403–433.
https://doi.org/10.1146/annurev.biochem.75.103004.142723 (2006). Article CAS PubMed Google Scholar * Struvay, C. & Feller, G. Optimization to low temperature activity in
psychrophilic enzymes. _Int. J. Mol. Sci._ 13, 11643–11665. https://doi.org/10.3390/ijms130911643 (2012). Article CAS PubMed PubMed Central Google Scholar * Necula-Petrareanu, G. _et
al._ Highly stable, cold-active aldehyde dehydrogenase from the Marine Antarctic _Flavobacterium_ sp. PL002. _Fermentation_ 8, 7 (2022). Article CAS Google Scholar * Titoiu, A. N. _et
al._ Carbon nanofiber and meldola blue based electrochemical sensor for NADH: Application to the detection of benzaldehyde. _Electroanalysis_ 30, 2676–2688.
https://doi.org/10.1002/elan.201800472 (2018). Article CAS Google Scholar * Yamanaka, Y. _et al._ Thermostable aldehyde dehydrogenase from psychrophile, _Cytophaga_ sp. KUC-1:
Enzymological characteristics and functional properties. _Biochem. Biophys. Res. Commun._ 298, 632–637. https://doi.org/10.1016/s0006-291x(02)02523-8 (2002). Article CAS PubMed Google
Scholar * Teoh, C. P. _et al._ Draft genome sequence of _Flavobacterium_ sp. strain PL002, isolated from Antarctic Porphyra Algae. _Microbiol. Resour. Announc._ 2021, 10.
https://doi.org/10.1128/MRA.00063-21 (2021). Article Google Scholar * D’Amico, S., Collins, T., Marx, J. C., Feller, G. & Gerday, C. Psychrophilic microorganisms: Challenges for life.
_EMBO Rep._ 7, 385–389. https://doi.org/10.1038/sj.embor.7400662 (2006). Article CAS PubMed PubMed Central Google Scholar * Marino, S. M. Protein flexibility and cysteine reactivity:
Influence of mobility on the H-bond network and effects on pKa prediction. _Protein J._ 33, 323–336. https://doi.org/10.1007/s10930-014-9564-z (2014). Article CAS PubMed Google Scholar *
Violot, S. _et al._ Structure of a full length psychrophilic cellulase from _Pseudoalteromonas haloplanktis_ revealed by X-ray diffraction and small angle X-ray scattering. _J. Mol. Biol._
348, 1211–1224. https://doi.org/10.1016/j.jmb.2005.03.026 (2005). Article CAS PubMed Google Scholar * Heim, R. & Strehler, E. E. Cloning an _Escherichia coli_ gene encoding a protein
remarkably similar to mammalian aldehyde dehydrogenases. _Gene_ 99, 15–23. https://doi.org/10.1016/0378-1119(91)90028-a (1991). Article CAS PubMed Google Scholar * Halavaty, A. S. _et
al._ Structural and functional analysis of betaine aldehyde dehydrogenase from _Staphylococcus aureus_. _Acta Crystallogr. D Biol. Crystallogr._ 71, 1159–1175.
https://doi.org/10.1107/s1399004715004228 (2015). Article CAS PubMed PubMed Central Google Scholar * Liu, T., Hao, L., Wang, R. & Liu, B. Molecular characterization of a
thermostable aldehyde dehydrogenase (ALDH) from the hyperthermophilic archaeon _Sulfolobus tokodaii_ strain 7. _Extremophiles_ 17, 181–190. https://doi.org/10.1007/s00792-012-0503-7 (2013).
Article CAS PubMed Google Scholar * Hayes, K. _et al._ The quaternary structure of Thermus thermophilus aldehyde dehydrogenase is stabilized by an evolutionary distinct C-terminal arm
extension. _Sci. Rep._ 8, 1 (2018). Article ADS Google Scholar * Bosshard, H. R., Marti, D. N. & Jelesarov, I. Protein stabilization by salt bridges: Concepts, experimental approaches
and clarification of some misunderstandings. _J. Mol. Recogn._ 17, 1–16. https://doi.org/10.1002/jmr.657 (2004). Article CAS Google Scholar * Chen, C. _et al._ Structure-based mutational
studies of substrate inhibition of betaine aldehyde dehydrogenase BetB from _Staphylococcus aureus_. _Appl. Environ. Microbiol._ 80, 3992–4002. https://doi.org/10.1128/aem.00215-14 (2014).
Article ADS PubMed PubMed Central Google Scholar * Steinman, C. R. & Jakoby, W. B. Yeast aldehyde dehydrogenase: II. Properties of the homogeneous enzyme preparations. _J. Biol.
Chem._ 243, 730–734. https://doi.org/10.1016/S0021-9258(19)81726-X (1968). Article CAS PubMed Google Scholar * Koppaka, V. _et al._ Aldehyde dehydrogenase inhibitors: A comprehensive
review of the pharmacology, mechanism of action, substrate specificity, and clinical application. _Pharmacol. Rev._ 64, 520–539. https://doi.org/10.1124/pr.111.005538 (2012). Article CAS
PubMed PubMed Central Google Scholar * Wasilewski, T., Kamysz, W. & Gębicki, J. Bioelectronic tongue: Current status and perspectives. _Biosens. Bioelectron._ 150, 111923.
https://doi.org/10.1016/j.bios.2019.111923 (2020). Article CAS PubMed Google Scholar * Li, X. _et al._ Characterization of a broad-range aldehyde dehydrogenase involved in alkane
degradation in _Geobacillus thermodenitrificans_ NG80-2. _Microbiol. Res._ 165, 706–712. https://doi.org/10.1016/j.micres.2010.01.006 (2010). Article CAS PubMed Google Scholar * Datta,
S., Annapure, U. S. & Timson, D. J. Different specificities of two aldehyde dehydrogenases from _Saccharomyces cerevisiae_ var. boulardii. _Biosci. Rep._ 37, 2.
https://doi.org/10.1042/bsr20160529 (2017). Article Google Scholar * Zhu, J., Wu, X. Y., Shan, D., Yuan, P. X. & Zhang, X. J. Sensitive electrochemical detection of NADH and ethanol at
low potential based on pyrocatechol violet electrodeposited on single walled carbon nanotubes-modified pencil graphite electrode. _Talanta_ 130, 96–102.
https://doi.org/10.1016/j.talanta.2014.06.057 (2014). Article CAS PubMed Google Scholar * Blandón-Naranjo, L. _et al._ Electrochemical behaviour of microwave-assisted oxidized MWCNTs
based disposable electrodes: Proposal of a NADH electrochemical sensor. _Electroanalysis_ 30, 509–516. https://doi.org/10.1002/elan.201700674 (2018). Article CAS Google Scholar * Bucur,
B., Purcarea, C., Andreescu, S. & Vasilescu, A. Addressing the Selectivity of enzyme biosensors: Solutions and perspectives. _Sensors (Basel)_ 21, 3038. https://doi.org/10.3390/s21093038
(2021). Article ADS CAS Google Scholar * Garcia, L. _et al._ Impact of acetaldehyde addition on the sensory perception of syrah red wines. _Foods_ 11, 1693 (2022). Article CAS Google
Scholar * Purcarea, C. _et al._ Aquifex aeolicus aspartate transcarbamoylase, an enzyme specialized for the efficient utilization of unstable carbamoyl phosphate at elevated temperature.
_J. Biol. Chem._ 278, 52924–52934. https://doi.org/10.1074/jbc.M309383200 (2003). Article CAS PubMed Google Scholar * Steinman, C. & Jakoby, W. Yeast aldehyde dehydrogenase. I.
Purification and crystallization. _J. Biol. Chem._ 242, 5019–5023 (1967). Article CAS Google Scholar * Gasteiger, E. _et al._ ExPASy: The proteomics server for in-depth protein knowledge
and analysis. _Nucleic Acids Res._ 31, 3784–3788. https://doi.org/10.1093/nar/gkg563 (2003). Article CAS PubMed PubMed Central Google Scholar * Madeira, F. _et al._ The EMBL-EBI search
and sequence analysis tools APIs in 2019. _Nucleic Acids Res._ 47, W636–W641. https://doi.org/10.1093/nar/gkz268 (2019). Article CAS PubMed PubMed Central Google Scholar * Katoh, K.,
Rozewicki, J. & Yamada, K. D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. _Brief Bioinform._ 20, 1160–1166.
https://doi.org/10.1093/bib/bbx108 (2019). Article CAS PubMed Google Scholar * Kuraku, S., Zmasek, C. M., Nishimura, O. & Katoh, K. aLeaves facilitates on-demand exploration of
metazoan gene family trees on MAFFT sequence alignment server with enhanced interactivity. _Nucleic Acids Res._ 41, W22-28. https://doi.org/10.1093/nar/gkt389 (2013). Article PubMed PubMed
Central Google Scholar * Letunic, I. & Bork, P. Interactive Tree Of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. _Nucleic Acids Res._ 49, W293-w296.
https://doi.org/10.1093/nar/gkab301 (2021). Article CAS PubMed PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS This study was fnancially supported by the UEFISCDI
PN-III-P2-2.1-PED2019-2746 and PN-III-P2-2.1-PED2019-2461 grants and Academia Româna (Grant no. RO1567-IBB05/2021). AUTHOR INFORMATION Author notes * These authors contributed equally: V. I.
Paun and R. M. Banciu. AUTHORS AND AFFILIATIONS * Department of Microbiology, Institute of Biology, 296 Splaiul Independentei, 060031, Bucharest, Romania V. I. Paun & C. Purcarea *
International Centre of Biodynamics, 1B Intrarea Portocalelor, 060101, Bucharest, Romania R. M. Banciu & A. Vasilescu * Departamento de Biotecnología, Facultad de Ciencias del Mar y
Recursos Biológicos, Universidad de Antofagasta, 1240000, Antofagasta, Chile P. Lavin * Metrohm DropSens, S.L.,Vivero de Ciencias de la Salud, C/Colegio Santo Domingo de Guzmán s/n, 33010,
Oviedo, Asturias, Spain P. Fanjul-Bolado Authors * V. I. Paun View author publications You can also search for this author inPubMed Google Scholar * R. M. Banciu View author publications You
can also search for this author inPubMed Google Scholar * P. Lavin View author publications You can also search for this author inPubMed Google Scholar * A. Vasilescu View author
publications You can also search for this author inPubMed Google Scholar * P. Fanjul-Bolado View author publications You can also search for this author inPubMed Google Scholar * C. Purcarea
View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS C.P. and A.V. performed the experimental design, data interpretation and wrote the
article; V.I.P. obtained and characterized the recombinant enzyme; R.M.B. and A.V. executed the electrochemical enzymatic tests and wine samples analyses; P.L. performed the sequence
analyses; P.F.B. characterized the CNT electrodes by Raman and SEM. All authors contributed to data interpretation and revised the manuscript. CORRESPONDING AUTHOR Correspondence to C.
Purcarea. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE Springer Nature remains neutral with regard to
jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under
a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate
credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article
are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons
licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of
this licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Paun, V.I., Banciu, R.M., Lavin, P. _et al._ Antarctic aldehyde
dehydrogenase from _Flavobacterium_ PL002 as a potent catalyst for acetaldehyde determination in wine. _Sci Rep_ 12, 17301 (2022). https://doi.org/10.1038/s41598-022-22289-8 Download
citation * Received: 10 August 2022 * Accepted: 12 October 2022 * Published: 15 October 2022 * DOI: https://doi.org/10.1038/s41598-022-22289-8 SHARE THIS ARTICLE Anyone you share the
following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer
Nature SharedIt content-sharing initiative