Improving the efficiency and effectiveness of an industrial sars-cov-2 diagnostic facility
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT On 11th March 2020, the UK government announced plans for the scaling of COVID-19 testing, and on 27th March 2020 it was announced that a new alliance of private sector and academic
collaborative laboratories were being created to generate the testing capacity required. The Cambridge COVID-19 Testing Centre (CCTC) was established during April 2020 through collaboration
between AstraZeneca, GlaxoSmithKline, and the University of Cambridge, with Charles River Laboratories joining the collaboration at the end of July 2020. The CCTC lab operation focussed on
the optimised use of automation, introduction of novel technologies and process modelling to enable a testing capacity of 22,000 tests per day. Here we describe the optimisation of the
laboratory process through the continued exploitation of internal performance metrics, while introducing new technologies including the Heat Inactivation of clinical samples upon receipt
into the laboratory and a Direct to PCR protocol that removed the requirement for the RNA extraction step. We anticipate that these methods will have value in driving continued efficiency
and effectiveness within all large scale viral diagnostic testing laboratories. SIMILAR CONTENT BEING VIEWED BY OTHERS LATERAL FLOW TEST ENGINEERING AND LESSONS LEARNED FROM COVID-19 Article
19 January 2023 A ROLE FOR BIOFOUNDRIES IN RAPID DEVELOPMENT AND VALIDATION OF AUTOMATED SARS-COV-2 CLINICAL DIAGNOSTICS Article Open access 08 September 2020 FASTER DETECTION OF
ASYMPTOMATIC COVID-19 CASES AMONG CARE HOME STAFF IN ENGLAND THROUGH THE COMBINATION OF SARS-COV-2 TESTING TECHNOLOGIES Article Open access 29 March 2024 INTRODUCTION Following the
declaration of SARS-CoV-2 as a pandemic by the World Health Organisation on 11th March 20201, governments across the world announced unprecedented measures to mitigate the spread of the
virus through their populations. Alongside the reduction of social contacts and isolation of symptomatic individuals, a key tool in the pandemic response was the expansion of diagnostic
facilities to detect the spread of the disease and then to contain it in an effort to mitigate healthcare infrastructure being overwhelmed—a strategy that has proved effective in countries
including New Zealand, South Korea, and Iceland, among others2,3,4,5,6,7. In the UK, the Secretary of State for Health announced plans for the creation of a national network of new
laboratories (so-called Lighthouse Laboratories) that would rapidly scale-up capacity for delivering RT-qPCR analysis of clinical samples8. The Cambridge COVID-19 Testing Centre (CCTC) was
established during April 2020 as part of this network, through collaboration between AstraZeneca (AZ), GlaxoSmithKline (GSK), and the University of Cambridge. The initial challenges of
rapidly deploying smaller-scale facilities have been documented by others9,10,11,12 however the scale of testing required at the CCTC necessitated a different way of thinking to ensure
capacity and throughput could be achieved in a relatively small footprint, whilst ensuring the quality of the process was maintained or improved. The CCTC partner groups built on their
expertise in automated laboratory screening/profiling processes to achieve this taking the CCTC from concept to operational testing facility within the Anne McLaren Building, Cambridge in
just 6 weeks (Supplementary Fig. S1). The CCTC is unique in many ways to other local and national COVID-19 diagnostic laboratories, as in addition to processing the normal single swab
samples, the centre also evolved a process for pooled patient swab samples from the University of Cambridge Asymptomatic Testing Programme for its students13,14 and testing of staff samples
from the local NHS Trust hospital (Addenbrooke’s Hospital—the largest in the East of England). The centre also employed a dedicated technology development team involved in research
activities leading to pioneering innovations for improving the safety, efficiency, robustness, and portability of the COVID-19 screening process. Initial deployment with a focus on rapidly
expanding capacity meant that the CCTC adopted the standard RT-qPCR process already established in many facilities, but from the outset condensed the PCR reaction into a 384-well format,
thereby reducing the PCR machine requirement by fourfold. Whilst this assay process delivered high quality in a robust manner there was still room for continued optimisation in terms of
efficiency. As the initial volunteer workforce began to return to their home organisations, Charles River Laboratories entered the CCTC collaboration in July 2020, providing a large-scale
sustainable scientific workforce to continue diagnostic testing. All partners worked together thereafter to further develop technologies and iteratively improve performance as an analytical
facility. RESULTS ANALYSING AND IMPROVING THE OPERATIONAL PROCESS THROUGH MODELLING Following the establishment of the facility in May 2020, the focus turned to enhancing our efficiency and
effectiveness of operation. The critical first step in this journey was to establish and agree Key Performance Indicators (KPI) setting a baseline against which to measure performance. These
KPI focused on four areas: * 1. _Quality_ In-Process (IP) void rate ≤ 0.5% of samples voided through process errors in the laboratory * 2. _Capacity_ The ability to process up to 22,000
samples in a 24-h period * 3. _Turn Around Time (TAT)_ > 80% of samples having results reported within 24 h of bio-sampling (in response to the target set by the UK Prime Minister)7,15. *
4. _Safety_ No reportable Safety, Health and Environmental incidents of any description. In comparison to other testing facilities within the Lighthouse Laboratory Network and globally9,10,
the CCTC had a relatively constrained footprint of separate rooms within an already operational laboratory facility. With this restricted footprint, process modelling was essential to
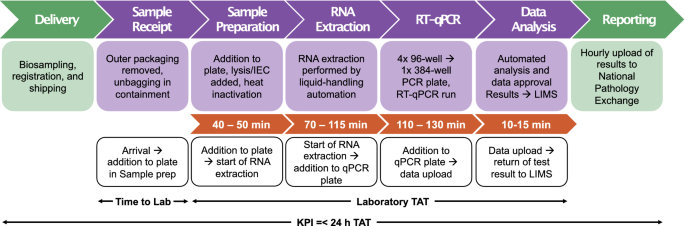
correctly ascertain the optimal number of automated platforms and staffing deployment to deliver the workflow at each station across the facility (Fig. 1), first basing the model on best
estimates. As the laboratory process matured over the first months of operation, the model was refined through the feedback of real-world empirical data, building in more rulesets that
served to highlight weaknesses in the logic and iteratively improved the predictive power of the model16. The initial CCTC laboratory process was able to achieve a capacity of more than
10,000 samples/day. Over the summer months in 2020, the CCTC strived to double its daily capacity to 22,000 samples/day. We were however conscious that simply adding more staff would not be
the most efficient solution to the problem of delivering against our four KPI. Social distancing, both within the laboratory and the wider site, was crucial to maintain, in addition to the
understanding that once a certain team size is reached, the addition of further resource can make processes less efficient due to sub-optimal communication and reporting17,18. Our modelling
determined that for continuous CCTC operation, the optimal staffing should be as described in Table 1a and equipped with 17 class-II Biological Safety Cabinets (BSC), 11 Beckman Coulter
Biomek liquid handlers (i5/i7), and 9 Roche RT-qPCR Light Cycler®480 II instruments. We initially deployed two nine-hour shifts of ~ 40 staff, across all days of the week, however after
refining our model based on the changes in sample delivery regime to a ‘start/stop’ model, we calculated performance would achieve an average of 17,000 samples/day with a maximum of 20,000
samples/day. This difference from our target capacity was tightly linked to the assumption within our initial modelling that a constant flow of samples would be maintained, providing an
endless stream of work during operating hours. In reality, this was rarely achieved due to the varying time of sample delivery to the lab; for example, Testing Sites would perform a large
amount of bio-sampling towards the end of each day, and therefore large consignments of samples would be received by the lab at the end of the evening shift, leaving no time for them to be
fully processed during that working day. In our focus on efficiency, we exploited our modelling to identify bottlenecks in our laboratory process and strategically implement improvements on
that process. The introduction of a night shift allowed a 24-h operation that avoided in-process samples being held overnight, and therefore our process model could be adapted back from
‘start/stop’ to ‘continuous’—now predicting a maximum operating capacity of 24,000 samples/day (Table 1b). The modelling continued to highlight a major bottleneck in the process at the RNA
extraction step driven by the fixed number of liquid-handling robots in the RNA extraction lab. Removing the requirement for RNA extraction altogether would both reduce the laboratory
footprint and make the process more economical, transferring the bottleneck to the labour-intensive step of removing secondary packaging within a BSC. A theoretical removal of the
requirement for BSC containment at the stage of secondary packaging, allowing this to occur on the open bench, was predicted to expand our capacity to an average of 29,000 samples/day.
Intrinsically linked to capacity the theoretical TAT of a sample was calculated as: 3 h 50 min–5 h 10 min. However empirical data on timings gathered through the initial phase of the CCTC
operation showed that our mean end-to-end laboratory processing time was in fact 8 h 35 min. To address both the capacity and TAT bottlenecks, our technology development focussed towards two
key innovations: * 1. The removal of RNA extraction (so called Direct to PCR; D2PCR) to create a more economical and efficient process whilst reducing the laboratory footprint. * 2. Heat
Inactivation of viable samples upon receipt prior to entry into the laboratory environment to circumvent the requirement of BSC containment at the point of secondary packaging removal19. We
describe the validation of Heat Inactivation of viral samples at scale, within a separate manuscript currently under preparation, and it is not discussed further here. DECREASING TURNAROUND
TIME THOUGH ASSAY MODIFICATION Alongside the introduction of Heat Inactivation, we also explored experimentally the scope for a Direct to PCR assay (D2PCR) that removed the requirement for
RNA extraction shown through our modelling to be a capacity and rate-limiting step due to the physical laboratory space available to accommodate the required liquid-handling robotic
platforms and the long run-time of the protocol (70–115 min). The RNA extraction step of the COVID-19 testing workflow achieves two purposes: first the Guanidinium Isothiocyanate (GITC)
content of the RNA extraction lysis buffer serves to inactivate potentially viable virus. At the time of establishing the CCTC, GITC-mediated virus inactivation was the most well understood,
and therefore the preferred method20,21. Second, RNA extraction purifies and concentrates viral RNA in advance of RT-qPCR detection. However, advances in RT-qPCR reagents lead to the
possibility of performing RT-qPCR directly on crude samples, and when coupled with heat inactivation, the very real possibility of removing any RNA extraction step completely. In our
workflow, the omission of RNA extraction was calculated to reduce the laboratory TAT on samples by 2 h, whilst simultaneously increasing overall capacity of the laboratory by repurposing
staff and facilities into sample receipt and preparation. This D2PCR approach has other significant advantages, in particular a reduction in the use of laboratory consumables, including a
50% reduction in the number of pipette tips, further reducing the cost of the assay, reducing waste and at a point when the global supply-chain for reagents, labware, and equipment could not
keep up with demand, facilitating centre operation. Further to this, RNA extraction methods use large amounts of solvents that require bespoke storage and disposal. Use of D2PCR for
detection of COVID-19 has been previously demonstrated22,23,24. Herein we present a clinically approved high-throughput methodology, developed using the _Genesig® Real Time PCR COVID-19 High
Throughput HT-CE kit V2.0_ targeting the same ORF1ab region of SARS-Cov-2 and containing an optimised buffer formulation which overcomes sample-mediated PCR inhibition. Validation of the
D2PCR process for clinical testing was carried out as described in the methods, comparing the D2PCR method directly with the standard RNA extraction-based protocol. All samples with a
positive result in the standard assay, with a Cq value of 33 or lower, tested positive using the D2PCR assay (Fig. 2a,b) with a concordance rate of 100%. For weaker positive samples with Cq
values of between 33 and 36, the concordance rate was 52.6%, while very weak positives (Cq > 36 in standard assay) were mostly not detected (6.25% positive to positive detection rate)
(Fig. 2b). This shift in the limit of detection was expected based on the D2PCR using fourfold less RNA input than the standard assay (due to the lack of concentration effect from RNA
extraction), as well as some likely impact of interference on PCR efficiency from the crude sample matrix. The significance of individuals with high Cq positive results within wider public
health response is a matter of current debate, however it is likely that this is reflective of low-level viral RNA relating to individuals early or late in their course of infection, even
when they are no longer infectious to others25. Data were reviewed by our Clinical Lead and wider Public Health England boards, where it was agreed that the reduced sensitivity at extremely
low viral-load levels was acceptable and the D2PCR methodology was formally approved for clinical sample testing. Beyond the benefits of cost, reagents, footprint, and waste reduction we
assessed the effect that the D2PCR method would have on TAT in our operational laboratory. When we examined the laboratory TAT in this pilot study, we found the samples had a median time to
completion of 3 h 32 min. When compared against all other samples processed in the same month (March 2021) using the standard laboratory process, we found this represented a median time
saving of 1 h 52 min (Fig. 2c). As mentioned above, we have also developed and deployed a method for heat inactivation of samples before they enter the lab. The combination of D2PCR with
heat inactivation led to a further median in lab time saving of 33 min (Fig. 2d). EXPLOITING OPERATIONAL DATA USING AN INFORMATICS-BASED APPROACH Across the Lighthouse Laboratory Network,
the end-to-end laboratory process was supported by a Laboratory Information Management System (LIMS) that provided the backbone of data management within the labs. A LIMS is fundamental to
management of data flow within a testing laboratory such as the CCTC dealing with several thousand samples per day—mapping the lifecycle of individual patient samples as they progress
through the physical laboratory process. As patient samples undergo transformation and compression from individual vials to multiwell microtitre plates, onwards through plate-plate
transfers, the LIMS records that lineage and captures various timestamps throughout the process (Fig. 1). These timestamps are not only imperative to the detailed tracking of individual
samples based on an anonymised barcode, but also provide a rich data set with which to view performance of the process in a real-time fashion. However due to the nature of the LIMS
environment and requirement to ensure change-control was centralised across the lab network, the ability to be agile with development of aligned local IT tools to exploit the data was
crucial. The combination of a core _Customisable Off The Shelf_ product aligned with associated tools developed in an agile methodology to bring immediate benefit in exploitation of
operational data is well proven to deliver results quickly26. To this end we targeted two user bases who we thought would be best placed to interact with these data—delivering tools
appropriate for each (Supplementary Fig. S2). Firstly, we provided the laboratory management team with data regarding past performance to examine areas for improvement (Centre Performance
Overview tool & Shift Lead dashboard; Supplementary Figs. S3, S4). Secondly, we provided the scientists in the laboratory with dashboards to enable real-time feedback on performance
against key performance indicators (Supplementary Fig. S5). VISUALIZATION OF RETROSPECTIVE AND REAL-TIME OPERATIONAL DATA The _Centre Performance Overview_ tool provides a retrospective view
of the laboratory TAT, broken down by station and time of day, with multiple interactive methods of viewing the data. Visualisation of where and when samples were being delayed focussed our
attention, enabling adoption of working practices aimed at reducing any bottleneck. Key process inefficiencies were quickly identified at the handovers between stations and shifts, which
could be addressed through process change without requiring significant modification to the SOPs for the individual workstations. Visualising TAT data in this fashion also highlighted the
importance of maintaining staff levels at defined minimum numbers in certain teams to avoid new process bottlenecks arising—ensuring that the CCTC management team could work with operational
Shift Leads to rebalance resource appropriately. Viewing the flow of data through the centre in this holistic fashion also enabled informed discussion with the upstream Department of Health
& Social Care (DHSC) logistics teams around optimal sample delivery schedules to achieve the best TAT. To complement the retrospective executive view, it was crucial to provide
real-time, non-interactive dashboards, providing quantitative feedback to the teams on each shift regarding their performance in real-time. This approach has previously been documented for
the receipt of samples and result reporting at an in-house hospital diagnostic facility, with operational improvements made considering this visualisation of data, but our efforts
concentrated on the laboratory process27. Information was broken down for each station in three streams (Supplementary Figs. S4, S5): * 1. The incoming workload from the previous station to
prepare reagents and equipment. * 2. A real-time view of the workload at the station, where plates experiencing a delay beyond expected process time are highlighted in red. * 3. A 24-h
analysis of the day’s performance, allowing instant feedback. The visualisation of current workflow was particularly important in stations containing automated platforms, where dashboards
were configured to highlight automation end-times so that plates/data could be expedited to the next step. These tools were specifically designed to ensure completed plates were swiftly
moved to the next stage, striving towards a steady flow through the lab. To investigate any effect on TAT through use of these data management tools, we monitored the time a sample plate
spent within Sample Preparation before and after implementation. Here we observed a notable decrease in the time a sample spent at this stage within a few days of the introduction (Fig. 3)
and an overall significant reduction was observed across the time points studied with a median reduction of 8.46 min (10.6% improvement). In particular, the number of plates spending over
three hours in Sample Preparation were substantially reduced through introduction of this tool, indicating that staff are not necessarily working at a higher speed, but rather that delayed
plates are being identified and expedited, thus reducing the variance in time spent at this station. REVIEWING CCTC PERFORMANCE To review performance of the CCTC against our established KPI,
we plotted the seven-day rolling mean of the process timing data collected to quantify our progress (Fig. 4). The laboratory TAT understandably had a direct relation to the number of
samples processed, however, after implementing the strategies described in our paper (minus D2PCR) the CCTC sustained a high workload with peaks in both January 2021 and March 2021 without
an aligned detrimental effect on TAT. Indeed, our mean TAT for March–April 2021 was below 6 h, in line with the theoretical time for the process of 3 h 50 min–5 h 10 min (Fig. 1). This focus
on exploitation of our operational data to continually drive efficiency of process has led to the CCTC consistently achieving its KPI of > 80% of samples processed within 24 h (achieved
on > 73% of days in 2021). Heat Inactivation upon receipt was formally adopted into the CCTC process in early February 2021 and quickly showed positive impact by helping to smooth the
flow of samples from receipt into the lab – along with the other advantages described earlier. Whilst our efforts in reducing laboratory TAT had an observable impact, this would be
counter-productive if these improvements were detrimental to quality. The seven-day rolling mean of the centre’s In-Process (IP) voids is plotted in Fig. 4c and shows that there has been no
increase in IP voids throughout our drive towards efficiency and effectiveness of process (the average rate remaining below our target KPI at 0.45%). DISCUSSION A comprehensive clinical
testing infrastructure is a critical component in combatting disease outbreaks. At the CCTC we deployed a functional industrial scale diagnostics facility and have continued to strive for
improved efficiency and effectiveness of our process, to ensure that the centre delivered its objectives to as high a standard as possible. In the establishment and continued management of
any process in an efficient manner, it is important to ensure that the baseline for establishing what ‘good’ looks like is agreed by all stakeholders. Of equal importance is that all those
delivering the process understand these baseline metrics and how their own efforts can impact them (both positively and negatively). Our efforts in progression of the CCTC from initial
establishment to fully optimised operation has been underpinned by our ability to use internal process metrics to highlight bottlenecks and improve consistency of flow through our end-to-end
process. From point of sample receipt into the CCTC we are able to track progress of samples through each of the different ‘stations’ (Fig. 1) and display that progress in almost real-time
through use of our comprehensive suite of dashboards (Supplementary Figs. S4, S5). These data drawn from several underlying sources were computationally wrangled into a consistent set of
data objects which could then be displayed to various consumers of that data, ensuring that display was tailored to their needs. Through regular management review of operational performance
data and translation of that data into operational improvements through management on the ground, the CCTC was able to exploit those data in near real-time to enable the CCTC to meet all our
KPI and further drive efficiency of our processes. In this report we have documented the informatics tools that we have used to attain our objectives on TAT and quality, whilst capacity
modelling, heat inactivation upon receipt, and D2PCR have exemplified our efforts to reduce the footprint, increase the safety of our process, lower our dependence on multiple supply chains,
and reduce the burden of labour-intensive steps, making a more effective and economical diagnostics facility. In addition to the optimisation noted above we further optimised the assay set
up and quality control as detailed in the Supplemental Information including changes in assay volume, instrument performance tracking and data integrity. This has resulted in what we believe
is the optimised process that future pathogen screening laboratories can follow (Fig. 5). In the first year of operation the CCTC processed 3,264,596 clinical samples. Over this time,
continuous process monitoring has been applied to highlight areas of focus for technology advancement including heat inactivation and D2PCR, with the impact of those advancements being
observed in real-time In situations such as emerging pandemic threats, the speed at which a highly efficient response can be implemented is key to minimising impact to human health and
society. Adoption of the processes and improvements described here, as learned through the COVID-19 pandemic, will enhance efficiency of process and effectiveness of delivery for future
clinical diagnostics facilities. METHODS ETHICS STATEMENT This study was conducted as part of the Lighthouse Laboratories surveillance for COVID-19 infections set up under the auspices of
Section 251 of the National Health Service Act 2006 and/or Regulation 3 of The Health Service (Control of Patient Information) Regulations 2002. The study therefore did not require
individual patient consent or ethical approval. No Patient Identifiable information (PII) was received by the Centre. Authors only had access to anonymised data in the form of sample
barcodes. Approval for the operation of the CCTC and improvements to the procedures used therein was granted by the Department for Health and Social Care under the emergency provisions
granted by the Secretary of State under Section 251 of the National Health Service Act. CAPACITY MODELLING A stochastic simulation of the sample preparation and screening processing steps
was developed by Dr Michael Allen (University of Exeter Medical School) using the _SymPy_ library associated with the Python development environment16. The simulation was developed to
consider the processing times at each step with associated human and equipment resources. Variation in time for either human or equipment related processing was applied using a triangular
distribution. Workforce break times, shift routines and estimates for equipment breaking down were incorporated into the model. Simulations were run over 30 iterations and the results
aggregated to provide an output for capacity, test-sample queuing, and resource utilisation at each step and for the overall process. The simulation data was used to identify potential
processing bottlenecks so that solutions could be explored by adjust the screening process and/or applied human and equipment resources. SARS-COV-2 RT-QPCR DIAGNOSTIC ASSAY Methodology for
the standard SARS Cov-2 diagnostic test used at the CCTC is described in detail in the Supplementary Information, where we have presented the Standard Operating Procedures in abridged form,
focusing on transferable aspects and removing any CCTC-specifics that would be irrelevant to other laboratories. In brief, clinical oropharyngeal/nasopharyngeal (OP/NP) samples were received
at the CCTC in leakproof UN3373 packaging containing a screw capped sample tube with a swab stick immersed in viral transport medium (VTM). OP/NP sab vials were unpacked and racked within a
microbiological safety cabinet and following Containment Level 2 precautions. For each sample, 200 µl of VTM was transferred to a 96 deep well sample plate, followed by the addition of RNA
extraction lysis buffer, Proteinase K and Internal Extraction Control (IEC) RNA. The sample plate was sealed and double contained, and placed at 65 °C for 10 min for heat inactivation of
viable SARS-Cov-2, followed by incubation at room temperature for 10 min. RNA was extracted using the RNAdvance Viral Kit (C63510) on Biomek i5 or i7 automated platforms from Beckman
Coulter. Viral RNA was eluted in nuclease-free water and then used for RT-qPCR using the Genesig Real-Time COVID-19 PCR High Throughput assay kit (Primer Design Ltd, Geneisg
Z-Path-COVID-19-CE HT1.0) as described by the manufacturer except that 10 µl (i.e. half) reaction volumes were used. RT-PCR reactions were prepared in white, 384-well LightCycler 480
Multiwell Plates (Roche #04729749001), 6 µl RT-qPCR master mix was added using a ThermoFisher Multidrop Combi and 4 μl extracted RNA sample was added using an Agilent Bravo. RT-qPCR
reactions were run on a Light Cycler 480 II and data was analysed using a bespoke algorithm (FastFinder software, UgenTec) to define Cq values and assign test results following
interpretation of controls (positive, negative, IEC) and according to a defined decision tree. DIRECT TO PCR (D2PCR) VALIDATION In the D2PCR assay, the standard process described previously
is shortened by omission of the initial sample lysis and RNA extraction steps. Instead, 100 μl of each OP/NP swab sample was transferred to a _Hard-Shell Low-Profile Skirted 96-Well PCR
Plate_ (Biorad #HSP9601), sealed with an _Aluminium Foil Seal_ (Beckman Coulter #538619) and heated in a _PCR Max AlphaCycler_, set to 65 °C for 20 min. Heat inactivation could also be
performed at the stage of sample receipt, which has the advantage of allowing subsequent steps to be performed without Containment Level 2 working, or by another suitable method that
achieves the required heat exposure for viral inactivation prior to PCR set-up outside of biological containment. All equipment used for heat inactivation should be suitably calibrated to
verify the required heating of samples28. RT-PCR reactions were prepared in _White, 384-well LightCycler 480 Multiwell Plates_ (Roche #04729749001) by sequential addition of 3.5 μl of
_Genesig Real Time PCR COVID-19 High Throughput HT-CE kit V2.0_ (Genesig #Z-Path-COVID-19-CE) PCR master mix using a _ThermoFisher Multidrop Combi_, followed by 2.5 μl of a 50-fold dilution
of IEC in nuclease-free water using an _Agilent Bravo_ (omitting positive and negative control wells where IEC was not included). The RT-qPCR assay was initiated through addition of 4 μl
heat inactivated sample to the 384-well microplate containing PCR master mix and IEC using an _Agilent Bravo_, and loading of the 384-well microplate into a _Roche LightCycler 480 II_.
Initial D2PCR experiments were performed using OP/NP swab samples with known SARS-CoV-2 status (samples already tested using the approved CCTC standard laboratory process) to determine
suitable conditions for heat inactivation of potential viable SARS-Cov-2 and RT-qPCR set-up. In the absence of an RNA extraction step, the IEC was included to confirm successful RT-PCR in
every sample (therefore highlighting any unexpected sample-mediated reaction inhibition). IEC was added to PCR master mix in 384-well microplates to avoid weak or variable IEC signals that
are seen when IEC is added to samples themselves, likely a result of RNA degradation. Validation of the D2PCR methodology for clinical testing was carried out by testing of 1100 OP/NP swab
samples in parallel to the standard RNA extraction-based methodology, testing over three separate days (Fig. 2). OPERATIONAL INFORMATICS To enable rapid development, we based our solution
around an RStudio connect server deployed on a virtual machine connected to a data lake containing output from our LIMS. Architecture of the system was set up as shown in Supplementary Fig.
S2. Automated queries generated reports from the LIMS containing all active and recently completed (archived) plates on a 15–30 min cycle. These reports were output in CSV format and
deposited into an Azure file share, forming the basis of our data lake. The Azure file share was mapped to a virtual machine running RStudio connect. A series of scheduled R markdown scripts
regularly imported the CSV reports, calculated required statistics, and appended the new data to existing RDS files which contained all the processed data required. Data was visualised and
exploited via several applications coded in _Shiny_ (an RStudio package) which read the RDS files and provided web-based visualisations (Supplementary Figs. S3, S4, S5). REFERENCES * WHO.
_WHO Director-General's opening remarks at the media briefing on COVID-19—11 March 2020_,
https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 (2020). * Li, R. _et al._ Substantial
undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV-2). _Science_ 368, 489–493 (2020). Article ADS CAS Google Scholar * Shim, E., Tariq, A., Choi,
W., Lee, Y. & Chowell, G. Transmission potential and severity of COVID-19 in South Korea. _Int. J. Infect. Dis._ 93, 339–344 (2020). Article CAS Google Scholar * Gudbjartsson, D. F.
_et al._ Spread of SARS-CoV-2 in the Icelandic population. _N. Engl. J. Med._ 382, 2302–2315 (2020). Article CAS Google Scholar * Geoghegan, J. L. _et al._ Genomic epidemiology reveals
transmission patterns and dynamics of SARS-CoV-2 in Aotearoa New Zealand. _Nat. Commun._ 11, 1–7 (2020). Article Google Scholar * Torres, I., Sippy, R. & Sacoto, F. Assessing critical
gaps in COVID-19 testing capacity: The case of delayed results in Ecuador. _BMC Public Health_ 21, 1–8 (2021). Article Google Scholar * Larremore, D. B. _et al._ Test sensitivity is
secondary to frequency and turnaround time for COVID-19 screening. _Sci. Adv._ 7, 5393 (2021). Article ADS Google Scholar * DHSC. _Health Secretary launches biggest diagnostic lab network
in British history to test for coronavirus_, https://www.gov.uk/government/news/health-secretary-launches-biggest-diagnostic-lab-network-in-british-history-to-test-for-coronavirus (2020). *
Richter, A. _et al._ How to establish an academic SARS-CoV-2 testing laboratory. _Nat. Microbiol._ 5, 1452–1454 (2020). Article CAS Google Scholar * Gillam, T. B. _et al._ Norwich
COVID-19 testing initiative pilot: Evaluating the feasibility of asymptomatic testing on a university campus. _J. Public Health_ 43, 82–88 (2021). Article Google Scholar * Amen, A. M. _et
al._ Blueprint for a pop-up SARS-CoV-2 testing lab. _Nat. Biotechnol._ 38, 791–797. https://doi.org/10.1038/s41587-020-0583-3 (2020). Article CAS Google Scholar * Nascimento Junior, J. A.
C. _et al._ Trends in MERS-CoV, SARS-CoV, and SARS-CoV-2 (COVID-19) diagnosis strategies: A patent review. _Front. Public Health_ https://doi.org/10.3389/fpubh.2020.563095 (2020). Article
PubMed PubMed Central Google Scholar * Warne, B. _et al. Research Square_,https://doi.org/10.21203/rs.3.rs-520626/v1 (2021). * Aggarwal, D. _et al. Nature
Portfolio_,https://doi.org/10.21203/rs.3.rs-520627/v1 (2021). * Woodcock, A. _Coronavirus: Boris Johnson pledges to get all test results out within 24 hours by end of June_,
https://www.independent.co.uk/news/uk/politics/coronavirus-boris-johnson-test-results-24-hours-june-lockdown-a9546556.html) (2020). * Allen, M. _Covid Testing Lab simulation_,
https://github.com/MichaelAllen1966/2004_covid_testing_lab_simulation (2020). * Brooks, F. P. _The Mythical Man-Month: Essays on Software Engineering_ (Addison-Wesley, 1975). Google Scholar
* Brooks, F. P. Jr. The mythical man-month after 20 years. _IEEE Softw._ 12, 57 (1995). Article Google Scholar * Batéjat, C., Grassin, Q. & Manuguerra, J.-C. Heat inactivation of the
severe acute respiratory syndrome coronavirus 2. _J. Biosafety Biosecurity_ 3, 1–3 (2021). Article Google Scholar * PHE. _COVID-19: PHE laboratory assessments of inactivation methods_,
https://www.gov.uk/government/publications/covid-19-phe-laboratory-assessments-of-inactivation-methods (2020). * Pastorino, B. _et al._ Evaluation of chemical protocols for inactivating
SARS-CoV-2 infectious samples. _Viruses_ 12, 624 (2020). Article CAS Google Scholar * Arumugam, A. _et al._ A rapid SARS-CoV-2 RT-PCR assay for low resource settings. _Diagnostics_ 10,
739 (2020). Article CAS Google Scholar * Smyrlaki, I. _et al._ Massive and rapid COVID-19 testing is feasible by extraction-free SARS-CoV-2 RT-PCR. _Nat. Commun._ 11, 4812.
https://doi.org/10.1038/s41467-020-18611-5 (2020). Article ADS CAS PubMed PubMed Central Google Scholar * Bruce, E. A. _et al._ Direct RT-qPCR detection of SARS-CoV-2 RNA from patient
nasopharyngeal swabs without an RNA extraction step. _PLoS Biol._ https://doi.org/10.1371/journal.pbio.3000896 (2020). Article PubMed PubMed Central Google Scholar * Mina, M. J., Peto,
T. E., García-Fiñana, M., Semple, M. G. & Buchan, I. E. Clarifying the evidence on SARS-CoV-2 antigen rapid tests in public health responses to COVID-19. _Lancet_ 397, 1425–1427 (2021).
Article CAS Google Scholar * Clark, R. & Wingfield, J. LIMS deployment in an assay service environment: Improving efficiency and effectiveness through information management. _J.
Cases Inf. Technol._ 14, 14–34. https://doi.org/10.4018/jcit.2012070102 (2012). Article Google Scholar * Maury, E. _et al._ An automated Dashboard to improve laboratory COVID-19
diagnostics management. _medRxiv_ https://doi.org/10.1101/2021.03.20.21253624 (2021). Article Google Scholar * Oona, D. _et al. Research Square_,https://doi.org/10.21203/rs.3.rs-763230/v1
(2021). Download references ACKNOWLEDGEMENTS We thank the Scientific and Logistics leadership at the CCTC (including Shift Leaders and discipline leaders not named as authors on this paper):
Krystal LaBonte, Brian Moroney, Jean Rhodes, Abbigail Nolan, Dr. Jamie Ware, Susan Shanahan, Rob Vickers, Dr. Mark Burrell, Cameron Fergusson, Brett Wyman, Dr. Cleo Paule, Dr. Peri Tate. We
thank Dr. Michael Allen (University of Exeter) for development of the capacity simulation model used. We thank all the staff (both volunteers and employed) who worked as part of the CCTC
over its lifetime along with those from AZ, CRL, GSK, and the University of Cambridge that supported every aspect of our operation. We thank Sheena Lanagan, Simon Mellor, Rich Ingle, Dan
Cioffi (AZ), George Pickering and James Wheatcroft (GSK) for leading the finance, legal and procurement efforts aligned to the CCTC. We thank Karl Wilson, Jon Holgate, Steve Hoensch
(University of Cambridge) and all staff at the Anne McLaren Building who accommodated the CCTC operation. We thank Dr. Becky Scott, Dr. Kathryn Kenworthy (GSK), Penny James and Jon Elliott
(AZ) for their advice as part of the Joint Steering Committee. We thank Pat McDonald, Ellie Weston (AZ) Rahul Kandhal and Beth Mavin (GSK) for HR support over the initial phase of the
project. We thank Crystal Chum, Nicola Port, Frances Earle, Jessica Bradley and Jodie Sissons (CRL) for their support in recruitment and HR guidance during the second phase of the project.
We thank Dr. Nick Brown (Cambridge University Hospitals) for his discussions and clinical advice to support our process changes. We thank the Department of Health and Social Care for funding
and regulatory approvals. We also thank NHS Test and Trace. AUTHOR INFORMATION Author notes * These authors contributed equally: Julie A. Douthwaite, Christopher A. Brown, John R. Ferdinand
and Rahul Sharma. * These authors jointly supervised this work: Rob Howes and Roger Clark. * Chris Abell is deceased. AUTHORS AND AFFILIATIONS * BioPharmaceuticals R&D, AstraZeneca,
Cambridge, UK Julie A. Douthwaite, Jane Elliott, Molly A. Taylor, Nancy T. Malintan, Hannah Duvoisin, Oona Delpuech, Alexandra L. Orton, Haidee Pitt, David J. Nicholls, Anna Cuthbert,
Abhishek Upadhyay, Craig Hewitt, Douglas Ross-Thriepland, Christopher Brankin, Matthieu Chodorge, Gareth Browne, Clive Green, Steve Rees, Menelas N. Pangalos & Rob Howes * Charles River
Laboratories, Chesterford Research Park, Saffron Walden, UK Christopher A. Brown, John R. Ferdinand, Rahul Sharma, Thomas Hill, Fred Kuenzi, Simon Fish, Ian Richards, Giles Ratcliffe,
Abigail Marklew, Ian Waddell & Roger Clark * Department of Medicine, University of Cambridge, Cambridge, UK John R. Ferdinand & Rahul Sharma * GSK R&D Tech, Stevenage, UK Simon
Fish, Shane Weaver & Penny A. Smee * GSK R&D, Stevenage, UK Palwinder K. Mander, Ruud M. DeWildt, Joost van Kempen, Jon G. Bartlett, Paula M. Allen, Emma L. Koppe, Charlotte A.
Ashby, Julian D. Phipps, Nalini Mehta, David J. Brierley, David G. Tew, Melanie V. Leveridge & Stuart M. Baddeley * Division of Virology, Department of Pathology, University of
Cambridge, Cambridge, UK Ian G. Goodfellow * Vice Chancellor’s Office, University of Cambridge, Cambridge, UK Chris Abell & Andy Neely * School of Clinical Medicine, University of
Cambridge, Cambridge, UK Patrick H. Maxwell Authors * Julie A. Douthwaite View author publications You can also search for this author inPubMed Google Scholar * Christopher A. Brown View
author publications You can also search for this author inPubMed Google Scholar * John R. Ferdinand View author publications You can also search for this author inPubMed Google Scholar *
Rahul Sharma View author publications You can also search for this author inPubMed Google Scholar * Jane Elliott View author publications You can also search for this author inPubMed Google
Scholar * Molly A. Taylor View author publications You can also search for this author inPubMed Google Scholar * Nancy T. Malintan View author publications You can also search for this
author inPubMed Google Scholar * Hannah Duvoisin View author publications You can also search for this author inPubMed Google Scholar * Thomas Hill View author publications You can also
search for this author inPubMed Google Scholar * Oona Delpuech View author publications You can also search for this author inPubMed Google Scholar * Alexandra L. Orton View author
publications You can also search for this author inPubMed Google Scholar * Haidee Pitt View author publications You can also search for this author inPubMed Google Scholar * Fred Kuenzi View
author publications You can also search for this author inPubMed Google Scholar * Simon Fish View author publications You can also search for this author inPubMed Google Scholar * David J.
Nicholls View author publications You can also search for this author inPubMed Google Scholar * Anna Cuthbert View author publications You can also search for this author inPubMed Google
Scholar * Ian Richards View author publications You can also search for this author inPubMed Google Scholar * Giles Ratcliffe View author publications You can also search for this author
inPubMed Google Scholar * Abhishek Upadhyay View author publications You can also search for this author inPubMed Google Scholar * Abigail Marklew View author publications You can also
search for this author inPubMed Google Scholar * Craig Hewitt View author publications You can also search for this author inPubMed Google Scholar * Douglas Ross-Thriepland View author
publications You can also search for this author inPubMed Google Scholar * Christopher Brankin View author publications You can also search for this author inPubMed Google Scholar * Matthieu
Chodorge View author publications You can also search for this author inPubMed Google Scholar * Gareth Browne View author publications You can also search for this author inPubMed Google
Scholar * Palwinder K. Mander View author publications You can also search for this author inPubMed Google Scholar * Ruud M. DeWildt View author publications You can also search for this
author inPubMed Google Scholar * Shane Weaver View author publications You can also search for this author inPubMed Google Scholar * Penny A. Smee View author publications You can also
search for this author inPubMed Google Scholar * Joost van Kempen View author publications You can also search for this author inPubMed Google Scholar * Jon G. Bartlett View author
publications You can also search for this author inPubMed Google Scholar * Paula M. Allen View author publications You can also search for this author inPubMed Google Scholar * Emma L. Koppe
View author publications You can also search for this author inPubMed Google Scholar * Charlotte A. Ashby View author publications You can also search for this author inPubMed Google
Scholar * Julian D. Phipps View author publications You can also search for this author inPubMed Google Scholar * Nalini Mehta View author publications You can also search for this author
inPubMed Google Scholar * David J. Brierley View author publications You can also search for this author inPubMed Google Scholar * David G. Tew View author publications You can also search
for this author inPubMed Google Scholar * Melanie V. Leveridge View author publications You can also search for this author inPubMed Google Scholar * Stuart M. Baddeley View author
publications You can also search for this author inPubMed Google Scholar * Ian G. Goodfellow View author publications You can also search for this author inPubMed Google Scholar * Clive
Green View author publications You can also search for this author inPubMed Google Scholar * Chris Abell View author publications You can also search for this author inPubMed Google Scholar
* Andy Neely View author publications You can also search for this author inPubMed Google Scholar * Ian Waddell View author publications You can also search for this author inPubMed Google
Scholar * Steve Rees View author publications You can also search for this author inPubMed Google Scholar * Patrick H. Maxwell View author publications You can also search for this author
inPubMed Google Scholar * Menelas N. Pangalos View author publications You can also search for this author inPubMed Google Scholar * Rob Howes View author publications You can also search
for this author inPubMed Google Scholar * Roger Clark View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS J.A.D. led the Technology Development
for the CCTC. C.A.B. was Operational Excellence Lead for the CCTC. J.R.F. was Operational Informatics lead for the CCTC. R.S. was a scientific Team Lead at the CCTC. R.C. held
accountability for operational delivery within the CCTC. C.G. led the CCTC through initial setup and operation. R.H. led the CCTC as Director (succeeding C.G.). I.G.F. provided scientific
guidance in setup of the CCTC. S.M.B., A.N., I.W., S.R., C.A., P.H.M .provided oversight and steering. N.M., H.D., T.H., A.O., and H.P. generated the D2PCR data. J.F. performed analysis on
the operational data. F.K., S.F. delivered key elements of informatics tools. D.N. performed capacity & TAT modelling. All authors assisted with implementation and operational management
of the CCTC. C.A.B., J.F., J.D., R.S. and R.C. drafted the manuscript, with input from A.M., A.U. and editing and approval from all authors. CORRESPONDING AUTHORS Correspondence to Julie A.
Douthwaite or Christopher A. Brown. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE Springer Nature remains
neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION 1. SUPPLEMENTARY INFORMATION 2. RIGHTS AND
PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any
medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The
images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is
not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission
directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE
Douthwaite, J.A., Brown, C.A., Ferdinand, J.R. _et al._ Improving the efficiency and effectiveness of an industrial SARS-CoV-2 diagnostic facility. _Sci Rep_ 12, 3114 (2022).
https://doi.org/10.1038/s41598-022-06873-6 Download citation * Received: 29 July 2021 * Accepted: 07 February 2022 * Published: 24 February 2022 * DOI:
https://doi.org/10.1038/s41598-022-06873-6 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative