A scaffold-free approach to cartilage tissue generation using human embryonic stem cells
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
Articular cartilage functions as a shock absorber and facilitates the free movement of joints. Currently, there are no therapeutic drugs that promote the healing of damaged articular
cartilage. Limitations associated with the two clinically relevant cell populations, human articular chondrocytes and mesenchymal stem cells, necessitate finding an alternative cell source
for cartilage repair. Human embryonic stem cells (hESCs) provide a readily accessible population of self-renewing, pluripotent cells with perceived immunoprivileged properties for cartilage
generation. We have developed a robust method to generate 3D, scaffold-free, hyaline cartilage tissue constructs from hESCs that are composed of numerous chondrocytes in lacunae, embedded in
an extracellular matrix containing Type II collagen, sulphated glycosaminoglycans and Aggrecan. The elastic (Young’s) modulus of the hESC-derived cartilage tissue constructs (0.91 ± 0.08
MPa) was comparable to full-thickness human articular cartilage (0.87 ± 0.09 MPa). Moreover, we have successfully scaled up the size of the scaffold-free, 3D hESC-derived cartilage tissue
constructs to between 4.5 mm and 6 mm, thus enhancing their suitability for clinical application.
Hyaline articular cartilage covers the ends of bones and, by providing a smooth lubricated surface for joint articulation, acts as a low-friction shock absorber in the joints. Chondrocytes
are the primary cell type present in cartilage1. Chondrocytes have an essential role in cartilage maintenance by producing an extracellular matrix consisting primarily of Type II collagen
and proteoglycans, namely Aggrecan1. Being avascular and hypocellular, adult articular cartilage has a limited capacity for self-repair.
Articular cartilage is susceptible to damage from daily wear and tear and trauma due to falls, sports injuries, etc. Currently, there are no pharmacological agents that promote comprehensive
healing of articular cartilage defects. Hence, regenerative medicine approaches for articular cartilage repair have focused on the generation of cartilage tissue primarily from clinically
relevant cell populations, namely human articular chondrocytes (HACs), chondroprogenitor cells (CPCs) and bone marrow-derived mesenchymal stem cells (MSCs). Although HACs are widely used in
restorative approaches for articular cartilage repair, several limitations are associated with the use of this cell population. For example, invasive surgeries are required to harvest the
cartilage biopsies, which, in turn, cause donor site morbidity; a limited number of chondrocytes can be isolated from the cartilage biopsies, thus requiring expensive cell culture for
expansion of chondrocyte numbers in vitro; dedifferentiation of chondrocytes due to 2D monolayer culture and their limited lifespan in vitro2.
MSCs have attracted much attention because of their extended self-renewal potential and ability to differentiate into multiple stromal lineages including cartilage; invasive techniques are
however required to obtain bone marrow samples. Additionally, high variability in the chondrogenic differentiation potential of MSCs from different individuals, debatable immunoprivileged
characteristics, reports of inferior fibrocartilaginous repair tissue generation and the propensity of MSC-derived chondrocytes for hypertrophic differentiation, have limited the use of this
adult stem cell population for cartilage regeneration3,4,5.
In contrast, human embryonic stem cells (hESCs) constitute a readily accessible population of self-renewing, pluripotent cells with perceived immunoprivileged properties that have the
ability to grow indefinitely and thus provide an unlimited source of cells for regenerative medicine applications6. hESCs have been differentiated into chondrogenic cells via different
methods including co-culture7,8, directed differentiation9, embryoid body formation10,11,12 and MSC intermediates13,14. However, previous attempts to generate cartilage from hESCs using
scaffold-free approaches have been hampered by the generation of constructs that are approximately 1 mm in size8,15,16,17. In addition, limited testing has been performed to determine the
mechanical properties of these constructs. This study aims to generate and scale up the size of 3D, scaffold-free hyaline cartilage tissue constructs from hESCs that are structurally and
mechanically analogous to native human hyaline cartilage.
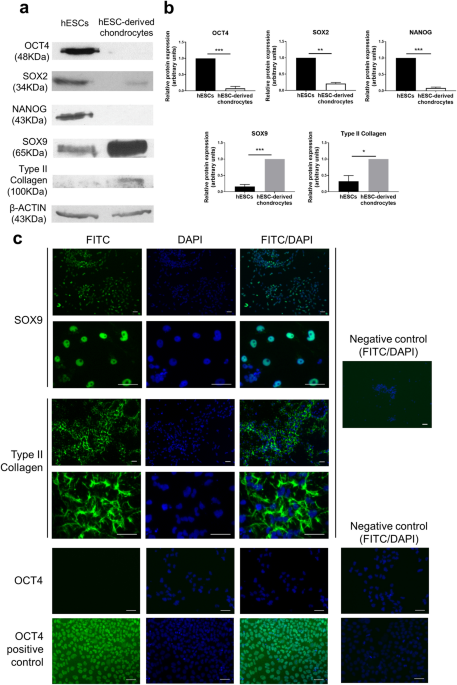
hESCs were differentiated over a 14-day period into chondrocytes at 5% O2. The hESC-derived chondrocytes displayed a dramatic reduction in the expression of the pluripotency proteins, OCT4
(p