Securing genetic integrity in freshwater pearl mussel propagation and captive breeding
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Securing genetic integrity is of key importance in conservation-oriented captive breeding programs releasing juveniles into the wild. This is particularly true for species such as
the endangered freshwater pearl mussel (_Margaritifera margaritifera_) for which a number of captive breeding facilities has been established in Europe. The core objective of this study was
to compare the genetic constitution of 29 cohorts of captive-bred freshwater pearl mussels from five different breeding facilities in Austria, France, Luxembourg and Germany, with their
original 14 source populations from nine major European drainages, based on microsatellite markers. Captive-bred mussels represented 11 different genetic clusters, suggesting an important
contribution of the breeding stations to securing the genetic diversity of the species. In almost all cases, the cultured offspring closely resembled the genetic constitution of the source
mussels as revealed from the STRUCTURE analysis and the generally high assignment of offspring to the original source populations. The majority of captive-bred cohorts had an increased
inbreeding coefficient and decreased genetic variability compared to their source populations as measured by AR and HO. Highest numbers of deformed juveniles coincided with very low levels
of HO < 0.05. Since erosion of genetic diversity in captive breeding was mostly evident in individual year-cohorts, long-term breeding over multiple years can minimize such effects. The
systematic selection of priority populations for conservation, effective breeding strategies avoiding effects of in- and outbreeding by genetically informed selection of parent individuals,
and a network of collaboration among the different breeding facilities would be very useful to increase resilience and effectiveness. SIMILAR CONTENT BEING VIEWED BY OTHERS SHEDDING LIGHT ON
VARIATION IN REPRODUCTIVE SUCCESS THROUGH STUDIES OF POPULATION GENETIC STRUCTURE IN A SOUTHEAST PACIFIC COAST MUSSEL Article 06 April 2023 EVALUATING THE EFFECT OF OVERHARVESTING ON
GENETIC DIVERSITY AND GENETIC POPULATION STRUCTURE OF THE COCONUT CRAB Article Open access 22 June 2020 TEMPORAL ANALYSIS SHOWS RELAXED GENETIC EROSION FOLLOWING IMPROVED STOCKING PRACTICES
IN A SUBARCTIC TRANSNATIONAL BROWN TROUT POPULATION Article Open access 30 August 2021 INTRODUCTION Freshwater biodiversity is globally in decline, with freshwater mussels being among the
most affected taxonomic groups1,2. One of the species that receives greatest attention is the freshwater pearl mussel (_Margaritifera margaritifera_) which simultaneously fulfills the
criteria of indicator, keystone, flagship and umbrella species3. Freshwater pearl mussels can reach ages of more than 100 years and have an exceptional life cycle that involves a parasitic
life stage on a fish host that can either be Atlantic salmon (_Salmo salar_), brown trout (_Salmo trutta_), or both in European populations4,5. Degradation of pearl mussel habitats, mostly
related to siltation and colmation of interstitial spaces in the stream bed where juvenile pearl mussels burrow, have resulted in a lack of recruitment and severe declines of many pearl
mussel populations throughout their European range6,7. Since restoration of stream beds requires consideration of entire catchments and the restoration of natural flow dynamics8, this
approach is time-consuming and costly. At the same time, many pearl mussel populations are overaged and expected to die out in the near future. Since the first descriptions of the ongoing
threats and population declines9, the downward trend of populations has even become worse7. This development has prompted the creation of a first European CEN standard for a single species10
as well as several captive breeding efforts in different countries11,12,13,14. Captive breeding of freshwater pearl mussel was first established in the Czech Republic by Jaroslav Hruška11
and is mostly based on induced release of glochidia larvae from parent mussels, a subsequent infestation and holding of fish hosts as well as collection and raising of dropped-off
juveniles14. Due to the strong conservation interest in freshwater pearl mussel, its genetic constitution has been studied in depth both throughout its European15,16,17,18,19,20,21,22 as
well as its North American23 distributions, facilitating the consideration of priority populations for conservation in captive breeding. A wise selection of parents or an annual replacement
of breeders can help secure the genetic diversity in captive breeding efforts. Still, there are multiple factors that may compromise the genetic constitution of captive-bred mussels compared
to their parent populations. For instance, the selection of small numbers of parent mussels that are not representative for the genetic constitution of the entire population can already
narrow the basis of genetic variability within the breeding program. During infestation of host fish, the compatibility of the used species and strains among mussel and fish as well as the
age of the fish all can have effects on attachment rates, survival and growth5,24,25. Physiology of the host fish immune system related to temperature26, the effects of different loading
densities of mussel larvae on swimming performance of the host27 as well as the duration of the parasitic phase28,29 and associated nutrient transfer from the host to the mussel30 all have a
potential selective effect on the mussels during the parasitic phase. In the subsequent post-parasitic (i.e. juvenile) phase, stock origin and environmental conditions31, food quantity as
well as water and sediment quality32,33,34, but also changing of culture systems and the cleaning regimes35 were found to affect survival and growth. Generally, mortalities are highest until
the juveniles reach the first millimeter36. In order to make the most of recent advances in freshwater mussel propagation and restoration37, consideration of genetic effects during the
breeding process and objective evaluation of ongoing breeding activities is needed yet still lacking. In addition to the technical optimization of breeding facilities for freshwater
mussels38, such information can be useful in identifying the most suitable captive breeding techniques for retaining a maximum of the genetic-evolutionary potential of mussels3. The core
objective of this study thus was to analyze the genetic constitution of captive-bred freshwater pearl mussels from different European breeding facilities and compare their genetic diversity
and differentiation with the original populations. These served as a source for the parent mussels and captive-bred juveniles have been, or are intended to be released into the same streams.
Specifically, we hypothesized that (1) breeding efforts would be suitable to secure the genetic identity of the captive bred mussels as indicated by low genetic differentiation between
original populations and captive-bred juveniles, (2) there would be no decrease of the genetic variability in pearl mussel during the captive breeding as evident from highly similar genetic
diversity indices such as Ho and AR between original populations and captive-bred juveniles. MATERIAL AND METHODS STUDY DESIGN Our study design was based on the comparison of population
genetic parameters of natural freshwater pearl mussel populations and corresponding cohorts of juveniles propagated at specialized breeding stations. We included five facilities from four
European countries (Germany, Austria, Luxembourg, France) in our sampling regime. In these, freshwater pearl mussels were propagated from a total of 14 different source populations
representing nine main drainage systems: Elbe (Weiße Elster, Wolfsbach/Zinnbach), Danube (Kleine Ohe, Naarn, Wolfertsrieder Bach), Rhine (Sûre), Maas (Rulles, Anlier), Loire (Le Sarthon),
Orne (La Rouvre), Sienne (L’Airou), Blavet (Le Bonne Chère, Le Loc’h) and Aulne (L’Elez) (Table 1). Source Populations of the streams Sûre, Rulles and Anlier were located on Belgian
territory and juveniles were reared in Luxembourg. For genetic analyses we used data of 382 adult specimens from respective source populations collected between 2003 and 201915,16,17 in
their natural habitats (based on representative sample collection over multiple locations per stream) or directly from individuals of the broodstock held at the breeding station as practiced
in Austria39,40. Genetic constitution of those mussels was compared to results of 897 propagated juveniles comprising 29 different cohorts which were selected from different age classes
ranging from 0 + to ca. 16 years. They were collected either while being held at the breeding facility, or from gravel-filled cage-boxes submerged in the streams of intended release, or from
already released and marked individuals. SAMPLING AND DNA EXTRACTION We collected 40–100 µL haemolymph from the foot tissue of adult individuals and juveniles exceeding 30 mm total shell
length by following the methodology of Geist & Kuehn16. This sampling technique is not harmful for the mussels which were returned alive to their original sites immediately after
sampling. Juveniles of cohorts between 0 + and 5 years of age and a total shell length between < 1 mm and ca. 1 cm were sampled using whole individuals due to their small size. Obvious
deformations of juvenile mussels (e.g., irregular and compressed shell shapes and growth patterns) were additionally noted. Haemolymph was transferred into 1.7 mL Eppendorf tubes while
juveniles were kept alive in containers filled with water. Both were cooled at 4 °C and subsequently transported to the laboratories of the Technical University of Munich, Germany. Samples
were prepared for DNA extraction by centrifuging haemolymph at 14.000 g for 5 min and discarding the supernatant whereas juveniles were transferred to individual reaction tubes. To ensure
efficient lysis of the tissue, the smallest individuals (~ 1 mm) were crushed inside the reaction tube using a metal probe, or one shell valve with adhering tissue was used. Genomic DNA was
then isolated from cellular pellets and juveniles using the NucleoSpin Tissue Kit (Macherey–Nagel GmbH, Düren, Germany) according to the manufacturer’s protocol for tissue samples and eluted
in 80 µL of BE buffer. PCR AND GENOTYPING DNA samples were genotyped at nine standard species-specific microsatellite markers (MarMa2671, MarMa3050, MarMa3621, MarMa4143, MarMa4322,
MarMa4726, MarMa5167, MarMa5280 and MarMa5023) as described by Geist et al.15 and Geist & Kuehn16,17. Polymerase chain reactions (PCRs) were performed in a total volume of 12.5 µL
containing 25 ng genomic DNA, 0.2 µM of each primer, 0.2 mM of each dNTP, 3 mM MgCl2 for eight Loci (2 mM MgCl2 for Locus 5280), 1 × FirePol® PCR buffer BD (0.8 M Tris–HCl, 0.2 M (NH4)SO4
and 0.5 U FirePol® Taq DNA polymerase (Solis Biodyne, Tartu, Estonia) under the cycling conditions described in Geist et al.15 and Geist & Kuehn16. Forward primers were end-labelled with
Cy5 fluorescent dye and PCR products were separated on 5% denaturing 19:1 acrylamid:bisacrylamid gels on an ALFexpressII DNA analyser (Amersham Pharmacia Biotech) and allele lengths were
scored using ALLELELINKS 1.02 software. To ensure consistent allele scoring between individual lanes and among gels, two internal size standards were included per lane42 as well as 11 size
standards and one previously genotyped reference sample in two separate lanes. STATISTICAL ANALYSES Microsatellite allele frequencies, the mean number of alleles per locus (A), allelic
richness (AR) as a standardized measure of the number of alleles corrected for sample size, expected and observed heterzygosities (HE, HO) and inbreeding coefficient (FIS) were calculated
using Fstat v. 2.9.343. We used Genepop v. 4.7.344 to test genotypic distributions for conformance with Hardy–Weinberg expectations using the probability test45, to calculate pairwise FST
values46 between source populations and offspring cohorts and to estimate the significance of genotypic differentiation between these populations pairs. All probability tests were based on
the Markov chain47,48 method using 10,000 dememorization steps, 100 batches and 5000 iterations per batch. The number of distinct multilocus genotypes (MLG) was determined using the
R-package Poppr v. 2.8.349. The R-package Adegenet v. 2.1.150 was used to determine mean individual inbreeding coefficients (Find) for each source population and offspring cohort by
calculating for each individual the probability of being homozygous at a locus: p(h) = F + (1 − F) \(\sum\nolimits_{i} {p_{i}^{2} }\) and summing up log-likelihoods over all microsatellite
loci to account for multilocus genotypes, where F refers to the probability of an individual to inherit two identical alleles from a single ancestor and _p__i_ refers to the frequency of
allele _i_ in a population. Additionally, we estimated relatedness between individuals within populations based on the F-value of the 2mod program51 which provides information on the
probability that two genes share a common ancestor within a population and is correlated with effective population size. The Markov chain Monte Carlo (MCMC) simulation was run for 200,000
iterations and the initial 10% of the data were discarded to avoid dependence on starting conditions. We used Structure v. 2.3.4 software52 to determine and visualize the number of genetic
clusters (K) present among source populations and to assign probabilities of cluster memberships to propagated individuals. We tested values of K ranging from 2 to 20 under the admixture
model and assuming correlated allele frequencies using 20,000 burn-ins, 200,000 MCMC repetitions and 10 iterations per K to assess the convergence of ln P(X│K). The software package Clumpak
v. 1.153 was used to infer the most likely number of K based on the ΔK method of Evanno et al.54. The implemented program Clumpp v. 1.155 was used to find the optimal individual alignments
of replicated cluster analyses using the LargeKGreedy algorithm and 2000 random input orders which were then visualized using Distruct v. 1.156. Bayesian clustering techniques may produce
biased results in terms of cluster recognition when working with unbalanced sample sizes57,58. We therefore validated Structure results using the multivariate approach of discriminant
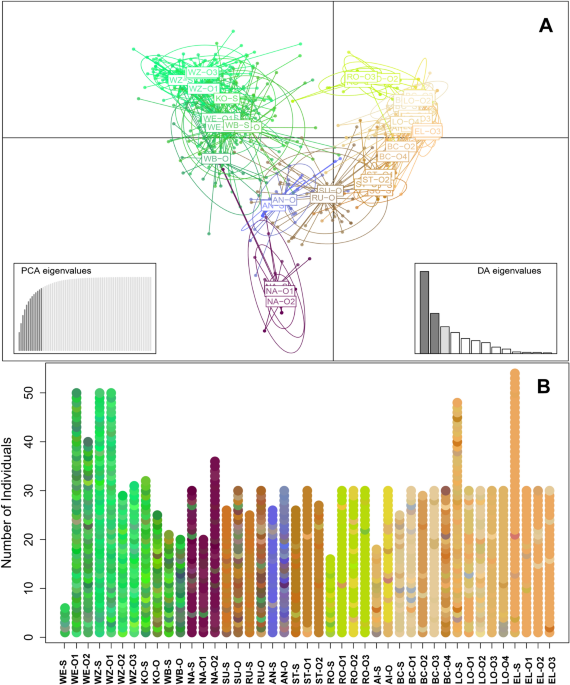
analysis of principal components (DAPC)59 implemented in the software package Adegenet v. 2.1.150 in R v. 3.6.260 which is less sensitive to uneven sampling58. DAPC first transforms the data
using principal component analysis (PCA) and then performs a Discriminant Analysis (DA) on the retained principal components (PC). We retained 13 PCs which explained 85% of the total
variation of the data set. Results of the DAPC were visualized by assigning the first three PCs to intensities of the color channels of the RGB system. Similar generated colors thus
correspond to similar genetic constitutions of respective individuals or populations59. RESULTS GENETIC INTEGRITY The captive breeding efforts over the five investigated rearing facilities
were found to represent a diversity of genetically differentiated clusters of freshwater pearl mussel. Overall, eleven genetic clusters were identified as the most probable outcome using the
ΔK method (Table 2, Fig. S2). In almost all cases, the cultured offspring closely resembled the genetic constitution of the source mussels as revealed from the Structure analysis and the
generally high assignment of offspring to the original source populations. In 13 out of 29 cases (45%), a 100% assignment to the original population was found, and in 25 out of the 29 cases
(86%), more than 80% of individuals were correctly assigned (Table 2). Otherwise, low levels of assignment occurred in populations (BC, LO, RU and SU) that generally had extremely low values
of genetic variability (Table 1) and where drift effects due to the limited availability of a small number of gravid broodstock mussels for the breeding were expected. The result of the
DAPC is consistent with the findings of the Structure analyses and graphically illustrates the genetic differentiation between source and captive-bred mussels based on color codes of
multilocus genotype frequencies of each individual sampled (Fig. 1). Most FST values between captive-bred mussels with their original populations from the wild were low to moderate,
indicating high similarity in the alleles present and their frequencies in both groups (Table 1). This was for instance obvious with the Austrian NA-O1 and NA-O2 compared to NA-S, a
culturing system where the parent mussels are permanently maintained in a flow-through system allowing for a near-natural glochidia attachment to host fish that are maintained in tanks fed
by the flow-through systems. In some cases, very high FST values of up to 0.638 between single year cohorts of offspring compared to the original populations were evident (e.g., ST-S with
ST-O1). In most cases, these pronounced differences were not consistently present over different year cohorts, especially in captive breeding situations where glochidia from gravid mussels
are annually collected in the wild to infest host fish in the rearing facility and where these source mussels differ from year to year. GENETIC VARIABILITY Mean values of observed
heterozygosity (HO) and allelic richness (AR) in source populations ranged from HO = 0.006 (EL) to HO = 0.460 (WB) and from AR = 1.1 (EL and RU) to AR = 2.9 (WB) while the global means of
source populations (HO = 0.131, AR = 1.7) were slightly higher than that of juvenile cohorts (HO = 0.112, AR = 1.6). In most cohorts of propagated juveniles, remarkably low levels of these
two diversity parameters were detected, but also in the majority of the source populations, especially the westernmost ones (Table 1). However, in comparison to the source populations both
AR and HO decreased in over half of the juvenile cohorts (Fig. 2) while respective individual inbreeding coefficients tended to increase (Fig. S1). Another remarkable finding relates to the
numbers of physically deformed juveniles. In all six cohorts with ≥ 3 deformed specimens (ST-O1, ST-O2, RO-O1, RO-O2, LO-O2, EL-O1), observed heterozygosities were below 0.05 (Tab. 1).
DISCUSSION The value and usefulness of captive breeding in fish populations has been subject to a great controversy61 and the increasing number of captive breeding efforts in freshwater
mussels37,38, particularly in freshwater pearl mussel11,12,13,14,35,36, makes it necessary to critically reflect on these measures. Our study provides a first analysis of 29 captive-bred
annual cohorts of freshwater pearl mussel in comparison to the genetic constitution of populations from their original streams considering four different European countries. Breeding efforts
were found to represent a broad, yet not complete spectrum of the genetic diversity of wild populations, and seem to be a valuable tool to at least partially secure the genetic and
evolutionary potential of populations at the brink of extinction until efforts of habitat restoration succeed and populations manage to sufficiently recruit in the wild. The findings also
illustrate the usefulness of genetic monitoring in captive breeding to identify alterations of the genetic constitution in terms of genetic identity as well as decreased genetic variability.
Most breeding stations are focused on local production of juveniles from nearby populations using local fish stocks. The findings of this study with eleven different genetic clusters
represented in those breeding efforts suggest that this approach appears generally useful in considering the overall pronounced degree of genetic differentiation among European populations16
as well as the co-evolutionary genetic patterning of freshwater pearl mussel and its hosts17. A main challenge lies in the simultaneous rearing of multiple populations and year cohorts in
the same rearing facility which always poses a certain risk of mixing different populations. Such confusion was also suspected in one of the rearing facilities in this study and the genetic
analyses provided a powerful tool in validating and re-assigning the offspring to the correct source population in the wild. Even though this was a rare incident, it may be generally useful
to ensure validation of the integrity of captive-bred mussels before they are being released into the wild, especially in situations when the source population is not yet extinct. Currently,
most breeding stations rely on different ways of collecting parent mussels or larvae from the wild versus completely maintaining a suite of parent mussels together with host fishes. Since
the primary objective of those breeding stations is to produce juvenile mussels and since there are many time-critical constraints in terms of finding sufficient numbers of gravid females,
handling of glochidia larvae and the infestation of host fish, there is typically no time for a documentation of the exact procedures and methods such as numbers of parent mussels and
glochidia collected as well as on individual host fish infestations and other rearing commodities that all may also affect the genetic constitution of the offspring. These constraints, along
with the very different genetic background of the pearl mussel source populations considered in this study prevent us from systematically linking certain genetic effects to specific
attributes of the respective breeding method. Still, the findings of this study suggest that gathering such information as regularly practiced in many state and federal breeding stations in
the US, would be very useful. If this cannot be realized, then at least females should be exchanged annually and progeny from a single female should not be used more than once at each
reintroduction locality. Moreover, a realistic evaluation of the effects of any captive breeding on the genetic constitution of populations should not look at single cohorts but rather at
the cumulative effects over time. Whilst the findings of this study revealed rather low genetic diversity and signs of inbreeding in some of the annual cohorts, pronounced differences were
typically observed when comparing the findings for cohorts from different years. Such differences can be explained by different availabilities of gravid mussels (in some populations only
single individuals in certain years) as well as different infestation, metamorphosis and rearing success. Variable levels of multiple paternity as previously observed in this species62,63
and the Louisiana Pearlshell, _Margaritifera hembeli_, may also play a role64. In any case, the results of this study allow drawing the consistent picture that maintaining rearing activities
for specific populations over multiple years is most beneficial since it reduces the risk of genetic bottlenecks, drift and selection effects. This is particularly true for a long-lived
species like _Margaritifera margaritifera_, where the reproductive period extends over more than 80 years65, allowing multiple generations of different age to jointly reproduce. Still, the
extremely low genetic diversity values also observed in most of the source populations for captive breeding suggest that the actual efforts to rescue genetically outstanding populations by
captive breeding should begin before an erosion of the genetic variability at small effective population sizes. In cases of populations which are already suffering from loss of genetic
diversity, an individual-based selection of specimens for the collection of glochidia is recommended, focusing on individuals which best represent the remaining gene pool of the original
population. Also, in any breeding effort it is useful to avoid possible selection and drift effects. In the case of freshwater pearl mussel, it is thus mandatory to use a full and diverse
suit of fish hosts and avoid fish strains that only result in metamorphosis of few specimens4,5,24. This also includes using older than the commonly utilized 0 + fish which were shown to
result in higher numbers of developing postparasitic juveniles under captive breeding situations25. The age and size at which captive-bred juvenile pearl mussels should be released into the
wild still remains controversial. The greater capability of adaptation31 as well as the lower risk of genetic erosion and die-offs in the rearing facility would suggest an early release.
Colmated stream beds and other adverse habitat conditions to which older and larger mussels are much more resistant than young ones, as well as the absence of a decreased genetic variability
of older cohorts compared to younger ones in this study (see e.g. BC-O1,2,3,4 and WE-O1, WE-O2 with ages of more than 10 years), along with the continuous improvements of survival in
rearing facilities32,35,36,38,66 clearly suggest that stocking with such cohorts is also a feasible option. Effective conservation of freshwater pearl mussel will likely depend on a
combination of habitat restoration and effective captive breeding to rescue genetically unique populations over time. An integration of scientific findings into the ecology and conservation
genetics of the species is thus likely to be most successful3. Given the well-established knowledge on the genetic constitution of freshwater pearl mussel throughout its European and North
American distribution15,16,17,18,19,20,21,22,23 and the large and increasing number of breeding facilities for this species, a more systematic approach of evidence-based conservation and
restoration67 can be recommended. Such an approach should also include systematic selection of priority populations for conservation, effective breeding strategies avoiding effects of in-
and outbreeding by genetically informed selection of parent individuals, and a network of collaboration among the different facilities. DATA AVAILABILITY The original data used for the study
will be available from the Dryad Digital Repository. REFERENCES * Geist, J. Integrative freshwater ecology and biodiversity conservation. _Ecol. Indic._ 11, 1507–1516 (2011). Article
Google Scholar * Lopes-Lima, M. _et al._ Conservation status of freshwater mussels in Europe: State of the art and future challenges. _Biol. Rev._ 92, 572–607 (2017). Article PubMed
Google Scholar * Geist, J. Strategies for the conservation of endangered freshwater pearl mussels (_Margaritifera margaritifera_ L.): A synthesis of conservation genetics and ecology.
_Hydrobiologia_ 644, 69–88 (2010). Article Google Scholar * Taeubert, J. E. & Geist, J. The relationship between the freshwater pearl mussel (_Margaritifera margaritifera_) and its
hosts. _Biol. Bull._ 44, 67–73 (2017). Article Google Scholar * Salonen, J. K. _et al._ Atlantic salmon (_Salmo salar_) and brown trout (_Salmo trutta_) differ in their suitability as a
host for the endangered freshwater pearl mussel (_Margaritifera margaritifera_) in northern Fennoscandian rivers. _Freshw. Biol._ 62, 1346–1358 (2017). Article Google Scholar * Geist, J.
& Auerswald, K. Physicochemical stream bed characteristics and recruitment of the freshwater pearl mussel (_Margaritifera margaritifera_). _Freshw. Biol._ 52, 2299–2316 (2007). Article
Google Scholar * Stoeckl, K., Denic, M. & Geist, J. Conservation status of two endangered freshwater mussel species in Bavaria, Germany: Habitat quality, threats, and implications for
conservation management. _Aquat. Conserv._ 30, 647–661 (2020). Article Google Scholar * Auerswald, K. & Geist, J. Extent and cause of siltation in a headwater stream bed: Catchment and
soil erosion is less important than internal stream processes. _Land Degrad. Dev._ 29, 737–748. https://doi.org/10.1002/ldr.2779 (2018). Article Google Scholar * Bauer, G. Threats to the
freshwater pearl mussel _Margaritifera margaritifera_ L. in central Europe. _Biol. Conserv._ 45, 239–253 (1988). Article Google Scholar * Boon, P. J. _et al._ Developing a standard
approach for monitoring freshwater pearl mussel (_Margaritifera margaritifera_) populations in European rivers. _Aquat. Conserv._ 29, 1365–1379 (2019). Article Google Scholar * Hruska, J.
Nahrungsansprüche der Flußperlmuschel und deren halbnatürliche Aufzucht in der Tschechischen Republik (Dietary requirements and semi-natural rearing of freshwater pearl mussel in the Czech
Republic). _Heldia_ 4, 69–79 (1999). Google Scholar * Preston, S. J., Keys, A. & Roberts, D. Culturing freshwater pearl mussel _Margaritifera margaritifera_: A breakthrough in the
conservation of an endangered species. _Aquat. Conserv._ 17, 539–549. https://doi.org/10.1002/aqc.799 (2007). Article Google Scholar * Thomas, G. R., Taylor, J. & de Leaniz, C. G.
Captive breeding of the endangered freshwater pearl mussel, _Margaritifera margaritifera_. _Endanger. Species Res._ 12, 1–9 (2010). Article Google Scholar * Gum, B., Lange, M. & Geist,
J. A critical reflection on the success of rearing and culturing juvenile freshwater mussels with a focus on the endangered freshwater pearl mussel (_Margaritifera margaritifera_ L.).
_Aquat. Conserv._ 21, 743–751 (2011). Article Google Scholar * Geist, J., Rottmann, O., Schröder, W. & Kühn, R. Development of microsatellite markers for the endangered freshwater
pearl mussel _Margaritifera margaritifera_ L. (Bivalvia: Unionoidea). _Mol. Ecol. Resour._ 3, 444–446 (2003). Article CAS Google Scholar * Geist, J. & Kühn, R. Genetic diversity and
differentiation of central European freshwater pearl mussel (_Margaritifera margaritifera_ L.) populations: Implications for conservation and management. _Mol. Ecol._ 14, 425–439 (2005).
Article CAS PubMed Google Scholar * Geist, J. & Kuehn, R. Host-parasite interactions in oligotrophic stream ecosystems: The roles of life history strategy and ecological niche. _Mol.
Ecol._ 17, 997–1008 (2008). Article PubMed Google Scholar * Marchordom, A., Araujo, R., Erpenbeck, D. & Ramos, M. A. Phylogeography and conservation genetics of the endangered
European Margaritiferidae (Bivalvia: Unionoidea). _Biol. J. Linn. Soc. Lond._ 78, 235–252 (2003). Article Google Scholar * Stoeckle, _et al._ Strong genetic differentiation and low genetic
diversity of the freshwater pearl mussel (_Margaritifera margaritifera_ L.) in the southwestern European distribution range. _Conserv. Genet._ 18, 147–157 (2017). Article Google Scholar *
Karlsson, S., Larsen, B. M. & Hindar, K. Host-dependent genetic variation in freshwater pearl mussel (_Margaritifera margaritifera_ L.). _Hydrobiologia_ 735, 179–190 (2014). Article
Google Scholar * Geist, J., Söderberg, H., Karlberg, A. & Kuehn, R. Drainage-independent genetic structure and high genetic diversity of endangered freshwater pearl mussels
(_Margaritifera margaritifera_) in northern Europe. _Conserv. Genet._ 11, 1339–1350 (2010). Article Google Scholar * Geist, _et al._ Genetic structure of Irish freshwater pearl mussels
(_Margaritifera margaritifera_ and _Margaritifera durrovensis_): Validity of subspecies, roles of host fish, and conservation implications. _Aquat. Conserv._ 28, 923–933 (2018). Article
Google Scholar * Zanatta, _et al._ High genetic diversity and low differentiation in North American Margaritifera margaritifera (Bivalvia: Unionida: Margaritiferidae). _Biol. J. Linn. Soc.
Lond._ 123, 850–863 (2018). Article Google Scholar * Taeubert, J. E., Denic, M., Gum, B., Lange, M. & Geist, J. Suitability of different salmonid strains as hosts for the endangered
freshwater pearl mussel (_Margaritifera margaritifera_). _Aquat. Conserv._ 20, 728–734 (2010). Article Google Scholar * Marwaha, _et al._ Host (_Salmo trutta_) age influences resistance to
infestation by freshwater pearl mussel (_Margaritifera margaritifera_) glochidia. _Parasitol. Res._ 118, 1519–1532 (2019). Article PubMed Google Scholar * Taeubert, J. E., Gum, B. &
Geist, J. Variable development and excystment of freshwater pearl mussel (_Margaritifera margaritifera_ L.) at constant temperature. _Limnologica_ 43, 319–322 (2013). Article Google Scholar
* Taeubert, J. E. & Geist, J. Critical swimming speed of brown trout (_Salmo trutta_) infested with freshwater pearl mussel (_Margaritifera margaritifera_) glochidia and implications
for artificial breeding of an endangered mussel species. _Parasitol. Res._ 112, 1607–1613 (2013). Article PubMed Google Scholar * Marwaha, J., Jensen, K. H., Jakobsen, P. J. & Geist,
J. Duration of the parasitic phase determines subsequent performance in juvenile freshwater pearl mussels (_Margaritifera margaritifera_). _Ecol. Evol._ 7, 1375–1383 (2017). Article PubMed
PubMed Central Google Scholar * Eybe, T., Thielen, F., Bohn, T. & Sures, B. Influence of the excystment time on the breeding success of juvenile freshwater pearl mussels
(_Margaritifera margaritifera_). _Aquat. Conserv._ 25, 21–30 (2015). Article Google Scholar * Denic, M., Taeubert, J. E. & Geist, J. Trophic relationships between the larvae of two
freshwater mussels and their fish hosts. _Invertebr. Biol._ 134, 129–135 (2015). Article Google Scholar * Denic, M. _et al._ Influence of stock origin and environmental conditions on the
survival and growth of juvenile freshwater pearl mussels (_Margaritifera margaritifera_) in a cross-exposure experiment. _Limnologica_ 50, 67–74 (2015). Article CAS Google Scholar *
Hyvärinen, H. S. H., Chowdhury, M. M. R. & Taskinen, J. Pulsed flow-through cultivation of _Margaritifera margaritifera_: Effects of water source and food quantity on the survival and
growth of juveniles. _Hydrobiologia_. 3219–3229 (2021). * Hyvärinen, H., Saarinen-Valta, M., Mäenpää, E. & Taskinen, J. Effect of substrate particle size on burrowing of the juvenile
freshwater pearl mussel _Margaritifera margaritifera_. _Hydrobiologia_ https://doi.org/10.1007/s10750-021-04522-z (2021). Article Google Scholar * Taskinen, J. _et al._ Effect of pH, iron
and aluminum on survival of early life history stages of the endangered freshwater pearl mussel, _Margaritifera margaritifera_. _Toxicol. Environ. Chem._ 93, 1764–1777 (2011). Article CAS
Google Scholar * Lavictoire, L., Moorkens, E., Ramsay, A. & Sweeting, R. Effects of substrate size and cleaning regime on growth and survival of captive-bred juvenile freshwater pearl
mussels, _Margaritifera margaritifera_ (Linnaeus, 1758). _Hydrobiologia_ 766, 89–102 (2016). Article Google Scholar * Eybe, T., Thielen, F., Bohn, T. & Sures, B. The first millimetre:
Rearing juvenile freshwater pearl mussels (_Margaritifera margaritifera_ L.) in plastic boxes. _Aquat. Conserv._ 23, 964–975 (2013). Article Google Scholar * Strayer, D. L., Geist, J.,
Haag, W. R., Jackson, J. K. & Newbold, J. D. Essay: Making the most of recent advances in freshwater mussel propagation and restoration. _Conserv. Sci. Pract._ 1, e53.
https://doi.org/10.1111/csp2.53 (2019). Article Google Scholar * Patterson, M. A. _et al._ _Freshwater Mussel Propagation for Restoration_ (Cambridge University Press, 2018). Book Google
Scholar * Gstöttenmayr, D., Scheder, C. & Gumpinger, C. Conservation de la mulette perlière d’eau douce en Autriche: un système d’élevage contrôlé en progrès. _Penn ar Bed_ 222, 45–49
(2015). Google Scholar * Gumpinger, C., Pichler-Scheder, C. & Huemer, D. Das oberösterreichische Artenschutzprojekt „Vision Flussperlmuschel“. _Österreichs Fischerei_ 69, 259–273
(2016). Google Scholar * Rice, W. R. Analyzing tables of statistical tests. _Evolution_ 43, 223–225 (1989). Article PubMed Google Scholar * DeWoody, J. A. _et al._ Universal method for
producing ROXlabeled size standards suitable for automated genotyping. _Biotechniques_ 37, 348–352. https://doi.org/10.2144/04373BM02 (2004). Article CAS PubMed Google Scholar * Goudet,
J. Fstat (Version 1.2): A computer program to calculate F-statistics. _J. Hered._ 86, 485–486 (1995). * Rousset, F. Genepop’007: A complete reimplementation of the Genepop software for
Windows and Linux. _Mol. Ecol. Resour._ 8, 103–106 (2008). Article PubMed Google Scholar * Haldane, J. B. S. An exact test for randomness of mating. _J. Genet._ 52, 631–635.
https://doi.org/10.1007/BF02981502 (1954). Article Google Scholar * Weir, B. S. & Cockerham, C. C. Estimating F-statistics for the analysis of population structure. _Evolution_ 38,
1358–1370 (1984). CAS PubMed Google Scholar * Guo, S. W. & Thompson, E. A. Performing the exact test of Hardy-Weinberg proportion for multiple alleles. _Biometrics_ 48, 361–372
(1992). Article CAS PubMed MATH Google Scholar * Raymond, M. & Rousset, F. An exact test for population differentiation. _Evolution_ 49, 1280–1283 (1995). Article PubMed Google
Scholar * Kamvar, Z. N., Tabima, J. F. & Grünwald, N. J. Poppr: An R package for genetic analysis of populations with clonal, partially clonal, and/or sexual reproduction. _PeerJ_ 2,
e281. https://doi.org/10.7717/peerj.281 (2014). Article PubMed PubMed Central Google Scholar * Jombart, T. Adegenet: A R package for the multivariate analysis of genetic markers.
_Bioinformatics_ 24, 1403–1405 (2008). Article CAS PubMed Google Scholar * Ciofi, C., Beaumont, M. A., Swingland, I. R. & Bruford, M. W. Genetic divergence and units for conservation
in the Komodo dragon _Varanus komodoensis_. _Proc. Royal Soc. B_ 266, 2269–2274 (1999). Article Google Scholar * Pritchard, J. K., Stephens, M. & Donnelly, P. Inference of population
structure using multilocus genotype data. _Genetics_ 155, 945–959 (2000). Article CAS PubMed PubMed Central Google Scholar * Kopelman, N. M., Mayzel, J., Jakobsson, M., Rosenberg, N. A.
& Mayrose, I. CLUMPAK: A program for identifying clustering modes and packaging population structure inferences across K. _Mol. Ecol. Resour._ 15, 1179–1191 (2015). Article CAS PubMed
PubMed Central Google Scholar * Evanno, G., Regnaut, S. & Goudet, J. Detecting the number of clusters of individuals using the software structure: A simulation study. _Mol. Ecol._
14, 2611–2620 (2005). Article CAS PubMed Google Scholar * Jakobsson, M. & Rosenberg, N. A. CLUMPP: A cluster matching and permutation program for dealing with label switching and
multimodality in analysis of population structure. _Bioinformatics_ 23, 1801–1806 (2007). Article CAS PubMed Google Scholar * Rosenberg, N. A. DISTRUCT: A program for the graphical
display of population structure. _Mol. Ecol. Notes_ 4, 137–138 (2004). Article Google Scholar * Kalinowski, S. T. The computer program STRUCTURE does not reliably identify the main genetic
clusters within species: Simulations and implications for human population structure. _Heredity_ 106, 625–632 (2011). Article CAS PubMed Google Scholar * Puechmaille, S. J. The program
structure does not reliably recover the correct population structure when sampling is uneven: Subsampling and new estimators alleviate the problem. _Mol. Ecol. Resour._ 16, 608–627 (2016).
Article PubMed Google Scholar * Jombart, T., Devillard, S. & Balloux, F. Discriminant analysis of principal components: A new method for the analysis of genetically structured
populations. _BMC Genet._ 11, 94 (2010). Article PubMed PubMed Central Google Scholar * R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical
Computing, Vienna, Austria. URL: https://www.R-project.org/ (2019). * Trushenski, J. T., Whelan, G. E. & Bowker, J. D. Why keep hatcheries? Weighing the economic cost and value of fish
production for public use and public trust purposes. _Fisheries_ 43, 285–293 (2018). Article Google Scholar * Wacker, S., Larsen, B. M., Jakobsen, P. & Karlsson, S. High levels of
multiple paternity in a spermcast mating freshwater mussel. _Ecol. Evol._ 8, 8126–8134 (2018). Article PubMed PubMed Central Google Scholar * Wacker, S., Larsen, B. M., Jakobsen, P.
& Karlsson, S. Multiple paternity promotes genetic diversity in captive breeding of a freshwater mussel. _Glob. Ecol. Conserv._ 17, e00564. https://doi.org/10.1016/j.gecco.2019.e00564
(2019). Article Google Scholar * Garrison, N. L., Johnson, P. D. & Whelan, N. V. Conservation genomics reveals low genetic diversity and multiple parentage in the threatened freshwater
mussel, _Margaritifera hembeli_. _Conserv. Genet._ https://doi.org/10.1007/s10592-020-01329-8 (2021). Article Google Scholar * Bauer, G. Reproductive strategy of the freshwater pearl
mussel _Margaritifera margaritifera_. _J. Anim. Ecol._ 56, 691–704 (1987). Article Google Scholar * McMurray, S. E. & Roe, K. J. Perspectives on the controlled propagation,
augmentation, and reintroduction of freshwater mussels (Mollusca: Bivalvia: Unionoida). _Freshw. Mollusk Biol. Conserv._ 20, 1–12 (2017). Article Google Scholar * Geist, J. Seven steps
towards improving freshwater conservation. _Aquat. Conserv._ 25, 447–453 (2015). Article Google Scholar Download references ACKNOWLEDGEMENTS We thank Christine Seidel and Ewa Kluka for
technical assistance with genotyping in the laboratory, as well as Clemens Gumpinger, Daniela Csar (Austria), Pierrick Dury, Marie Capoulade, Pierre-Yves Pasco (France), Grégory Motte
(Belgium), Frankie Thielen (Luxembourg), Marco Denic, Michael Lange, Thomas Schiller and Felix Grunicke (Germany) for providing access to their captive-bred mussels and for their support of
this study. Analyses of the German populations were financially jointly supported by the Federal Agency for Nature Conservation with funds of the Federal Ministry for the Environment, Nature
Conservation and Nuclear Safety as well as the Federal Ministry of Education and Research (FKZ 01LC1313C). FUNDING Open Access funding enabled and organized by Projekt DEAL. AUTHOR
INFORMATION AUTHORS AND AFFILIATIONS * Aquatic Systems Biology Unit, Department of Life Science Systems, Technical University of Munich, 85354, Freising, Germany Juergen Geist, Helmut Bayerl
& Bernhard C. Stoeckle * Molecular Zoology Unit, Department of Molecular Life Sciences, Technical University of Munich, 85354, Freising, Germany Helmut Bayerl & Ralph Kuehn *
Department of Fish, Wildlife and Conservation Ecology, New Mexico State University, 2980 South Espina, Box 30003, Las Cruces, NM, 88003-8003, USA Ralph Kuehn Authors * Juergen Geist View
author publications You can also search for this author inPubMed Google Scholar * Helmut Bayerl View author publications You can also search for this author inPubMed Google Scholar *
Bernhard C. Stoeckle View author publications You can also search for this author inPubMed Google Scholar * Ralph Kuehn View author publications You can also search for this author inPubMed
Google Scholar CONTRIBUTIONS The study was conceived by J.G., R.K. and H.B. Sampling was conducted by J.G. and H.B. The molecular genetic analysis was led by H.B., B.C.S. and R.K. The
manuscript was primarily written by J.G., supported by H.B. and continuous input from all other authors. All authors read and approved the final manuscript. CORRESPONDING AUTHOR
Correspondence to Juergen Geist. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE Springer Nature remains
neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION. RIGHTS AND PERMISSIONS OPEN ACCESS This
article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as
you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party
material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the
article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the
copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Geist, J., Bayerl, H.,
Stoeckle, B.C. _et al._ Securing genetic integrity in freshwater pearl mussel propagation and captive breeding. _Sci Rep_ 11, 16019 (2021). https://doi.org/10.1038/s41598-021-95614-2
Download citation * Received: 25 February 2021 * Accepted: 28 July 2021 * Published: 06 August 2021 * DOI: https://doi.org/10.1038/s41598-021-95614-2 SHARE THIS ARTICLE Anyone you share the
following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer
Nature SharedIt content-sharing initiative