Cardiometabolic profile and leukocyte telomere length in a black south african population
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Several studies have reported a possible association between leucocyte telomere length (LTL) and cardio-metabolic diseases (CMDs). However, studies investigating such association
are lacking in South Africa despite having a very high prevalence of CMDs. We investigated the association between LTL and CMD risk profile in a black South African population. This was a
cross-sectional study with participants > 21 years of age and residing in five townships in Cape Town. CMD markers were compared between men and women and across quartiles of LTL. Linear
and logistic regressions relate increasing quartile and Log10LTL with CMD risk profile, with appropriate adjustment. Among 676-participants, diabetes, obesity and hypertension prevalence
were 11.5%, 23.1% and 47.5%. Waist-circumference, hip-circumference and highly sensitive c-reactive protein values were significantly higher in women (all _p_ < 0.001), while HDL-C (_p_ =
0.023), creatinine (_p_ = 0.005) and gamma glutamyl transferase (_p_ < 0.001) values were higher in men. In age, sex and BMI adjusted linear regression model, Log10 of LTL was associated
with low HDL-C (beta = 0.221; _p_ = 0.041) while logistic regression showed a significant association between Log10LTL and prevalent dyslipidaemia characterised by high LDL-C. In this
population, the relationship between LTL and CMD is weak given its association with only HDL-C and LDL-C. SIMILAR CONTENT BEING VIEWED BY OTHERS RELATIONSHIP BETWEEN AGING AND CONTROL OF
METABOLIC SYNDROME WITH TELOMERE SHORTENING: A CROSS-SECTIONAL STUDY Article Open access 19 October 2023 SARCOPENIC OBESITY IS ASSOCIATED WITH TELOMERE SHORTENING: FINDINGS FROM THE NHANES
1999–2002 Article Open access 04 November 2021 COMPARISON OF TRIGLYCERIDE-GLUCOSE RELATED INDICES IN PREDICTION OF CARDIOMETABOLIC DISEASE INCIDENCE AMONG US MIDLIFE WOMEN Article Open
access 03 June 2025 INTRODUCTION Telomeres are specialized chromosomal DNA–protein structures that make up the terminal regions of chromosomes and are formed of a highly conserved hexameric
(TTAGGG) tandem repeat DNA sequence. Their primary function is to protect and stabilize the genetic material carried by chromosomes in several ways. They shield the chromosomal termini from
recognition by the DNA damage response system of the cell, cap chromosome ends thereby preventing them from being degraded or fused together. The length of the telomere is longest at birth
and begins to decline within the first 6 weeks of life. In addition to chronological age, there is a wide range of genetic and environmental factors modulating telomere length (TL). Gender1,
ethnicity2 and level of physical activity3 have been shown to modulate TL. Moreover, short TL has been shown to be associated with type 2 diabetes in Caucasian, South Asian and
Afro-Caribbean4, Insulin Dependent Diabetes Mellitus (IDDM) in white American men5, gestational diabetes mellitus in Chinese population6, coronary artery disease in Chinese men7, vascular
dementia in German population8, cardiovascular disease9,10 and hypertension11 in the Adult United State population. This large body of evidence therefore suggests that there is a possible
relationship between TL and cardiometabolic diseases (CMDs). South Africa is facing a quadruple burden of diseases including infectious diseases such as HIV and tuberculosis; maternal and
child conditions; non-communicable diseases (NCDs); and violence, injuries and trauma12. CMDs, the leading NCDs, are among the top ten causes of death in South Africa12. Accordingly,
identification of biomarkers of CMDs is essential for early diagnosis and appropriate management of these conditions especially in black South Africans marked by higher prevalence of CMDs.
Given that CMDs have been shown to be associated with leucocyte telomere length (LTL) in European, American and Asian populations, we therefore undertook this study to investigate the
association between LTL and cardio-metabolic profile in a black South African population. RESULTS Out of the 1116 participants examined in this study, DNA extracted from stored samples for
analysis were available for 676 participants. This number resulted from samples with LTL values which could only be quantified on good quality DNA sample (absorbance 260 nm/280 nm ratio
between 1.7 and 2.0). The prevalence of the cardiometabolic disorders was 47.5% for obesity, 23.1% for hypertension and 11.5% for type 2 diabetes (Table 1). Weight, BMI, heart rate, WC, HC
and hs-CRP (all _p_ < 0.001) median values were higher in women than men while height (_p_ < 0.001), HDL-C (_p_ = 0.023), creatinine (_p_ = 0.005) and GGT (_p_ < 0.001) values were
significantly higher in men than women (Table 1). Moreover, more women than men were obese by all parameters measured [BMI (_p_ < 0.001), WC (_p_ < 0.001), WHR (_p_ = 0.012) and WHtR
(_p_ < 0.001)] and had dyslipidaemia [high triglycerides (_p_ = 0.003), high LDL-C values (_p_ = 0.009) and high non-HDL-C values (_p_ = 0.03)]. The distribution of TL was skewed to the
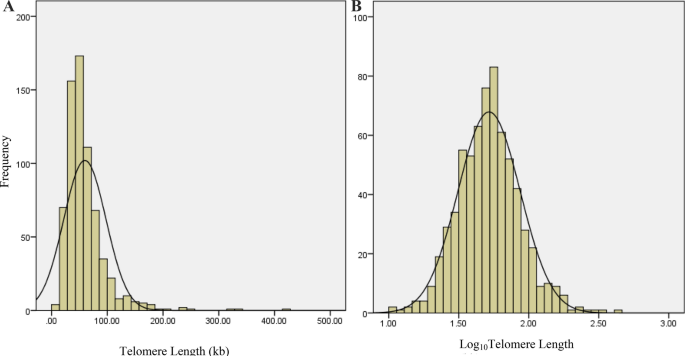
right (skewness = 3.45), indicating that TL values were not normally distributed (Fig. 1A). In order to have a normal distribution of TL, it was Log10 transformed (Fig. 1B). Correlation
analysis showed a significant positive association between LTL and HDL-C (r = 0.082, _p_ = 0.034) (Fig. 2A). In women, LTL was negatively correlated with LDL-C (r = − 0.1, _p_ = 0.037)
(Fig. 2B) and non-HDL-C (r = − 0.101, _p_ = 0.034) (Fig. 2C). In men, there was a significant positive correlation between LTL and Urea (r = 0.179, _p_ = 0.007) (Fig. 2D). The association
between LTL and cardiometabolic profile was investigated by categorizing the LTL values into quartiles with the first being the lowest and the fourth being the highest (Table 2). Amongst the
different cardio-metabolic parameters investigated, quartiles of LTL were shown to be positively and significantly associated with HDL-C (Table 3). Linear regression carried out in age and
sex adjusted model showed a significant association between Log10 LTL and HDL-C (beta = 0.028; _p_ = 0.041) (Table 4) while increasing quartiles of LTL was not associated with HDL-C in
linear regression models with similar levels of adjustment. Neither increasing quartiles of LTL nor Log10 LTL was associated with other cardio-metabolic parameters in age and sex adjusted
linear regression models. In logistic regressions, there were no associations between quartiles of LTL and diabetes, hypertension, any dyslipidaemia and obesity variables. However, when LTL
was Log transformed, there was a significant association between Log10 LTL and prevalent dyslipidaemia with High LDL-C > 3.0 mmol/L (OR = 0.41, _p_ = 0.045) (Table 5). DISCUSSION This
study examined the associations of LTL with cardiometabolic variables of adiposity, hypertension, type 2 diabetes and dyslipidaemia in a black urban South African population. Linear
regression carried out in age and sex adjusted model showed a significant association between Log10LTL and HDL-C as continuous variable. In logistic regression model, Log10LTL was associated
with prevalent dyslipidaemia characterized by high LDL-C. Correlation analysis showed that LTL was associated with Total Cholesterol and non-HDL-C in women and urea in men. However, neither
quartiles of LTL nor Log transformed values were associated with hypertension, obesity and type 2 diabetes, which was surprising and warrants further exploration in this population. The
association between LTL and some lipid parameters in the study suggests a possible but weak relationship between shortened TL and dyslipidaemia. Similar results were obtained in the United
States13 and in Iran14. Dyslipidaemia, characterised by altered serum lipid levels is associated with several disease conditions including coronary heart disease, hypertension, diabetes,
obesity and oxidative stress which is related with LTL shortening. Although most published literature reported a positive association between short telomere length and a high prevalence of
diabetes, obesity, hypertension and other cardiovascular diseases, in this study there were no associations between LTL with diabetes, hypertension or obesity. A meta-analysis of multiple
studies found a significant negative association between LTL and diabetes15 while a systematic review reported a weak to moderate association between obesity and telomere length16. Short TL
was also positively correlated with high SBP and DBP11, high fasting glycaemia17, altered lipid profile markers6 and cardiovascular diseases10,18,19,20. The proposed pathway of the
association between shortened TL and CMDs reported is bi-directional with cardio-metabolic diseases causing shortening of telomere length and short telomere length increasing the risk of
cardio-metabolic diseases. However, these studies have all been carried out in European, Asian and American population, with no studies from Africa, suggesting that the lack of association
in our study could be as a result of population differences. Moreover, the sample size of these cross-sectional studies and systematic reviews/meta-analysis was large enough (minimum >
5000) compared to 676 in our study. Therefore, their studies had more power to detect differences compared to our study. It is possible that LTL could also be determined by the origin and
evolution of individuals. Hansen reported shorter TL in Europeans and African Americans originating from Western Africa compared to those living in Africa originating from Tanzania (Eastern
Africa)21. These results are consistent with other studies reporting longer telomere length in Black Africans compared to white Europeans and Americans in both children and
adult22,23,24,25,26,27. However, TL was observed to be longer in white compared to black teachers in South Africa28. In this South African population, the risk of cardiovascular disease was
higher in black teachers that white teachers28. These results shows that genetic differences between ethnic groups and environmental factors ‘contribute’ to overall telomere length. Unlike
this study findings showing no association between TL and age, several studies have reported a significant negative correlation between TL and chronological age. TL shortens with age and age
associated disorders. A systematic review showed an average decrease of 21.91 base pair TL/year across cross-sectional studies and 32.2–45.5 base pair TL/year in 5 longitudinal studies29.
Another important factor that affects TL is sex with several studies showing TL to be longer in women than men9,30,31,32,33. However, in the present study there was no association between
LTL and gender. Even though the present study was carried out in an African population which is different from studies reporting an association between TL and age/gender (Asia, Europe and
USA) and could probably explain the difference in the results, further research is needed to explore these associations in Africans. Moreover, the black South African population in which the
study was carried out is genetically diverse with some having gene flow from Europe, East Africa and South Asia34. This genetic diversity in the study population could be responsible for
the difference in the results obtained. The cross-sectional design of the study prevents conclusions on a causal relationship between LTL and the CMD risk factors investigated. The small
sample size of the study and the low sample realisation in men (34%) characteristic of epidemiological studies in this country and probably due to their reluctance to participate,
particularly for the drawing of blood samples is another limitation. In this study, multiple hypothesis testing (MHT) was not performed. As such, the results would not withstand a stringent
MHT based correction which constitute a possible limitation. In a black urban South African population, LTL was weakly associated with HDL-C and LDL-C, but not with diabetes, hypertension or
obesity. Although the association of LTL with cardiometabolic risk factors have been reported in many populations, there is a paucity of data in African populations. Further research,
particularly in longitudinal studies, is required to clearly elucidate the relationship between LTL and CMDs in Africans. MATERIALS AND METHODS STUDY SITE AND POPULATION Participants
consisted of > 21 years old black men and women residing in Cape Town. This cross-sectional study titled Cardiovascular Risk in Black South Africans (CRIBSA) was conducted in 2008–2009
with data collected by a 3-stage cluster sampling as previously described35. This sampling technique was used with quotas, which were pre-specified by age and sex categories to ensure a
representative sample, Recruitment took place during office hours and those excluded were the following: pregnant and lactating women, individuals who were bedridden, unable to give consent,
on tuberculosis treatment, on antiretroviral therapy, cancer patients having received treatment within the last year and individuals residing in Cape Town for less than three months. Ethics
approval was obtained from the South African Medical Research Council’s Human Research Ethics Committee (EC026-9/2016) and the University of Cape Town’s Research and Ethics Committee
(224/2006), and informed consent was obtained from all participants. The research was performed in accordance with the declaration of Helsinki and all methods carried out with relevant
guidelines and regulations. DATA COLLECTION Data, which were collected by trained fieldworkers, included administered questionnaires, clinical examinations and biochemical analyses. Clinical
examinations comprised anthropometry (height, weight, and waist and hip circumferences) and blood pressure (BP) measured using standard techniques36. A calibrated scale was used to measure
weight to the nearest 0.5 kg with each participant barefoot and in light clothing. A stadiometer was used to measure height to the nearest 0.1 cm. A flexible tape measured waist and hip
circumferences to the nearest 0.1 cm. For waist circumference (WC), the tape was placed approximately 2 cm above the umbilicus while hip circumference (HC) was measured at the maximum
posterior protuberance of the buttocks with the participant standing upright with feet together. Three BP measurements were taken at intervals of 2 min, using an Omron BP monitor after the
participant had been rested for at-least 5 min. The average of the second and third BP measurements was used for analysis. After an overnight fast of approximately 10 h, blood samples were
collected by venepuncture into EDTA and dry tubes and a portion processed for biochemical analysis. Plasma glucose (hexokinase) was measured using a colorimetric method according to the
manufacturer’s protocol. Total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C) and triglycerides were measured in serum using standard enzymatic techniques37,38,39.
Low-density lipoprotein cholesterol (LDL-C) was calculated using the Friedewald formula40, while non-HDL-C was calculated using the formula: TC–HDL-C. An oral glucose tolerance test (OGTT)
was administered with blood samples collected 2 h after a glucose load41. All colorimetric measurements were conducted using a Beckman Coulter AU 500 spectrophotometer. Serum creatinine
(CAYMAN CHEMICAL), gamma glutamyl transferase (Abcam) and highly sensitive c-reactive protein (hs-CRP) (BIOMATIK ELISA) measurements were conducted on stored serum samples according to the
manufacturer’s protocol. TL assay was conducted from DNA samples extracted from whole blood stored at −80 °C in EDTA tubes using the salt extraction technique. Briefly, 5 mL blood samples in
EDTA tubes were defrosted to room temperature and poured into a 50 mL centrifuge tube. Thirty mL lysis buffer (see supplementary material) was added, and red blood cells were lysed by
incubation on ice and vortexing. After lysis of red blood cells, the pellets were washed thrice with phosphate buffered saline (see supplementary material) which was later discarded. The
pellets were then incubated with nuclear lysis buffer (see supplementary material) overnight at 60 °C. The next day, the supernatant was collected, and the proteins precipitated using 1 mL
saturated sodium chloride (6 M) solution. The supernatant containing the DNA was collected into new 15 mL centrifuge tubes and absolute ethanol added to precipitate the DNA by inversion.
Precipitated DNA was removed and washed with 70% ethanol. After washing, the precipitate was dissolved in Tris Ethylene Diamine Tetra-Acetate buffer (see supplementary material) and the
concentration and quality of the DNA measured using a Nano drop. All samples with absorbance 260 nm/280 nm ratio from 1.7 to 2 were diluted to 5 mg/mL using polymerase chain reaction (PCR)
grade water and TL measured by quantitative real time PCR using the method described by O’Callaghan and Fenech42. Serial dilutions of the telomere standard and the single copy gene (36B4)
standard were made as described by O’Callaghan and Fenech42. A master mix solution containing Power SYBR I (AmpliTaq Gold DNA polymerase, dNTPs, SYBR I Green Dye, optimised buffers and
passive reference dye (ROX) (10μL, 1×)), forward primer (1μL, 0.1 μM), reverse primer (1μL, 0.1 μM) and ddH2O (4uL) was prepared, mixed well and briefly centrifuged. Using a multichannel
pipetted, 16 μL master mix solution were pipetted into each well of a 96 well plate. Into the corresponding wells were added 4 μL each of DNA sample, standards, positive and non-template
control (distilled water) in duplicates. The plate was sealed with an optical clear film, centrifuged briefly and run in a QuantStudio 7 Flex Real Time PCR Thermocycler using the following
PCR conditions; 10 min at 95 °C, followed by 40 cycles of 95 °C for 15 s 60 °C for 1 min, followed by a dissociation (or melt) curve. At the end of the run, the plate was removed and
discarded. Each sample was amplified twice, using telomere forward and reverse primers and the single copy gene forward and reverse primers. After amplification was completed the AB software
produced a value for each reaction that is equivalent to kb/reaction based on the telomere standard curve values. The kb/reaction for telomere and genome copies/reaction for diploid genome
copy values were exported and used to calculate the LTL in kilobase (kb) as follows; LTL =
\(\frac{{{\text{telomere}}\;{\text{kilobase}}\;{\text{per}}\;{\text{reaction}}\;{\text{value}}}}{{{\text{diploid}}\;{\text{genome}}\;{\text{copy}}\;{\text{number}}}}\). DEFINITIONS Body mass
index (BMI), calculated as weight (kg)/height (m) squared, classified participants into three categories of generalised adiposity: normal weight (18 ≤ BMI < 25 kg/m2), overweight (25 ≤
BMI < 30 kg/m2) and obese (BMI ≥ 30 kg/m2)43. Central obesity was determined using the following criteria: WC > 94 cm in men and > 80 cm in women, waist-to-hip ratio (WHR) ≥ 0.9 in
men and ≥ 0.85 in women44 or waist-to-height ratio (WHtR) > 0.545. Hypertension was defined as systolic BP (SBP) ≥ 140 mmHg or diastolic BP (DBP) ≥ 90 mmHg or known hypertension on
treatment46. Dyslipidaemia was defined as TC > 5 mmol/l, triglycerides > 1.5 mmol/L, HDL-C < 1.2 mmol/L, LDL-C > 3.0 mmol/L and non-HDL-C > 3.37 mmol/L or taking anti-lipid
agents47. Diabetes was defined as fasting plasma glucose ≥ 7.0 mmol/L and/ or 2-h post glucose load ≥ 11.1 mmol/L, previously diagnosed or taking antidiabetic medications41. STATISTICAL
ANALYSIS Data analysis was carried out using SPSS Version 21 software. Continuous variables are presented as medians (25th to 75th percentiles) and categorical variables as counts
(percentages). Mann Whitney U test was used to compare baseline characteristics by sex. TL was categorised into quartiles and the linear trend in CMD profile (continuous variables) across
the different quartiles of TL was computed using the median test. Similarly, chi square test was computed and the linear-by-linear association used to compare the trend in proportions of
disease conditions (categorical variable) across the quartiles of TL. Spearman correlation was used to assess the association between quartile of TL and cardio-metabolic parameters. The
interactions between TL categories and cardio-metabolic risk profile were tested using linear and logistic regressions, by incorporating in the same model the main effects of the variables
of interest as well as their interaction term with TL. In linear and logistic regression analyses, TL was log transformed. A _p_ value < 0.05 was considered statistically significant.
DATA AVAILABILITY The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request. REFERENCES * Willeit, P. _et al._
Cellular aging reflected by leukocyte telomere length predicts advanced atherosclerosis and cardiovascular disease risk. _Arterioscler. Thromb. Vasc. Biol._ 30, 1649–1656 (2010). Article
CAS PubMed Google Scholar * Diez Roux, A. V. _et al._ Race/ethnicity and telomere length in the multi-ethnic study of atherosclerosis. _Aging Cell_ 8, 251–257 (2009). Article CAS PubMed
Google Scholar * Puterman, E. _et al._ The power of exercise: Buffering the effect of chronic stress on telomere length. _PLoS ONE_ 5, e10837 (2010). Article ADS PubMed PubMed Central
Google Scholar * Salpea, K. D. _et al._ Association of telomere length with type 2 diabetes, oxidative stress and ucp2 gene variation. _Atherosclerosis_ 209, 42–50 (2010). Article CAS
PubMed PubMed Central Google Scholar * Jeanclos, E. _et al._ Shortened telomere length in white blood cells of patients with iddm. _Diabetes_ 47, 482–486 (1998). Article CAS PubMed
Google Scholar * Weng, Q. _et al._ Leukocyte telomere length, lipid parameters and gestational diabetes risk: A case-control study in a Chinese population. _Sci. Rep._ 9, 8483.
https://doi.org/10.1038/s41598-019-44968-9 (2019). Article ADS CAS PubMed PubMed Central Google Scholar * Wang, Y. Y. _et al._ Association of shorter mean telomere length with large
artery stiffness in patients with coronary heart disease. _Aging Male_ 14, 27–32 (2011). Article PubMed Google Scholar * Von Zglinicki, T. _et al._ short telomeres in patients with
vascular dementia: An indicator of low antioxidative capacity and a possible risk factor?. _Lab. Investig._ 80, 1739–1747 (2000). Article Google Scholar * Fitzpatrick, A. L. _et al._
Leukocyte telomere length and cardiovascular disease in the cardiovascular health study. _Am. J. Epidemiol._ 165, 14–21 (2007). Article PubMed Google Scholar * Xu, C. _et al._ Association
between leucocyte telomere length and cardiovascular disease in a large general population in the united states. _Sci. Rep._ https://doi.org/10.1038/s41598-019-57050-1 (2020). Article
PubMed PubMed Central Google Scholar * Huang, Y.-Q. _et al._ The relationship between mean telomere length and blood pressure: Results from the National Health and Nutrition Examination
Surveys. _Ann. Transl. Med._ 8(8), 535. https://doi.org/10.21037/atm.2020.03.205 (2020). Article PubMed PubMed Central Google Scholar * STATISTICS SOUTH AFRICA. STATISTICAL RELEASE
P0309.3 Mortality and causes of death in South Africa: Findings from death notification (2017). * Chen, Y. F., Zhou, K. W., Yang, G. Z. & Chen, C. Association between lipoproteins and
telomere length in us adults: Data from the NHANES 1999–2002. _Lipids Health Dis._ https://doi.org/10.1186/s12944-019-1030-7 (2019). Article PubMed PubMed Central Google Scholar *
Karimi, B., Yunesian, M., Nabizadeh, R. & Mehdipour, P. Serum level of total lipids and telomere length in the male population: A cross-sectional study. _Am. J. Mens Health_
https://doi.org/10.1177/1557988319842973 (2019). Article PubMed PubMed Central Google Scholar * Wang, J. _et al._ Association between telomere length and diabetes mellitus: A
meta-analysis. _J. Int. Med. Res._ 44, 1156–1173 (2016). Article PubMed PubMed Central Google Scholar * Mundstock, E. _et al._ Effect of obesity on telomere length: Systematic review and
meta-analysis. _Obesity_ 23, 2165–2174 (2015). Article PubMed Google Scholar * Grunnet, L. G. _et al._ Leukocyte telomere length is associated with elevated plasma glucose and HbA1c in
young healthy men independent of birth weight. _Sci. Rep._ 9, 7639. https://doi.org/10.1038/s41598-019-43387-0 (2019). Article ADS CAS PubMed PubMed Central Google Scholar *
Raschenberger, J. _et al._ association of relative telomere length with cardiovascular disease in a large chronic kidney disease cohort: The GCKD study. _Atherosclerosis_ 242, 529–534
(2015). Article CAS PubMed Google Scholar * Brouilette, S. W. _et al._ Telomere length, risk of coronary heart disease, and statin treatment in the West of Scotland Primary Prevention
Study: A nested case-control study. _Lancet_ 369, 107–114 (2007). Article CAS PubMed Google Scholar * Ding, H. _et al._ Telomere length and risk of stroke in Chinese. _Stroke_ 43,
658–663 (2012). Article CAS PubMed Google Scholar * Hansen, M. E. B. _et al._ Shorter telomere length in Europeans than in Africans due to polygenetic adaptation. _Hum. Mol. Genet._ 25,
2324–2330 (2016). Article CAS PubMed PubMed Central Google Scholar * Hunt, S. C. _et al._ Leukocyte telomeres are longer in African Americans than in whites: The National Heart, Lung,
and Blood Institute Family Heart Study and the Bogalusa Heart Study. _Aging Cell_ 7, 451–458 (2008). Article CAS PubMed Google Scholar * Zhu, H. _et al._ Leukocyte telomere length in
healthy Caucasian and African-American adolescents: Relationships with race, sex, adiposity, adipokines, and physical activity. _J. Pediatr._ 158, 215–220 (2011). Article PubMed Google
Scholar * Adler, N. _et al._ Educational attainment and late life telomere length in the Health, Aging and Body Composition Study. _Brain Behav. Immun._ 27, 15–21 (2013). Article PubMed
Google Scholar * Needham, B. L. _et al._ Socioeconomic status, health behavior, and leukocyte telomere length in the National Health and Nutrition Examination Survey, 1999–2002. _Soc. Sci.
Med._ 85, 1–8 (2013). Article PubMed PubMed Central Google Scholar * Factor-Litvak, P. _et al._ Leukocyte telomere length in newborns: Implications for the role of telomeres in human
disease. _Pediatrics_ 137, e20153927 (2016). Article PubMed PubMed Central Google Scholar * Needham, B. L., Hicken, M. T., Govia, I. O., Mitchell, C. & Abdou, C. M. Maternal social
disadvantage and newborn telomere length in archived dried blood spots from the Michigan neonatal biobank. _Biodemogr. Soc. Biol._ 63, 221–235 (2017). Article Google Scholar * Kanel, R.
V., Malan, N. T., Hamer, M. & Malan, L. Comparison of telomere length in black and white teachers from South Africa: The sympathetic activity and ambulatory blood pressure in Africans
study. _Psychosom. Med._ 77, 26–32 (2015). Article Google Scholar * Müezzinler, A., Zaineddin, A. K. & Brenner, H. A systematic review of leukocyte telomere length and age in adults.
_Ageing Res. Rev._ 12, 509–519 (2013). Article PubMed Google Scholar * Bekaert, S. _et al._ Telomere length and cardiovascular risk factors in a middle-aged population free of overt
cardiovascular disease. _Aging Cell_ 6, 639–647 (2007). Article CAS PubMed Google Scholar * Benetos, A. _et al._ Telomere length as an indicator of biological aging—The gender effect and
relation with pulse pressure and pulse wave velocity. _Hypertension_ 37, 381–385 (2001). Article CAS PubMed Google Scholar * Gardner, M. _et al._ Gender and telomere length: Systematic
review and meta-analysis. _Exp. Gerontol._ 51, 15–27 (2014). Article CAS PubMed Google Scholar * Nawrot, T. S., Staessen, J. A., Gardner, J. P. & Aviv, A. Telomere length and
possible link to x chromosome. _The Lancet_ 363, 507–510 (2004). Article CAS Google Scholar * Sengupta, D. _et al._ Genetic substructure and complex demographic history of South African
Bantu speakers. _Nat. Commun._ 12, 2080. https://doi.org/10.1038/s41467-021-22207 (2021). Article ADS CAS PubMed PubMed Central Google Scholar * Peer, N. _et al._ Rising diabetes
prevalence among urban-dwelling Black South Africans. _PLoS ONE_ 7(9), e43336 (2012). Article ADS MathSciNet CAS PubMed PubMed Central Google Scholar * Alberti, K. G., Zimmet, P.
& Shaw, J. Metabolic syndrome—A new world-wide definition. A consensus statement from the international diabetes federation. _Diabet. Med._ 23, 469–480 (2006). Article CAS PubMed
Google Scholar * Allain, C. C., Poon, L. S., Chan, C. S., Richmond, W. & Fu, P. C. Enzymatic determination of total serum cholesterol. _Clin. Chem._ 20, 470–475 (1974). Article CAS
PubMed Google Scholar * Jabbar, J., Siddique, I. & Qaiser, R. Comparison of two methods (precipitation manual and fully automated enzymatic) for the analysis of HDL and LDL
cholesterol. _J. Pak. Med. Assoc._ 56, 59–61 (2006). PubMed Google Scholar * Mcgowan, M. W., Artiss, J. D., Strandbergh, D. R. & Zak, B. A. Peroxidase-coupled method for the
colorimetric determination of serum triglycerides. _Clin. Chem._ 29, 538–542 (1983). Article CAS PubMed Google Scholar * Friedewald, W. T., Levy, R. I. & Fredrickson, D. S.
Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. _Clin. Chem._ 18, 499–502 (1972). Article CAS PubMed
Google Scholar * WHO. Definition, Diagnosis and Classification of Diabetes Mellitus and its Complications: Report of a WHO Consultation. Geneva: World Health Org. (1999). * O’Callaghan, N.
J. & Fenech, M. A quantitative PCR method for measuring absolute telomere length. _Biol. Proc. Online_ 13, 3. https://doi.org/10.1186/1480-9222-13-3 (2011). Article CAS Google Scholar
* WHO. Obesity: Preventing and Managing the Global Epidemic: Report of a WHO Consultation. Geneva: World Health Organization. (2000). * WHO. Waist Circumference and Waist–Hip Ratio: Report
of a WHO Expert Consultation. Geneva, 8–11 December 2008. Geneva: World Health Organization. (2011). * Ashwell, M. & Hsieh, S. D. Six reasons why the waist-to-height ratio is a rapid
and effective global indicator for health risks of obesity and how its use could simplify the international public health message on obesity. _Int. J. Food. Sci. Nutr._ 56, 303–307 (2005).
Article PubMed Google Scholar * Williams, B. _et al._ ESC scientific document group. esc/esh guidelines for the management of arterial hypertension. _Eur. Heart J._ 39, 3021–3104 (2018).
Article PubMed Google Scholar * Diagnosis, management and prevention of the common dyslipidaemias in South Africa-clinical guideline. South African Medical Association and Lipid and
Atherosclerosis Society of Southern Africa Working Group. _S. Afr. Med. J._ 90, 164–174, 176–178 (2000). Download references ACKNOWLEDGEMENTS The authors would like to acknowledge the
participants, fieldworkers, SAMRC research nurse fieldworkers Debbie Jonathan and Theresa Gogela, fieldwork coordinator Erica April, study manager Serena van Haght, and statisticians Nomonde
Gwebushe, Rebecca Shanmugam and Ria Laubscher. FUNDING This work was supported by the South African Medical Research Council (SAMRC): SAMRC-RFA-IRF-02-2016; an unrestricted grant from
Servier Laboratories (South Africa); the Initiative for Cardiovascular Health Research in Developing Countries (IC Health) Foundation Council; and Brigham and Women’s Hospital, Harvard
University. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS *
Laboratory for Molecular Medicine and Metabolism, Biotechnology Center, University of Yaoundé 1, Yaoundé, Cameroon Ndonwi Elvis Ngwa & Eugene Sobngwi * South African Medical Research
Council/Cape Peninsula University of Technology Cardio-Metabolic Health Research Unit, Department of Biomedical Sciences, Faculty of Health and Wellness Sciences, Cape Peninsula University
of Technology, Cape Town, South Africa Ndonwi Elvis Ngwa & Tandi E. Matsha * Biostatistics Unit, South African Medical Research Council, Cape Town, South Africa Carl Lombard * Chronic
Disease Initiative for Africa, Department of Medicine, UCT, Cape Town, South Africa Naomi Levitt * Non-Communicable Diseases Research Unit, South African Medical Research Council, Durban,
Cape Town, South Africa Andre-Pascal Kengne & Nasheeta Peer * Department of Medicine, University of Cape Town, Cape Town, South Africa Andre-Pascal Kengne & Nasheeta Peer * Division
of Epidemiology and Biostatistics, Department of Global Health, University of Stellenbosch, Cape Town, South Africa Carl Lombard Authors * Ndonwi Elvis Ngwa View author publications You can
also search for this author inPubMed Google Scholar * Tandi E. Matsha View author publications You can also search for this author inPubMed Google Scholar * Carl Lombard View author
publications You can also search for this author inPubMed Google Scholar * Naomi Levitt View author publications You can also search for this author inPubMed Google Scholar * Eugene Sobngwi
View author publications You can also search for this author inPubMed Google Scholar * Andre-Pascal Kengne View author publications You can also search for this author inPubMed Google
Scholar * Nasheeta Peer View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS The authors confirm contribution to the paper as follows:
conception and design; N.P. and A.P.K. conceived and designed the study, E.N.N. carried out the research work, prepared the draft manuscript, analysis and interpretation of results, T.M. and
A.P.K. supervised the research work, T.M., C.L., N.L. reviewed the results and all authors approved the final version of the manuscript. CORRESPONDING AUTHOR Correspondence to Ndonwi Elvis
Ngwa. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE Springer Nature remains neutral with regard to
jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under
a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate
credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article
are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons
licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of
this licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Ngwa, N.E., Matsha, T.E., Lombard, C. _et al._ Cardiometabolic
profile and leukocyte telomere length in a Black South African population. _Sci Rep_ 12, 3323 (2022). https://doi.org/10.1038/s41598-022-07328-8 Download citation * Received: 15 July 2021 *
Accepted: 31 January 2022 * Published: 28 February 2022 * DOI: https://doi.org/10.1038/s41598-022-07328-8 SHARE THIS ARTICLE Anyone you share the following link with will be able to read
this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative