Progression detection capabilities of circumpapillary and macular vessel density in advanced glaucomatous eyes
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT This study investigated the progression detection capabilities of circumpapillary and macular vessel density (cpVD and mVD) in advanced primary open angle glaucoma (POAG) eyes using
the rates of change in VD (trend-based analysis) and variability limits derived from healthy eyes. (event-based analysis) This study included 75 POAG eyes [visual field (VF) mean deviation
< − 10 decibels, mean follow-up; 2.3 years] and 33 healthy eyes. Of 75 POAG eyes, 17 (22.7 %) and 58 eyes (77.3 %) were classified into the VF progression and stable groups, respectively.
The VF progression group showed significantly faster VD loss than the stable group. (cpVD; − 1.76 vs. − 0.84 %/year, mVD; − 1.10 vs. − 0.47 %/year, P < 0.05) However, the rates of change
in circumpapillary retinal nerve fiber layer and macular ganglion cell complex thickness were similar between the groups. (cpRNFLT; − 0.67 vs. − 0.53 \(\mu\)m/year, GCCT; − 0.48 vs. − 0.12
\(\mu\)m/year, P > 0.05) Event-based analysis showed stronger agreement between VD and VF progression (cpVD; kappa value (k) = 0.630, mVD; k = 0.667, P < 0.05) than that between
structure and VF progression. (cpRNFLT; k = 0.111, GCCT; k = 0.194, P > 0.05). In conclusion, VD loss showed better progression detection capabilities than structural loss in advanced
POAG eyes. Detection of cpVD and mVD loss may be useful for detecting progression in the advanced stages of POAG to complement other reference standard strategies. SIMILAR CONTENT BEING
VIEWED BY OTHERS FOVEAL AVASCULAR ZONE VESSEL DENSITY IS ASSOCIATED WITH VISUAL FIELD PROGRESSION IN EARLY-STAGE GLAUCOMA EYES WITH CENTRAL VISUAL FIELD DAMAGE Article Open access 25 October
2023 ASSOCIATION OF SUPERFICIAL MACULAR VESSEL DENSITY WITH VISUAL FIELD PROGRESSION IN OPEN-ANGLE GLAUCOMA WITH CENTRAL VISUAL FIELD DAMAGE Article Open access 03 May 2023 DETECTION AND
AGREEMENT OF EVENT-BASED OCT AND OCTA ANALYSIS FOR GLAUCOMA PROGRESSION Article 11 November 2023 INTRODUCTION Glaucoma is defined as progressive optic neuropathy which accompanies
characteristic visual field (VF) deterioration1. Detecting glaucomatous progression is essential to prevent irreversible vision loss. Generally, progressive retinal nerve fiber layer (RNFL)
atrophy or optical coherence tomography (OCT) measured RNFL thinning earlier than the detectable reduction of sensitivity in standard automated perimetry (SAP)2,3,4. Therefore, OCT is
currently the standard method for early detection and has been widely used for glaucoma monitoring based on structural abnormalities such as RNFL and macular ganglion cell-inner plexiform
layer (GC-IPL) thinning. Unfortunately, however, detecting glaucomatous progression using OCT in advanced glaucoma is challenging because RNFL is not sensitive enough to detect glaucomatous
progression as it already reaches the measurement floor5,6. Functional assessment usually fails to accurately detect glaucomatous progression due to greater VF fluctuation in the advanced
stages7,8,9. The recent introduction of OCT angiography (OCTA) has allowed clinicians to noninvasively image the microvasculature of the optic nerve head (ONH), retina, and choroid and
evaluate the perfusion status of these structures10,11,12. Vessel density (VD) assessed by OCTA may be a useful method for diagnosing glaucoma and monitoring glaucomatous
progression13,14,15. VD assessment showed good diagnostic performance, reproducibility and repeatability, which enhances reliability in progression monitoring16. In addition, circumpapillary
and macular VD (cpVD and mVD) are less likely to reach the measurement floor than thickness parameters in advanced glaucoma, suggesting that VD assessment may be more useful than RNFL or
GC-IPL measurements for detecting glaucomatous progression in advanced stages17,18,19,20. Both event- and trend-based analyses are widely used to detect glaucomatous progression. Event
analysis can detect glaucomatous progression with a relatively smaller number of tests compared to trend-based analyses. Further to this, in clinical practice, whether structural and
functional loss exceeds the stable glaucoma population-derived variability limits is important in judging glaucoma progression. The trend-based approach has the advantage of providing the
progression rate. In this study, we investigated the progression detection capabilities of circumpapillary and macular VD in advanced primary open angle glaucoma (POAG) eyes with the rate of
change in VD measurements and via an event-based analysis using the variability limit derived from real-world data. METHODS STUDY SUBJECTS We retrospectively recruited participants by
reviewing the medical records of patients who visited the glaucoma clinic at Asan Medical Center from January of 2017 to April of 2021. This study was approved by the Institutional Review
Board of Asan Medical Center and is compliant with the Declaration of Helsinki. Informed consent from the study subjects was waived by the IRB of Asan Medical Center due to the retrospective
study design. Both healthy controls and POAG patients were recruited for the study. All participants underwent comprehensive ophthalmic examination, including a review of their medical
history, measurement of best-corrected visual acuity (BCVA), intraocular pressure (IOP, Goldmann applanation tonometry), refractive error, axial length (IOL Master version 5; Carl Zeiss
Meditec, Dublin, CA, USA), ultrasound pachymetry for central corneal thickness (CCT) (DGH-550; DGH Technology, Inc., Exton, PA, USA), and slit-lamp microscopy. POAG was diagnosed after an
initial glaucoma workup including dilated fundus ophthalmoscopy, gonioscopy, optic disc stereophotography and red-free RNFL photography (Canon, Tokyo, Japan), VF testing with SAP (Humphrey
field analyzer with Swedish interactive threshold algorithm standard 24–2 test; Carl Zeiss Meditec), spectral-domain optical coherence tomography (SD-OCT) (Spectralis HRA OCT; Heidelberg
Engineering, Heidelberg, Germany), and OCTA (Angiovue; Optovue Inc., Fremont, CA, USA). Inclusion criteria for both healthy and POAG patients were (1) age \(\ge\) 18 years; (2) BCVA \(\ge\)
20/30 and spherical equivalent between − 8.0 and + 3.0 diopters (D), and cylinder correction within \(\pm\) 3 D; and (3) normal anterior chamber and open angle on slit lamp and gonioscopy.
The study exclusion criteria were (1) any history of intraocular surgery (including any cataract/glaucoma), any history of trauma or laser treatment during follow-up; (2) severe media
opacities, including cataracts of more than C2, N2, or P2 based on the Lens Opacities Classification System III21, which obscure the scan image; and (3) ocular diseases other than glaucoma,
such as severe myopic maculopathy, diabetic retinopathy, retinal venous occlusive disease, optic neuritis, or systemic/neurologic disease that could influence the ONH and VF tests. Other
exclusion criteria included unreliable VF results (fixation loss > 20%, false-positive error > 15%, and false-negative error > 15%) and poor-quality OCT/OCTA images (more details on
these exclusion criteria are provided in the OCT/OCTA sections). If both eyes were eligible in any subject, one eye was randomly selected for the study. Inclusion criteria for glaucoma
patients were (1) the presence of a glaucomatous optic disc finding (i.e., the presence of focal neuroretinal rim thinning, notching, localized or diffuse atrophy of RNFL) and glaucomatous
VF defects according to Anderson’s criteria as confirmed by at least two reliable VF examinations [three or more adjacent points on a pattern deviation probability map with P < 0.05 and
one abnormal point with P < 0.01, a glaucoma hemifield test outside the normal limits, or a pattern standard deviation (PSD) of P < 0.05 on two consecutive reliable VF tests
(false-positive errors < 15%, false-negative errors < 15%, and fixation loss < 20%)]22; (2) an average of the initial two VFs mean deviation (MD) measurements of less than − 10
decibels (dB) to include advanced-stage of POAG eyes; and (3) at least five serial VF tests and at least four serial SD-OCT and OCTA scans every 6–12 months during a minimum of two years.
Patients with VF defects resulting from general, neurologic, or any other ophthalmic condition were excluded. The first VF result was excluded to obviate any learning effect. Healthy
controls were individuals who visited our glaucoma clinic for a regular health examination. They were matched to OAG eyes by age (\(\le\) 10 years) and axial length (\(\le\) 1 mm) and were
required to have (1) an IOP < 21 mmHg with no history of elevated IOP; (2) normal appearance of the optic disc and an intact neuroretinal rim; (3) normal RNFL thickness (i.e., average and
quadrant RNFL thickness within 99% confidence limits) based on SD-OCT; (4) normal VFs (i.e., a PSD within 95% confidence limits and a GHT result within the normal limit)22; and (5) OCT and
OCTA scans at least three times every 6–12 months during the follow-up. VESSEL DENSITY, GANGLION CELL COMPLEX THICKNESS, AND RETINAL NERVE FIBER LAYER THICKNESS ACQUISITION All OCTA scans of
macular and ONH were performed using the AngioVue OCTA system (software version 2018.1.0.43, Angiovue; Optovue Inc., Fremont, CA, USA) in the high-definition (HD) retinal, HD optic disc
scan and ganglion cell complex (GCC) scan. This OCTA system utilized the split-spectrum amplitude-decorrelation angiography method to capture the dynamic motion of red blood cells and
provide high-resolution three-dimensional visualization of vascular structures23. Macular whole image vessel density measurements (mVD) were calculated from images of 6 × 6-mm2 scans
centered on the fovea. An automated segmentation algorithm of the AngioVue software was applied to visualize the superficial retinal capillary plexuses [from the internal limiting membrane
(ILM) to the posterior boundary of the inner plexiform layer (IPL)]. The capillary vessel was evaluated from images of 4.5 × 4.5-mm2 scans centered on the ONH within the radial peripapillary
capillary slab from the ILM to the nerve fiber layer after automated removal of any large vessels. cpVD measurements were the percentage of measured area occupied by small vessels within an
instrument-defined 1000-µm-wide elliptical annulus from the optic disc boundary. Macular ganglion cell complex (GCCT) measurements consisting of the ganglion cell layer, IPL, and RNFL were
obtained from a 6-mm-diameter area on the macular centered 1 mm temporal to the fovea. Spectralis SD-OCT (Spectralis HRA OCT; Heidelberg Engineering, Heidelberg, Germany) was used to measure
circumpapillary RNFL thickness (cpRNFLT). Spectralis uses a dual-beam, a confocal laser-scanning ophthalmoscopy, to acquire reference scans for eye-movement tracking and OCT images. cpRNFLT
was measured from a high-resolution 3.46-mm-diameter circular scan centered on the optic disc. All participants underwent OCT, OCTA and VF tests on the same day during follow-up. Three
glaucoma specialists (A.L., J.W.S., and K.R.S.) reviewed all OCT and OCTA images to evaluate image quality. OCT/OCTA images were excluded if they had (1) poor image quality with quality
score < 12 for OCT scans and a signal strength index < 30 for OCTA scans; (2) motion artifacts (i.e., significant residual motion line); (3) localized weak signal intensity caused by
vitreous floaters; (4) poor clarity (i.e., media opacity); (5) fixation error; or (6) segmentation failure. ASSESSMENT OF GLAUCOMA PROGRESSION VISUAL FIELD PROGRESSION Only reliable VFs with
\(\le\) 15% false positives, \(\le\) 15% false negatives, and \(\le\) 20% fixation loss were included in the study. Progression was defined if there was a significant deterioration from the
baseline pattern deviation at more than three of the same test points evaluated on three consecutive examinations based on the Early Manifest Glaucoma Trial (EMGT) criteria (event-based
analysis)24. Only “likely progression” was defined as VF progression. In the case which had too advanced VF MD to detect VF progression based on the EMGT criteria (VF MD < − 16 dB), VF
progression was defined if the linear regression of the MD slope was negative with a P-value less than 0.05. (trend-based analysis) Each eye was classified in the VF progression or stable
groups, respectively. OCT/OCTA-DERIVED PARAMETER PROGRESSION VD, cpRNFLT, and GCCT progression were defined if two consecutive OCT/OCTA tests decreased more than the tolerance limit compared
with the baseline test. For this event-based analysis, the tolerance limit was obtained using the longitudinal variability measurements in healthy eyes, which were the same as the
within-subject standard deviation (Sw) from a single healthy eye if tested multiple times. The tolerance limit was defined as 1.96 × \(\surd 2\) × Sw. Additionally, we determined the
intraclass correlation coefficient (ICC) and coefficient of variation (COV) to assess reproducibility. We calculated ICC by the two-way random effects model using the absolute agreement
definition. The COV, expressed as a percentage (%), was calculated as the square root of the variance divided by the mean of repeated measurements. ICC values ranged from 0–1, where 0
indicates no agreement and 1 perfect agreement between repeated measurements25. The rate of changes in VD, cpRNFLT, GCCT, and VF MD over time were estimated by the trend-based approach using
age-adjusted linear mixed-effects model analysis with random intercepts to account for within-participant variability. STATISTICAL ANALYSIS The distribution normality was assessed with the
Kolmogorov–Smirnov test. Results are demonstrated as a mean and standard deviation or frequency and percentage. The demographic and clinical characteristics of the study subjects were
compared between the VF progression and stable groups and the healthy control group. Comparisons among groups were performed using a one-way analysis of variance with Tukey’s post hoc test
for quantitative variables. Fisher’s exact test was used for categorical variables. Agreement between VF progression and OCT/OCTA-derived parameter progression was assessed by Kappa (k)
statistics. The strength of agreement was categorized per Landis and Koch26: 0 = poor, 0–0.20 = slight, 0.21–0.40 = fair, 0.41–0.60 = moderate, 0.61–0.80 = substantial, and 0.81–1.00 =
almost perfect. All statistical analyses were performed using SPSS ver. 21.0 program (SPSS Inc., Chicago, IL, USA). P-values less than 0.05 were considered statistically significant. RESULTS
GENERAL PARTICIPANT CHARACTERISTICS After an initial review, we included 132 POAG eyes of 132 subjects who met the initial inclusion criteria (92 POAG eyes of 92 participants and 40 healthy
eyes of 40 participants). Of these, we excluded 11 eyes with unreliable VF tests, 10 eyes with poor OCT/OCTA scan quality, one eye with proliferative diabetic retinopathy, and two eyes with
epiretinal membranes. Therefore, 108 eyes consisting of 75 advanced POAG eyes and 33 healthy eyes were included in the final analysis. Mean follow-up duration and the number of visits were
2.3 \(\pm\) 1.0 years and 4.5 \(\pm\) 1.0 visits, respectively, for glaucoma eyes and 1.8 \(\pm\) 0.9 years and 3.3 \(\pm\) 0.6 visits, respectively, for healthy eyes. The baseline VF MD of
the 75 POAG eyes was − 16.39 \(\pm\) 5.02 dB. Table 1 summarizes the demographic and baseline clinical characteristics of the participants with POAG and healthy controls. Among the 75 POAG
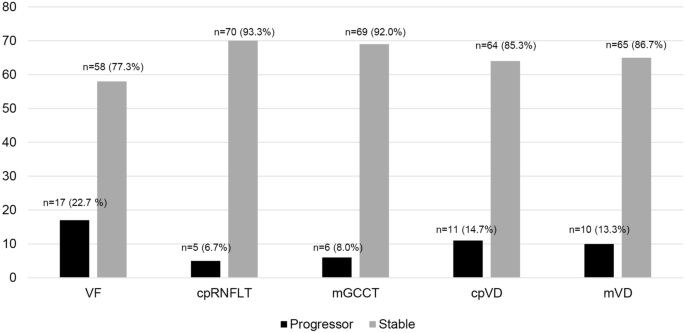
eyes, VF progression was detected in 17 eyes (22.7%) by either event (8 eyes, 10.7%) or trend analysis (9 eyes, 12.0%), and 58 eyes (77.3%) showed stable VF. All OCT/OCTA parameters were
significantly lower in both VF progression and stable groups than in the healthy group (P < 0.05). There was no statistical difference between the VF progression and stable groups in all
parameters, except for the number of glaucoma medications at the first visit (2.2 \(\pm\) 0.7 vs. 1.2 \(\pm\) 1.0, P < 0.001) and final VF MD (− 19.22 \(\pm\) 5.98 vs. − 16.51 \(\pm\)
4.42, P = 0.048). TREND-BASED ANALYSIS Table 2 demonstrates the comparison of the rate of VF MD and OCT/OCTA-derived parameters determined by age-adjusted linear mixed-effects models between
the VF progression and stable groups. Both groups showed a significant reduction in cpVD and mVD (P < 0.05), but there was no significant reduction in cpRNFLT and GCCT (P > 0.05). The
reduction rate of MD (− 1.01 dB/year vs. − 0.19 dB/year, P < 0.001), cpVD (− 1.76%/year vs. − 0.84%/year, P = 0.019), and mVD (− 1.10%/year vs. − 0.47%/year, P = 0.018) were faster in
the VF progression group than in the stable group. EVENT-BASED ANALYSIS All OCT/OCTA-derived parameters in the 33 healthy eyes showed excellent long-term reproducibility with high ICC values
ranging between 0.926–0.991 (Table 3). The tolerance limits of cpRNFLT, GCCT, cpVD, and mVD were \(\pm\) 6.50 \(\mathrm{\mu m}\), \(\pm\) 4.12 \(\mathrm{\mu m}\), \(\pm\) 5.16%, and \(\pm\)
4.96%, respectively. Based on those tolerance limits, progression was determined by each cpRNFLT, GCCT, cpVD, and mVD measurement, respectively. The number of eyes with VF progression [n =
17 (22.7%)] was greater than that of cpRNFLT, GCCT, cpVD, and mVD progression [n = 5 (6.7%), n = 6 (8.0%), n = 11 (14.7%), and n = 10 (13.3%), respectively, Fig. 1]. Agreement between VF
progression and OCT or OCTA-derived parameter progression was determined by kappa statistics (Table 4). The cpVD and mVD progressions showed substantial agreement with VF progression, with
kappa values of 0.630 and 0.667 (P < 0.05), respectively. However, cpRNFLT and GCCT progression showed slight agreement with VF progression, with kappa values of 0.111 and 0.194 (P >
0.05), respectively. Among the 17 eyes which showed VF progression, eight showed VD reduction when VF progression was detected. In one patient (12.5 %), both cpVD and mVD reductions were
detected on the same day visual field progression was detected. Five eyes (62.5 %) showed both mVD and VF progression at the same visit. and this was higher than those showing concurrent
cpVD and VF progression. (two eyes, 25.0 %). REPRESENTATIVE CASES Figures 2 and 3 show representative cases. In Fig. 2A, a 76-year-old man with baseline VF MD, -10.95 dB at baseline,
demonstrated VF progression preceded by cpVD reduction. Significant VF progression (“likely progression”) was detected on September 14, 2018. Approximately four months later, two consecutive
OCTA tests (on September 14, 2018, and January 21, 2019) showed cpVD reduction \(\ge\) the tolerance limit (5.16 %) compared to baseline cpVD. Figure 2B demonstrates a 72-year-old female
with baseline VF MD, − 13.43 dB at baseline who showed significantly decreased cpVD before VF progression was detected. Two consecutive OCTA tests on February 21, 2019, and November 4, 2020,
showed cpVD reduction \(\ge\) the tolerance limit (5.16 %) compared to baseline cpVD. VF progression (“likely progression”) was detected on April 2, 2021, approximately five months later.
In Fig. 3, a 69-year-old female (baseline VF MD, − 11.46 dB) showed mVD reduction (“two consecutive OCTA showed mVD reduction \(\ge\) the tolerance limit [4.96 %] compared to baseline mVD”)
and significant VF progression (“likely progression”) on April 6, 2021. DISCUSSION Detecting progression in advanced glaucomatous eyes is a major challenge in glaucoma management. Previous
studies have assessed the ability of VD to detect progression in advanced stage glaucoma by calculating the long-term rate of change of VD (trend-based analysis)19,20. However, in clinical
practice, whether structural and functional loss exceeds the population-derived variability limits (event-based analysis) is important in determining glaucoma progression. Nonetheless, only
limited information was available on longitudinal VD loss in respect to the event-based analysis in advanced POAG. Therefore, our objective was to evaluate the progression detection
capability of VD parameters compared with OCT-derived parameters in advanced stages of glaucoma using both trend-and event-based analyses. Our trend-based analysis showed that the
perimetrically progressed group had faster rates of cpVD and mVD loss than the stable group in advanced glaucomatous eyes, whereas there was no significant difference in the cpRNFLT and GCCT
thinning rates between the two groups (Table 2). In addition, the event-based analysis showed substantial agreement between VF progression and both cpVD and mVD progression. On the
contrary, both cpRNFLT and GCCT progression showed weaker (“slight”) agreement with VF progression (Table 4). These findings are consistent with previous studies which revealed that
thickness parameters are already close to the end of the dynamic range, but OCTA-measured VD does not approach a measurement floor until an advanced stage of glaucoma. The dynamic range of
OCTA-measured VD is larger than that of the thickness parameters6,17,27,28. The measurement floor of cpRNFLT is around − 10 to − 15 dB of VF MD and that of GCCT is around − 8.3 to − 13.9 dB
of VF MD6,17,28,29,30. After reaching the measurement floor, thickness parameters are no longer sensitive enough to detect glaucomatous progression. Our previous study and others have
reported that macular GCCT measurements are superior to cpRNFLT measurements in monitoring glaucomatous progression5,31,32. However, GCCT was not as sensitive as VD measurements in our
results, which may be because our current POAG eyes were at a significantly more advanced (MD = − 16.39 dB) stage than our previous study31 (MD = − 12.27 dB). Moghimi et al.17 reported that
the OptoVue-measured GCC floor was 70.7 \(\mathrm{\mu m}\) thick and VF MD at the estimated GCC floor was − 13.9 dB. The baseline GCCT and VF MD of the current study exceeded these
measurement floors. However, several study results differed from our study result. Bowd et al.5 measured the mean rates of change in GCIPL thickness in 87 eyes with advanced glaucoma (MD = −
17.0 dB) using Spectralis SD-OCT and found them to be − 0.21 \(\mu\)m/y (P < 0.001), which was significantly different from zero. Belghith et al.32 measured the mean rate of GC-IPL
change using Spectralis SD-OCT and found that it reached statistical significance [− 0.18 \(\mu\)m/y (P = 0.02)] in very advanced cases of glaucoma, with an MD = − 28 dB. The differences in
the mean rate of GC-IPL change between our study and these two studies may be attributable to differences in SD-OCT devices (Spectralis SD-OCT vs. OptoVue SD-OCT). In addition, Bowd et al.5
obtained images only twice in approximately two years and Belghith et al.32 performed SD-OCT scanning annually over 3.5 years. However, only the patients who underwent SD-OCT scanning at
least four times during the follow-up period participated in this study. These differences in analysis strategies may contribute to the different study results. Recent longitudinal studies
reported that VD measurements are useful for evaluating glaucomatous progression, particularly in cases of severe disease19,20. Shin et al.19 revealed that monitoring the rate of
longitudinal cpVD loss may help detect glaucomatous progression in moderate-to-advanced glaucomatous eyes. They examined the rate of longitudinal cpVD and cpRNFLT and their association with
VF progression in 158 POAG eyes with different levels of glaucoma severity. Progressors had a faster rate of change in cpVD than non-progressors, regardless of glaucoma stage, whereas the
rate of cpRNFLT thinning in progressors did not differ with non-progressors at a moderate-to-advanced stage of glaucoma. Hou et al.20 compared the change rate of GCCT and mVD which was
measured within 3 × 3-mm2 scan area in healthy, pre-perimetric glaucoma and POAG eyes. They reported that POAG eyes in the advanced stage showed faster decreases in mVD than GCCT and mVD
loss were significantly correlated with glaucoma severity, in contrast to the GCCT thinning. In our event-based analysis, we used the practical tolerance limits derived from healthy eyes for
defining VD and thickness parameters progression. The tolerance limits were \(\pm\) 6.50 \(\mathrm{\mu m}\) for cpRNFLT, \(\pm\) 4.12 \(\mathrm{\mu m}\) for GCCT, \(\pm\) 5.16 % for cpVD,
and \(\pm\) 4.96 % for VD. These measurements showed excellent long-term test–retest reproducibility with ICCs ranging from 0.926–0.991 in all four parameters (Table 3). The agreement
between progression detection by OCTA-derived parameters-VF progression was stronger (“substantial”) than that of OCT-derived parameters-VF progression (“slight”) (Table 4). These findings
are in line with the results of our trend-based analysis in which VD parameters had a faster rate of change than structural parameters in advanced stages of glaucoma. Previous studies have
reported that various SD-OCT devices showed good repeatability and reproducibility. Benjamin et al.33 evaluated the test–retest variability of RNFL thickness measurements using Spectralis
SD-OCT in 50 normal eyes on a single day and found 0.97 for ICC, 1.7 % for COV, and 4.95 \(\mathrm{\mu m}\) for the test–retest variability. Garas et al.34 evaluated reproducibility of
cpRNFLT using RTVue-100 in 14 normal eyes and reported 0.99 for ICC, 2.6 % for COV and 4.3 \(\mathrm{\mu m}\) for the test–retest variability. Kim et al.35 investigated the long-term
reproducibility of RNFL and GCIPL thickness with Cirrus SD-OCT in clinically stable POAG patients and reported 0.969 (ICC), 3.1 % (COV), and 6.56 \(\mathrm{\mu m}\) (test–retest variability)
for RNFL thickness and 0.989 (ICC), 2.0 % (COV), and 4.02 \(\mathrm{\mu m}\) (test–retest variability) for GC-IPL thickness. Recent studies have reported repeatability and reproducibility
of OCTA-derived VD measurements. Jayasree et al.16 reported repeatability of peripapillary and macular VD in 30 normal eyes using OCTA performed the same day. The mean ICC of the
peripapillary vessel density was 0.86, and the ICC of whole enface macular vessel density was 0.87. The test–retest variability of OCT and OCTA-measured parameters varied in studies
depending on the study design, interval time, and study population. However, our study results showed ICC and COV levels similar with previous studies within an acceptable range. We found
that the number of eyes with VF progression (17 eyes, 22.7%) was higher than that of structural progression (five eyes, 6.7 % for cpRNFL thickness and six eyes, 8.0 % for GCC thickness) in
an event-based analysis. (Fig. 1) This is comparable to previous studies in which functional loss was faster than the structural loss at late-stage glaucoma36,37. In addition, the number of
eyes with VD progression (11 eyes, 14.7 % for cpVD and 10 eyes, 13.3 % for mVD) was higher than that of the thickness parameters (Fig. 1). This result implies that VD progression may be more
apparent than structural progression in advanced glaucoma. The temporal relationship between VD reduction and VF progression is noteworthy. Among the 17 patients with VF progression, eight
patients showed VD reduction during the follow-up. The coincidence of mVD reduction and VF progression (five eyes, 62.5 %) was greater than that of cpVD reduction and VF progression (two
eyes, 25.0 %). This implies that mVD change may have higher temporal concordance with VF progression than cpVD. However, the number of cases was too small for a conclusion, and this finding
should be investigated in future research with more subjects. Our study has several strengths. First, the present study included relatively larger number of advanced glaucoma eyes compared
to previous study of Hou et al.20 which included only 5 advanced OAG eyes. Moreover, our study investigated larger 6 × 6-mm2 macular scans, which have higher diagnostic accuracy and more
information about vascular change of macular area compared to 3 × 3-mm2 scans38,39. Lastly, our study explored the association between vascular and functional glaucomatous progression via
the event-based analyses. The trend-based analysis can provide the rate of change over time, but it requires a longer follow-up and a greater number of tests. Since OCT-A is a relatively
newly adapted device, assessment of accurate rates can be limited. In contrast, an event-based analysis is sensitive for detecting progression with fewer tests and greater variability during
the follow-up40,41. Moreover, the event-based analysis can reflect “real-world” longitudinal data. There are some limitations to this study. First, we had a short follow-up period (2.3
\(\pm\) 1.0 years) and a small number of OCT and OCTA tests (4.5 ± 1.0 visits). Second, the number of VF progressed eyes was small (17 eyes, 22.7 %). Analysis with greater number eyes with
VF progression may have provided more robust outcomes. Further large-scale study is warranted in the future. Third, we used different devices for measurements of cpRNFLT and cpVD. The
Spectralis OCT and AngioVue were used for cpRFNLT and cpVD measurements, respectively. There were differences in image areas and imaging conditions, which may make it difficult to interpret
and directly compare the results. However, cpRNFLT measurement area of the Angiovue (Optovue Inc) is also different with cpVD measurement area, which 3.45-mm radius ring for cpRNFLT and 4.5
× 4.5-mm2 scans for cpVD. Therefore, different image area derived from different devices might not have had a great impact on our results. Fourth, healthy eyes were included in a university
hospital setting, which may have different characteristics than eyes observed in the general population. The tolerance limit derived from healthy eyes should be cautiously applied to a
clinically relevant population. Fifth, we did not evaluate the potential confounding effects of systemic conditions, IOP, and blood pressure-lowering medications on VD measurements. As
ocular hypotensive eyedrops or systemic hypotensive medications may affect ocular blood flow, they may affect the OCTA measurements. Therefore, our results should be interpreted with a
possibility of confounding effects of topical and/or systemic hypotensive medications. Lastly, as our study evaluated a single ethnic group, our data may not be generalizable to the overall
population. Further large-scale studies involving various ethnicities will be needed. In conclusion, we explored glaucomatous progression in advanced-stage glaucoma by both trend and
event-based analysis. In the trend-based analysis, the circumpapillary and macular VD reduction rates in the VF progression group were significantly faster than in the stable group, whereas
the cpRNFLT and GCCT reduction rate did not show a statistical difference between progressors and non-progressors. In the event-based analysis, circumpapillary and macular VD showed stronger
agreement with VF than agreement between thickness parameter and VF progression. These findings suggest that OCTA-derived VD parameters have significantly greater progression detection
capabilities than OCT-derived structural parameters in advanced glaucomatous eyes. DATA AVAILABILITY The datasets generated and analyzed during the current study are available from the
corresponding author on reasonable request. REFERENCES * Weinreb, R. N. & Khaw, P. T. Primary open-angle glaucoma. _Lancet_ 363, 1711–1720 (2004). Article PubMed Google Scholar *
Zeyen, T. G. & Caprioli, J. Progression of disc and field damage in early glaucoma. _Arch. Ophthalmol._ 111, 62–65 (1993). Article CAS PubMed Google Scholar * Sommer, A. _et al._
Clinically detectable nerve fiber atrophy precedes the onset of glaucomatous field loss. _Arch. Ophthalmol._ 109, 77–83 (1991). Article CAS PubMed Google Scholar * Quigley, H. A., Katz,
J., Derick, R. J., Gilbert, D. & Sommer, A. An evaluation of optic disc and nerve fiber layer examinations in monitoring progression of early glaucoma damage. _Ophthalmology_ 99, 19–28
(1992). Article CAS PubMed Google Scholar * Bowd, C., Zangwill, L. M., Weinreb, R. N., Medeiros, F. A. & Belghith, A. Estimating optical coherence tomography structural measurement
floors to improve detection of progression in advanced glaucoma. _Am. J. Ophthalmol._ 175, 37–44 (2017). Article PubMed Google Scholar * Mwanza, J. C. _et al._ Residual and dynamic range
of retinal nerve fiber layer thickness in glaucoma: Comparison of three OCT platforms. _Invest. Ophthalmol. Vis. Sci._ 56, 6344–6351 (2015). Article PubMed PubMed Central Google Scholar
* Tattersall, C. L., Vernon, S. A. & Menon, G. J. Mean deviation fluctuation in eyes with stable Humphrey 24–2 visual fields. _Eye (Lond)_ 21, 362–366 (2007). Article CAS Google
Scholar * Heijl, A., Lindgren, A. & Lindgren, G. Test-retest variability in glaucomatous visual fields. _Am. J. Ophthalmol._ 108, 130–135 (1989). Article CAS PubMed Google Scholar *
Advanced Glaucoma Intervention Study Investigators. Advanced glaucoma intervention study. 2. Visual field test scoring and reliability. _Ophthalmology_ 101, 1445–1455 (1994). Article
Google Scholar * Jia, Y. _et al._ Optical coherence tomography angiography of optic disc perfusion in glaucoma. _Ophthalmology_ 121, 1322–1332 (2014). Article PubMed Google Scholar *
Park, H. Y., Shin, D. Y., Jeon, S. J. & Park, C. K. Association between parapapillary choroidal vessel density measured with optical coherence tomography angiography and future visual
field progression in patients with glaucoma. _JAMA Ophthalmol._ 137, 681–688 (2019). Article PubMed PubMed Central Google Scholar * Moghimi, S. _et al._ Macular and optic nerve head
vessel density and progressive retinal nerve fiber layer loss in glaucoma. _Ophthalmology_ 125, 1720–1728 (2018). Article PubMed Google Scholar * Chung, J. K., Hwang, Y. H., Wi, J. M.,
Kim, M. & Jung, J. J. Glaucoma diagnostic ability of the optical coherence tomography angiography vessel density parameters. _Curr. Eye Res._ 42, 1458–1467 (2017). Article PubMed
Google Scholar * Lu, P. _et al._ Quantitative analysis of microvasculature in macular and peripapillary regions in early primary open-angle glaucoma. _Curr. Eye Res._ 45, 629–635 (2020).
Article CAS PubMed Google Scholar * Rao, H. L. _et al._ Relationship of optic nerve structure and function to peripapillary vessel density measurements of optical coherence tomography
angiography in glaucoma. _J. Glaucoma_ 26, 548–554 (2017). Article PubMed Google Scholar * Venugopal, J. P. _et al._ Repeatability of vessel density measurements of optical coherence
tomography angiography in normal and glaucoma eyes. _Br. J. Ophthalmol._ 102, 352–357 (2018). Article PubMed Google Scholar * Moghimi, S. _et al._ Measurement floors and dynamic ranges of
OCT and OCT angiography in glaucoma. _Ophthalmology_ 126, 980–988 (2019). Article PubMed Google Scholar * Ghahari, E. _et al._ Association of macular and circumpapillary microvasculature
with visual field sensitivity in advanced glaucoma. _Am. J. Ophthalmol._ 204, 51–61 (2019). Article PubMed PubMed Central Google Scholar * Shin, J. W., Song, M. K. & Kook, M. S.
Association between progressive retinal capillary density loss and visual field progression in open-angle glaucoma patients according to disease stage. _Am. J. Ophthalmol._ 226, 137–147
(2021). Article PubMed Google Scholar * Hou, H. _et al._ Ganglion cell complex thickness and macular vessel density loss in primary open-angle glaucoma. _Ophthalmology_ 127, 1043–1052
(2020). Article PubMed Google Scholar * Chylack, L. T. Jr. _et al._ The lens opacities classification system III. The longitudinal study of cataract study group. _Arch. Ophthalmol._ 111,
831–836 (1993). Article PubMed Google Scholar * Anderson, D. R. Collaborative normal tension glaucoma study. _Curr. Opin. Ophthalmol._ 14, 86–90 (2003). Article PubMed Google Scholar *
Jia, Y. _et al._ Quantitative optical coherence tomography angiography of vascular abnormalities in the living human eye. _Proc. Natl. Acad. Sci. U. S. A._ 112, E2395–E2402 (2015). Article
CAS PubMed PubMed Central Google Scholar * Leske, M. C., Heijl, A., Hyman, L. & Bengtsson, B. Early manifest glaucoma trial: Design and baseline data. _Ophthalmology_ 106,
2144–2153 (1999). Article CAS PubMed Google Scholar * Bland, J. M. & Altman, D. G. Statistics notes: Measurement error. _BMJ_ 312, 1654 (1996). Article CAS PubMed PubMed Central
Google Scholar * Landis, J. R. & Koch, G. G. The measurement of observer agreement for categorical data. _Biometrics_ 33, 159–174 (1977). Article CAS PubMed MATH Google Scholar *
Blumenthal, E. Z. _et al._ Correlating structure with function in end-stage glaucoma. _Ophthalm. Surg. Lasers Imaging_ 37, 218–223 (2006). Article Google Scholar * Miraftabi, A. _et al._
Macular SD-OCT outcome measures: Comparison of local structure-function relationships and dynamic range. _Invest. Ophthalmol. Vis. Sci._ 57, 4815–4823 (2016). Article PubMed PubMed Central
Google Scholar * Mwanza, J. C. _et al._ Retinal nerve fibre layer thickness floor and corresponding functional loss in glaucoma. _Br. J. Ophthalmol._ 99, 732–737 (2015). Article PubMed
Google Scholar * Hood, D. C., Anderson, S. C., Wall, M. & Kardon, R. H. Structure versus function in glaucoma: An application of a linear model. _Invest. Ophthalmol. Vis. Sci._ 48,
3662–3668 (2007). Article PubMed Google Scholar * Shin, J. W., Sung, K. R., Lee, G. C., Durbin, M. K. & Cheng, D. Ganglion cell-inner plexiform layer change detected by optical
coherence tomography indicates progression in advanced glaucoma. _Ophthalmology_ 124, 1466–1474 (2017). Article PubMed Google Scholar * Belghith, A. _et al._ Structural change can be
detected in advanced-glaucoma eyes. _Invest. Ophthalmol. Vis. Sci._ 57, 511–518 (2016). Article Google Scholar * Tan, B. B., Natividad, M., Chua, K. C. & Yip, L. W. Comparison of
retinal nerve fiber layer measurement between 2 spectral domain OCT instruments. _J. Glaucoma_ 21, 266–273 (2012). Article PubMed Google Scholar * Garas, A., Vargha, P. & Holló, G.
Reproducibility of retinal nerve fiber layer and macular thickness measurement with the RTVue-100 optical coherence tomograph. _Ophthalmology_ 117, 738–746 (2010). Article PubMed Google
Scholar * Kim, K. E., Yoo, B. W., Jeoung, J. W. & Park, K. H. Long-term reproducibility of macular ganglion cell analysis in clinically stable glaucoma patients. _Invest. Ophthalmol.
Vis. Sci._ 56, 4857–4864 (2015). Article PubMed Google Scholar * Bartz-Schmidt, K. U., Thumann, G., Jonescu-Cuypers, C. P. & Krieglstein, G. K. Quantitative morphologic and functional
evaluation of the optic nerve head in chronic open-angle glaucoma. _Surv. Ophthalmol._ 44(Suppl 1), S41–S53 (1999). Article PubMed Google Scholar * Jonas, J. B. & Gründler, A. E.
Correlation between mean visual field loss and morphometric optic disk variables in the open-angle glaucomas. _Am. J. Ophthalmol._ 124, 488–497 (1997). Article CAS PubMed Google Scholar
* Takusagawa, H. L. _et al._ Projection-resolved optical coherence tomography angiography of macular retinal circulation in glaucoma. _Ophthalmology_ 124, 1589–1599 (2017). Article PubMed
Google Scholar * Rao, H. L. _et al._ Diagnostic abilities of the optical microangiography parameters of the 3x3 mm and 6x6 mm macular scans in glaucoma. _J. Glaucoma_ 27, 496–503 (2018).
Article PubMed Google Scholar * Caprioli, J. The importance of rates in glaucoma. _Am. J. Ophthalmol._ 145, 191–192 (2008). Article PubMed Google Scholar * Casas-Llera, P. _et al._
Visual field index rate and event-based glaucoma progression analysis: comparison in a glaucoma population. _Br. J. Ophthalmol._ 93, 1576–1579 (2009). Article CAS PubMed Google Scholar
Download references AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Ophthalmology, College of Medicine, University of Ulsan, Asan Medical Center, 388-1 Pungnap-2-dong, Songpa-gu,
Seoul, 138-736, Korea Anna Lee, Kyung Rim Sung & Joong Won Shin Authors * Anna Lee View author publications You can also search for this author inPubMed Google Scholar * Kyung Rim Sung
View author publications You can also search for this author inPubMed Google Scholar * Joong Won Shin View author publications You can also search for this author inPubMed Google Scholar
CONTRIBUTIONS A.L., K.R.S., and J.W.S. wrote the main manuscript text and A.L. and K.R.S. prepared all figures. All authors reviewed the manuscript. CORRESPONDING AUTHOR Correspondence to
Kyung Rim Sung. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE Springer Nature remains neutral with regard
to jurisdictional claims in published maps and institutional affiliations. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International
License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source,
provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative
Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not
permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Lee, A., Sung, K.R. & Shin, J.W. Progression detection capabilities of
circumpapillary and macular vessel density in advanced glaucomatous eyes. _Sci Rep_ 12, 12109 (2022). https://doi.org/10.1038/s41598-022-16083-9 Download citation * Received: 02 March 2022 *
Accepted: 04 July 2022 * Published: 15 July 2022 * DOI: https://doi.org/10.1038/s41598-022-16083-9 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this
content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative